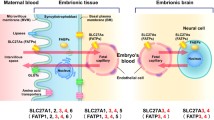
Abstract
The uptake and efflux of solutes across a plasma membrane is controlled by transporters. There are two main superfamilies of transporters, adenosine 5′-triphosphate (ATP) binding cassettes (ABCs) and solute carriers (SLCs). In the brain, SLC transporters are involved in transporting various solutes across the blood–brain barrier, blood–cerebrospinal fluid barrier, astrocytes, neurons, and other brain cell types including oligodendrocytes and microglial cells. SLCs play an important role in maintaining normal brain function. Hence, mutations in the genes that encode SLC transporters can cause a variety of neurological disorders. We identified the following SLC gene variants in 25 patients in our cohort: SLC1A2, SLC2A1, SLC5A1, SLC6A3, SLC6A5, SLC6A8, SLC9A6, SLC9A9, SLC12A6, SLC13A5, SLC16A1, SLC17A5, SLC19A3, SLC25A12, SLC25A15, SLC27A4, SLC45A1, SLC46A1, and SLC52A3. Eight patients harbored pathogenic or likely pathogenic mutations (SLC5A1, SLC9A6, SLC12A6, SLC16A1, SLC19A3, and SLC52A3), and 12 patients were found to have variants of unknown clinical significance (VOUS); these variants occurred in 11 genes (SLC1A2, SLC2A1, SLC6A3, SLC6A5, SLC6A8, SLC9A6, SLC9A9, SLC13A5, SLC25A12, SLC27A4, and SLC45A1). Five patients were excluded as they were carriers. In the remaining 20 patients with SLC gene variants, we identified 16 possible distinct neurological disorders. Based on the clinical presentation, we categorized them into genes causing intellectual delay (ID) or autism spectrum disorder (ASD), those causing epilepsy, those causing vitamin-related disorders, and those causing other neurological diseases. Several variants were detected that indicated possible personalized therapies: SLC2A1 led to dystonia or epilepsy, which can be treated with a ketogenic diet; SLC6A3 led to infantile parkinsonism-dystonia 1, which can be treated with levodopa; SLC6A5 led to hyperekplexia 3, for which unnecessary treatment with antiepileptic drugs should be avoided; SLC6A8 led to creatine deficiency syndrome type 1, which can be treated with creatine monohydrate; SLC16A1 led to monocarboxylate transporter 1 deficiency, which causes seizures that should not be treated with a ketogenic diet; SLC19A3 led to biotin-thiamine-responsive basal ganglia disease, which can be treated with biotin and thiamine; and SLC52A3 led to Brown-Vialetto-Van-Laere syndrome 1, which can be treated with riboflavin. The present study examines the prevalence of SLC gene mutations in our cohort of children with epilepsy and other neurological disorders. It highlights the diverse phenotypes associated with mutations in this large family of SLC transporter proteins, and an opportunity for personalized genomics and personalized therapeutics.





Similar content being viewed by others
References
Abdullah AM, El-Mouzan MI, El Shiekh OK, Al Mazyad A (1996) Congenital glucose-galactose malabsorption in Arab children. J Pediatr Gastroenterol Nutr 23(5):561–564. https://doi.org/10.1097/00005176-199612000-00008 (PMID: 8985845)
Al-Baradie RS, Chaudhary MW, Burshaid D, Mir A (2016) Unusual case of glucose-galactose malabsorption with infantile neuroaxonal dystrophy. Austin J Clin Neurol 3(2):1093 (ISSN: 2381-9154)
Alfadhel M, Tabarki B (2018) SLC19A3 gene defects sorting the phenotype and acronyms: review. Neuropediatrics 49(2):83–92. https://doi.org/10.1055/s-0037-1607191 (Epub 2017 Sep 29. PMID: 28962040)
Al Ghamdi AA, Al Shahwan S, Alshibani F, Melaiki B (2018) Recessive mutations in SLC13A5 result in a loss of citrate transport and cause refractory epilepsy/status epilepticus, developmental delay and progressive microcephaly. Neurology 90 (15 Supplement) P1.306
Al-Khawaga S, AlRayahi J, Khan F, Saraswathi S, Hasnah R, Haris B, Mohammed I, Abdelalim EM, Hussain K (2019) A SLC16A1 mutation in an infant with ketoacidosis and neuroimaging assessment: expanding the clinical spectrum of MCT1 deficiency. Front Pediatr 18(7):299. https://doi.org/10.3389/fped.2019.00299 (PMID: 31380330; PMCID: PMC6657212)
Amara SG, Fontana AC (2002) Excitatory amino acid transporters: keeping up with glutamate. Neurochem Int 41(5):313–318. https://doi.org/10.1016/s0197-0186(02)00018-9 (PMID: 12176072)
Anazi S, Maddirevula S, Faqeih E, Alsedairy H, Alzahrani F, Shamseldin HE, Patel N, Hashem M, Ibrahim N, Abdulwahab F, Ewida N, Alsaif HS, Al Sharif H, Alamoudi W, Kentab A, Bashiri FA, Alnaser M, AlWadei AH, Alfadhel M, Eyaid W, Hashem A, Al Asmari A, Saleh MM, AlSaman A, Alhasan KA, Alsughayir M, Al Shammari M, Mahmoud A, Al-Hassnan ZN, Al-Husain M, Osama Khalil R, Abd El Meguid N, Masri A, Ali R, Ben-Omran T, El Fishway P, Hashish A, Ercan Sencicek A, State M, Alazami AM, Salih MA, Altassan N, Arold ST, Abouelhoda M, Wakil SM, Monies D, Shaheen R, Alkuraya FS (2017) Clinical genomics expands the morbid genome of intellectual disability and offers a high diagnostic yield. Mol Psychiatry 22(4):615–624. https://doi.org/10.1038/mp.2016.113 (Epub 2016 Jul 19. PMID: 27431290)
Aoki Y, Cortese S (2016) Mitochondrial aspartate/glutamate carrier SLC25A12 and autism spectrum disorder: a meta-analysis. Mol Neurobiol 53(3):1579–1588. https://doi.org/10.1007/s12035-015-9116-3 (Epub 2015 Feb 10. PMID: 25663199)
Atabay B, Turker M, Ozer EA, Mahadeo K, Diop-Bove N, Goldman ID (2010) Mutation of the proton-coupled folate transporter gene (PCFT-SLC46A1) in Turkish siblings with hereditary folate malabsorption. Pediatr Hematol Oncol 27(8):614–619. https://doi.org/10.3109/08880018.2010.481705 (PMID: 20795774; PMCID: PMC3885236)
Auburger G, Ratzlaff T, Lunkes A, Nelles HW, Leube B, Binkofski F, Kugel H, Heindel W, Seitz R, Benecke R, Witte OW, Voit T (1996) A gene for autosomal dominant paroxysmal choreoathetosis/spasticity (CSE) maps to the vicinity of a potassium channel gene cluster on chromosome 1p, probably within 2 cM between D1S443 and D1S197. Genomics 31(1):90–94. https://doi.org/10.1006/geno.1996.0013 (PMID: 8808284)
Ayka A, Şehirli AÖ (2020) The role of the SLC transporters protein in the neurodegenerative disorders. Clin Psychopharmacol Neurosci 18(2):174–187. https://doi.org/10.9758/cpn.2020.18.2.174 (PMID:32329299;PMCID:PMC7236796)
Baroni MG, Alcolado JC, Pozzilli P, Cavallo MG, Li SR, Galton DJ (1992) Polymorphisms at the GLUT2 (beta-cell/liver) glucose transporter gene and non-insulin-dependent diabetes mellitus (NIDDM): analysis in affected pedigree members. Clin Genet 41(5):229–234. https://doi.org/10.1111/j.1399-0004.1992.tb03671.x (PMID: 1351429)
Bizzi A, Bugiani M, Salomons GS, Hunneman DH, Moroni I, Estienne M, Danesi U, Jakobs C, Uziel G (2002) X-linked creatine deficiency syndrome: a novel mutation in creatine transporter gene SLC6A8. Ann Neurol 52(2):227–231. https://doi.org/10.1002/ana.10246 (PMID: 12210795)
Bosch AM, Stroek K, Abeling NG, Waterham HR, Ijlst L, Wanders RJ (2012) The Brown-Vialetto-Van Laere and Fazio Londe syndrome revisited: natural history, genetics, treatment and future perspectives. Orphanet J Rare Dis 29(7):83. https://doi.org/10.1186/1750-1172-7-83 (PMID: 23107375; PMCID: PMC3517535)
Camacho JA, Obie C, Biery B, Goodman BK, Hu CA, Almashanu S, Steel G, Casey R, Lambert M, Mitchell GA, Valle D (1999) Hyperornithinaemia-hyperammonaemia-homocitrullinuria syndrome is caused by mutations in a gene encoding a mitochondrial ornithine transporter. Nat Genet 22(2):151–158. https://doi.org/10.1038/9658 (PMID: 10369256)
Christianson AL, Stevenson RE, van der Meyden CH, Pelser J, Theron FW, van Rensburg PL, Chandler M, Schwartz CE (1999) X linked severe mental retardation, craniofacial dysmorphology, epilepsy, ophthalmoplegia, and cerebellar atrophy in a large South African kindred is localised to Xq24-q27. J Med Genet 36(10):759–766. https://doi.org/10.1136/jmg.36.10.759.PMID:10528855;PMCID:PMC1734236
Clark JF, Cecil KM (2015) Diagnostic methods and recommendations for the cerebral creatine deficiency syndromes. Pediatr Res 77(3):398–405. https://doi.org/10.1038/pr.2014.203 (Epub 2014 Dec 18. PMID: 25521922)
Coorg R, Weisenberg JL (2015) Successful treatment of electrographic status epilepticus of sleep with felbamate in a patient with SLC9A6 mutation. Pediatr Neurol 53(6):527–531. https://doi.org/10.1016/j.pediatrneurol.2015.07.007 (Epub 2015 Jul 22. PMID: 26421989)
Corbeel L, Van den Berghe G, Jaeken J, Van Tornout J, Eeckels R (1985) Congenital folate malabsorption. Eur J Pediatr 143(4):284–290. https://doi.org/10.1007/BF00442302 (PMID: 3987728)
De Vivo DC, Trifiletti RR, Jacobson RI, Ronen GM, Behmand RA, Harik SI (1991) Defective glucose transport across the blood-brain barrier as a cause of persistent hypoglycorrhachia, seizures, and developmental delay. N Engl J Med 325(10):703–709. https://doi.org/10.1056/NEJM199109053251006 (PMID: 1714544)
Deleu D, Bamanikar SA, Muirhead D, Louon A (1997) Familial progressive sensorimotor neuropathy with agenesis of the corpus callosum (Andermann syndrome): a clinical, neuroradiological and histopathological study. Eur Neurol 37(2):104–109. https://doi.org/10.1159/000117419 (PMID: 9058066)
Delpire E, Kahle KT (2017) The KCC3 cotransporter as a therapeutic target for peripheral neuropathy. Expert Opin Ther Targets 21(2):113–116. https://doi.org/10.1080/14728222.2017.1275569. Epub 2017 Jan 5. PMID: 28019725
Epi4K Consortium (2016) De Novo mutations in SLC1A2 and CACNA1A are important causes of epileptic encephalopathies. Am J Hum Genet 99(2):287–298. https://doi.org/10.1016/j.ajhg.2016.06.003 (Epub 2016 Jul 28. PMID: 27476654; PMCID: PMC4974067)
Flatt JF, Guizouarn H, Burton NM, Borgese F, Tomlinson RJ, Forsyth RJ, Baldwin SA, Levinson BE, Quittet P, Aguilar-Martinez P, Delaunay J, Stewart GW, Bruce LJ (2011) Stomatin-deficient cryohydrocytosis results from mutations in SLC2A1: a novel form of GLUT1 deficiency syndrome. Blood 118(19):5267–5277. https://doi.org/10.1182/blood-2010-12-326645 (Epub 2011 Jul 26. PMID: 21791420)
Furihata T, Anzai N (2017) Functional expression of organic ion transporters in astrocytes and their potential as a drug target in the treatment of central nervous system diseases. Biol Pharm Bull 40(8):1153–1160. https://doi.org/10.1248/bpb.b17-00076 (PMID: 28768996)
Garcia CK, Li X, Luna J, Francke U (1994) cDNA cloning of the human monocarboxylate transporter 1 and chromosomal localization of the SLC16A1 locus to 1p13.2-p12. Genomics 23(2):500–503. https://doi.org/10.1006/geno.1994.1532 (PMID: 7835905)
Geller J, Kronn D, Jayabose S, Sandoval C (2002) Hereditary folate malabsorption: family report and review of the literature. Medicine (baltimore) 81(1):51–68. https://doi.org/10.1097/00005792-200201000-00004 (PMID: 11807405)
Gerards M, Kamps R, van Oevelen J, Boesten I, Jongen E, de Koning B, Scholte HR, de Angst I, Schoonderwoerd K, Sefiani A, Ratbi I, Coppieters W, Karim L, de Coo R, van den Bosch B, Smeets H (2013) Exome sequencing reveals a novel Moroccan founder mutation in SLC19A3 as a new cause of early-childhood fatal Leigh syndrome. Brain 136(Pt 3):882–890. https://doi.org/10.1093/brain/awt013 (Epub 2013 Feb 18. PMID: 23423671)
Gilfillan GD, Selmer KK, Roxrud I, Smith R, Kyllerman M, Eiklid K, Kroken M, Mattingsdal M, Egeland T, Stenmark H, Sjøholm H, Server A, Samuelsson L, Christianson A, Tarpey P, Whibley A, Stratton MR, Futreal PA, Teague J, Edkins S, Gecz J, Turner G, Raymond FL, Schwartz C, Stevenson RE, Undlien DE, Strømme P (2008) SLC9A6 mutations cause X-linked mental retardation, microcephaly, epilepsy, and ataxia, a phenotype mimicking Angelman syndrome. Am J Hum Genet 82(4):1003–1010. https://doi.org/10.1016/j.ajhg.2008.01.013 (Epub 2008 Mar 13. PMID: 18342287; PMCID: PMC2427207)
Hahn KA, Salomons GS, Tackels-Horne D, Wood TC, Taylor HA, Schroer RJ, Lubs HA, Jakobs C, Olson RL, Holden KR, Stevenson RE, Schwartz CE (2002) X-linked mental retardation with seizures and carrier manifestations is caused by a mutation in the creatine-transporter gene (SLC6A8) located in Xq28. Am J Hum Genet 70(5):1349–1356. https://doi.org/10.1086/340092 (Epub 2002 Mar 15. PMID: 11898126; PMCID: PMC447610)
Hardies K, de Kovel CG, Weckhuysen S, Asselbergh B, Geuens T, Deconinck T, Azmi A, May P, Brilstra E, Becker F, Barisic N, Craiu D, Braun KP, Lal D, Thiele H, Schubert J, Weber Y, van’t Slot R, Nürnberg P, Balling R, Timmerman V, Lerche H, Maudsley S, Helbig I, Suls A, Koeleman BP, De Jonghe P, autosomal recessive working group of the EuroEPINOMICS RES Consortium (2015) Recessive mutations in SLC13A5 result in a loss of citrate transport and cause neonatal epilepsy, developmental delay and teeth hypoplasia. Brain 138(Pt 11):3238–3250. https://doi.org/10.1093/brain/awv263 (Epub 2015 Sep 17. PMID: 26384929)
Hediger MA, Romero MF, Peng JB, Rolfs A, Takanaga H, Bruford EA (2004) The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteinsIntroduction. Pflugers Arch 447(5):465–468. https://doi.org/10.1007/s00424-003-1192-y (Epub 2003 Nov 18. PMID: 14624363)
Howard HC, Mount DB, Rochefort D, Byun N, Dupré N, Lu J, Fan X, Song L, Rivière JB, Prévost C, Horst J, Simonati A, Lemcke B, Welch R, England R, Zhan FQ, Mercado A, Siesser WB, George AL Jr, McDonald MP, Bouchard JP, Mathieu J, Delpire E, Rouleau GA (2002) The K–Cl cotransporter KCC3 is mutant in a severe peripheral neuropathy associated with agenesis of the corpus callosum. Nat Genet 32(3):384–392. https://doi.org/10.1038/ng1002 (Epub 2002 Oct 7. Erratum. In: Nat Genet 2002 Dec; 32(4):681. PMID: 12368912)
Hu C, Tao L, Cao X, Chen L (2020) The solute carrier transporters and the brain: physiological and pharmacological implications. Asian J Pharm Sci 15(2):131–144. https://doi.org/10.1016/j.ajps.2019.09.002 (Epub 2019 Nov 13. PMID: 32373195; PMCID: PMC7193445)
Ibarluzea N, Hoz AB, Villate O, Llano I, Ocio I, Martí I, Guitart M, Gabau E, Andrade F, Gener B, Tejada MI (2020) Targeted next-generation sequencing in patients with suggestive X-linked intellectual disability. Genes (basel) 11(1):51. https://doi.org/10.3390/genes11010051 (PMID: 31906484; PMCID: PMC7017351)
Kanai Y, Clémençon B, Simonin A, Leuenberger M, Lochner M, Weisstanner M, Hediger MA (2013) The SLC1 high-affinity glutamate and neutral amino acid transporter family. Mol Aspects Med 34(2–3):108–120. https://doi.org/10.1016/j.mam.2013.01.001 (PMID: 23506861)
Kavanaugh BC, Warren EB, Baytas O, Schmidt M, Merck D, Buch K, Liu JS, Phornphutkul C, Caruso P, Morrow EM (2019) Longitudinal MRI findings in patient with SLC25A12 pathogenic variants inform disease progression and classification. Am J Med Genet A 179(11):2284–2291. https://doi.org/10.1002/ajmg.a.61322 (Epub 2019 Aug 12. PMID: 31403263; PMCID: PMC6788951)
Klar J, Schweiger M, Zimmerman R, Zechner R, Li H, Törmä H, Vahlquist A, Bouadjar B, Dahl N, Fischer J (2009) Mutations in the fatty acid transport protein 4 gene cause the ichthyosis prematurity syndrome. Am J Hum Genet 85(2):248–253. https://doi.org/10.1016/j.ajhg.2009.06.021 (Epub 2009 Jul 23. PMID: 19631310; PMCID: PMC2725242)
Kondapalli KC, Hack A, Schushan M, Landau M, Ben-Tal N, Rao R (2013) Functional evaluation of autism-associated mutations in NHE9. Nat Commun 4:2510. https://doi.org/10.1038/ncomms3510 (PMID: 24065030; PMCID: PMC3815575)
Kurian MA, Zhen J, Cheng SY, Li Y, Mordekar SR, Jardine P, Morgan NV, Meyer E, Tee L, Pasha S, Wassmer E, Heales SJ, Gissen P, Reith ME, Maher ER (2009) Homozygous loss-of-function mutations in the gene encoding the dopamine transporter are associated with infantile parkinsonism-dystonia. J Clin Invest 119(6):1595–1603. https://doi.org/10.1172/JCI39060 (Epub 2009 May 26. PMID: 19478460; PMCID: PMC2689114)
Kurian MA, Li Y, Zhen J, Meyer E, Hai N, Christen HJ, Hoffmann GF, Jardine P, von Moers A, Mordekar SR, O’Callaghan F, Wassmer E, Wraige E, Dietrich C, Lewis T, Hyland K, Heales S Jr, Sanger T, Gissen P, Assmann BE, Reith ME, Maher ER (2011) Clinical and molecular characterisation of hereditary dopamine transporter deficiency syndrome: an observational cohort and experimental study. Lancet Neurol 10(1):54–62. https://doi.org/10.1016/S1474-4422(10)70269-6 (Epub 2010 Nov 25. PMID: 21112253; PMCID: PMC3002401)
Lepagnol-Bestel AM, Maussion G, Boda B, Cardona A, Iwayama Y, Delezoide AL, Moalic JM, Muller D, Dean B, Yoshikawa T, Gorwood P, Buxbaum JD, Ramoz N, Simonneau M (2008) SLC25A12 expression is associated with neurite outgrowth and is upregulated in the prefrontal cortex of autistic subjects. Mol Psychiatry 13(4):385–397. https://doi.org/10.1038/sj.mp.4002120 (Epub 2008 Jan 8. PMID: 18180767)
Lin L, Yee SW, Kim RB, Giacomini KM (2015) SLC transporters as therapeutic targets: emerging opportunities. Nat Rev Drug Discov 14(8):543–560. https://doi.org/10.1038/nrd4626 (Epub 2015 Jun 26. PMID: 26111766; PMCID: PMC4698371)
Lion-François L, Cheillan D, Pitelet G, Acquaviva-Bourdain C, Bussy G, Cotton F, Guibaud L, Gérard D, Rivier C, Vianey-Saban C, Jakobs C, Salomons GS, des Portes V (2006) High frequency of creatine deficiency syndromes in patients with unexplained mental retardation. Neurology 67(9):1713–1714. https://doi.org/10.1212/01.wnl.0000239153.39710.81 (PMID: 17101918)
Liu QR, López-Corcuera B, Mandiyan S, Nelson H, Nelson N (1993) Cloning and expression of a spinal cord- and brain-specific glycine transporter with novel structural features. J Biol Chem 268(30):22802–22808 (PMID: 8226790)
Liu J, Yang A, Zhang Q, Yang G, Yang W, Lei H, Quan J, Qu F, Wang M, Zhang Z, Yu K (2015) Association between genetic variants in SLC25A12 and risk of autism spectrum disorders: an integrated meta-analysis. Am J Med Genet B Neuropsychiatr Genet 168B(4):236–246. https://doi.org/10.1002/ajmg.b.32304 (Epub 2015 Apr 29. PMID: 25921325)
Liu J, Mo W, Zhang Z, Yu H, Yang A, Qu F, Hu P, Liu Z, Wang S (2017) Single nucleotide polymorphisms in SLC19A1 and SLC25A9 are associated with childhood autism spectrum disorder in the Chinese Han population. J Mol Neurosci 62(2):262–267. https://doi.org/10.1007/s12031-017-0929-6 (Epub 2017 May 24. PMID: 28536923)
Mathieu J, Bédard F, Prévost C, Langevin P (1990) Neuropathie sensitivo-motrice héréditaire avec ou sans agénésie du corps calleux: étude radiologique et clinique de 64 cas [Motor and sensory neuropathies with or without agenesis of the corpus callosum: a radiological study of 64 cases]. Can J Neurol Sci 17(2):103–108 (French. PMID: 2357646)
Mir A, Alhazmi R, Albaradie R (2018) Biotin–thiamine-responsive basal ganglia disease-a treatable metabolic disorder. Pediatr Neurol 87:80–81. https://doi.org/10.1016/j.pediatrneurol.2018.06.006 (Epub 2018 Jul 12. PMID: 30119991)
Moeschler JB, Shevell M, Committee on Genetics (2014) Comprehensive evaluation of the child with intellectual disability or global developmental delays. Pediatrics 134(3):e903–e918. https://doi.org/10.1542/peds.2014-1839 (PMID: 25157020)
Morrow EM, Yoo SY, Flavell SW, Kim TK, Lin Y, Hill RS, Mukaddes NM, Balkhy S, Gascon G, Hashmi A, Al-Saad S, Ware J, Joseph RM, Greenblatt R, Gleason D, Ertelt JA, Apse KA, Bodell A, Partlow JN, Barry B, Yao H, Markianos K, Ferland RJ, Greenberg ME, Walsh CA (2008) Identifying autism loci and genes by tracing recent shared ancestry. Science 321(5886):218–223. https://doi.org/10.1126/science.1157657 (Erratum in: Science. 2010 Dec 24; 330(6012):1746. PMID: 18621663; PMCID: PMC2586171)
Nałęcz KA (2017) Solute carriers in the blood–brain barier: safety in abundance. Neurochem Res 42(3):795–809. https://doi.org/10.1007/s11064-016-2030-x (Epub 2016 Aug 9. PMID: 27503090)
Nicolas-Jilwan M, Medlej R, Sulaiman RA, AlSayed M (2020) The neuroimaging findings of monocarboxylate transporter 1 deficiency. Neuroradiology 62(7):891–894. https://doi.org/10.1007/s00234-020-02435-7 (Epub 2020 Apr 21. PMID: 32318771)
Orlowski J, Grinstein S (2004) Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch 447(5):549–565. https://doi.org/10.1007/s00424-003-1110-3 (Epub 2003 Jul 4. PMID: 12845533)
Ozand PT, Gascon GG, Al Essa M, Joshi S, Al Jishi E, Bakheet S, Al Watban J, Al-Kawi MZ, Dabbagh O (1998) Biotin-responsive basal ganglia disease: a novel entity. Brain 121(Pt 7):1267–1279. https://doi.org/10.1093/brain/121.7.1267 (PMID: 9679779)
Patak J, Hess JL, Zhang-James Y, Glatt SJ, Faraone SV (2017) SLC9A9 co-expression modules in autism-associated brain regions. Autism Res 10(3):414–429. https://doi.org/10.1002/aur.1670 (Epub 2016 Jul 21. PMID: 27439572)
Puffenberger EG, Jinks RN, Sougnez C, Cibulskis K, Willert RA, Achilly NP, Cassidy RP, Fiorentini CJ, Heiken KF, Lawrence JJ, Mahoney MH, Miller CJ, Nair DT, Politi KA, Worcester KN, Setton RA, Dipiazza R, Sherman EA, Eastman JT, Francklyn C, Robey-Bond S, Rider NL, Gabriel S, Morton DH, Strauss KA (2012) Genetic mapping and exome sequencing identify variants associated with five novel diseases. PLoS ONE 7(1):e28936. https://doi.org/10.1371/journal.pone.0028936 (Epub 2012 Jan 17. PMID: 22279524; PMCID: PMC3260153)
Rees MI, Harvey K, Pearce BR, Chung SK, Duguid IC, Thomas P, Beatty S, Graham GE, Armstrong L, Shiang R, Abbott KJ, Zuberi SM, Stephenson JB, Owen MJ, Tijssen MA, van den Maagdenberg AM, Smart TG, Supplisson S, Harvey RJ (2006) Mutations in the gene encoding GlyT2 (SLC6A5) define a presynaptic component of human startle disease. Nat Genet 38(7):801–806. https://doi.org/10.1038/ng1814 (Epub 2006 Jun 4. PMID: 16751771; PMCID: PMC3204411)
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL, ACMG Laboratory Quality Assurance Committee (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17(5):405–424. https://doi.org/10.1038/gim.2015.30 (Epub 2015 Mar 5. PMID: 25741868; PMCID: PMC4544753)
Salomons GS, van Dooren SJ, Verhoeven NM, Cecil KM, Ball WS, Degrauw TJ, Jakobs C (2001) X-linked creatine-transporter gene (SLC6A8) defect: a new creatine-deficiency syndrome. Am J Hum Genet 68(6):1497–1500. https://doi.org/10.1086/320595 (Epub 2001 Apr 20. PMID: 11326334; PMCID: PMC1226136)
Schaller L, Lauschke VM (2019) The genetic landscape of the human solute carrier (SLC) transporter superfamily. Hum Genet 138(11–12):1359–1377. https://doi.org/10.1007/s00439-019-02081-x (Epub 2019 Nov 2. PMID: 31679053; PMCID: PMC6874521)
Shashidharan P, Huntley GW, Meyer T, Morrison JH, Plaitakis A (1994) Neuron-specific human glutamate transporter: molecular cloning, characterization and expression in human brain. Brain Res 662(1–2):245–250. https://doi.org/10.1016/0006-8993(94)90819-2 (PMID: 7859077)
Shin DS, Mahadeo K, Min SH, Diop-Bove N, Clayton P, Zhao R, Goldman ID (2011) Identification of novel mutations in the proton-coupled folate transporter (PCFT-SLC46A1) associated with hereditary folate malabsorption. Mol Genet Metab 103(1):33–37. https://doi.org/10.1016/j.ymgme.2011.01.008 (Epub 2011 Jan 25. PMID: 21333572; PMCID: PMC3081934)
Srour M, Shimokawa N, Hamdan FF, Nassif C, Poulin C, Al Gazali L, Rosenfeld JA, Koibuchi N, Rouleau GA, Al Shamsi A, Michaud JL (2017) Dysfunction of the cerebral glucose transporter SLC45A1 in individuals with intellectual disability and epilepsy. Am J Hum Genet 100(5):824–830. https://doi.org/10.1016/j.ajhg.2017.03.009 (Epub 2017 Apr 20. PMID: 28434495; PMCID: PMC5420346)
Striano P, Weber YG, Toliat MR, Schubert J, Leu C, Chaimana R, Baulac S, Guerrero R, LeGuern E, Lehesjoki AE, Polvi A, Robbiano A, Serratosa JM, Guerrini R, Nürnberg P, Sander T, Zara F, Lerche H, Marini C, EPICURE Consortium (2012) GLUT1 mutations are a rare cause of familial idiopathic generalized epilepsy. Neurology 78(8):557–562. https://doi.org/10.1212/WNL.0b013e318247ff54 (Epub 2012 Jan 25. PMID: 22282645)
Tabarki B, Al-Hashem A, Alfadhel M (2013) Biotin–thiamine-responsive Basal Ganglia disease. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mirzaa G, Amemiya A (eds) GeneReviews® [Internet]. University of Washington, Seattle (PMID: 24260777)
Tabarki B, Alfadhel M, AlShahwan S, Hundallah K, AlShafi S, AlHashem A (2015) Treatment of biotin-responsive basal ganglia disease: open comparative study between the combination of biotin plus thiamine versus thiamine alone. Eur J Paediatr Neurol 19(5):547–552. https://doi.org/10.1016/j.ejpn.2015.05.008 (Epub 2015 Jun 12. PMID: 26095097)
Tessa A, Fiermonte G, Dionisi-Vici C, Paradies E, Baumgartner MR, Chien YH, Loguercio C, de Baulny HO, Nassogne MC, Schiff M, Deodato F, Parenti G, Rutledge SL, Vilaseca MA, Melone MA, Scarano G, Aldamiz-Echevarría L, Besley G, Walter J, Martinez-Hernandez E, Hernandez JM, Pierri CL, Palmieri F, Santorelli FM (2009) Identification of novel mutations in the SLC25A15 gene in hyperornithinemia–hyperammonemia–homocitrullinuria (HHH) syndrome: a clinical, molecular, and functional study. Hum Mutat 30(5):741–748. https://doi.org/10.1002/humu.20930 (PMID: 19242930)
Thomas RH, Chung SK, Wood SE, Cushion TD, Drew CJ, Hammond CL, Vanbellinghen JF, Mullins JG, Rees MI (2013) Genotype-phenotype correlations in hyperekplexia: apnoeas, learning difficulties and speech delay. Brain 136(Pt 10):3085–3095. https://doi.org/10.1093/brain/awt207 (Epub 2013 Sep 11. PMID: 24030948)
Thurm A, Himelstein D, D’Souza P, Rennert O, Jiang S, Olatunji D, Longo N, Pasquali M, Swedo S, Salomons GS, Carrillo N (2016) Creatine transporter deficiency: screening of males with neurodevelopmental disorders and neurocognitive characterization of a case. J Dev Behav Pediatr 37(4):322–326. https://doi.org/10.1097/DBP.0000000000000299 (PMID: 27096572; PMCID: PMC4907372)
Turunen JA, Rehnström K, Kilpinen H, Kuokkanen M, Kempas E, Ylisaukko-Oja T (2008) Mitochondrial aspartate/glutamate carrier SLC25A12 gene is associated with autism. Autism Res 1(3):189–192. https://doi.org/10.1002/aur.25 (PMID: 19360665)
van Hasselt PM, Ferdinandusse S, Monroe GR, Ruiter JP, Turkenburg M, Geerlings MJ, Duran K, Harakalova M, van der Zwaag B, Monavari AA, Okur I, Sharrard MJ, Cleary M, O’Connell N, Walker V, Rubio-Gozalbo ME, de Vries MC, Visser G, Houwen RH, van der Smagt JJ, Verhoeven-Duif NM, Wanders RJ, van Haaften G (2014) Monocarboxylate transporter 1 deficiency and ketone utilization. N Engl J Med 371(20):1900–1907. https://doi.org/10.1056/NEJMoa1407778 (PMID: 25390740)
Weber YG, Storch A, Wuttke TV, Brockmann K, Kempfle J, Maljevic S, Margari L, Kamm C, Schneider SA, Huber SM, Pekrun A, Roebling R, Seebohm G, Koka S, Lang C, Kraft E, Blazevic D, Salvo-Vargas A, Fauler M, Mottaghy FM, Münchau A, Edwards MJ, Presicci A, Margari F, Gasser T, Lang F, Bhatia KP, Lehmann-Horn F, Lerche H (2008) GLUT1 mutations are a cause of paroxysmal exertion-induced dyskinesias and induce hemolytic anemia by a cation leak. J Clin Invest 118(6):2157–2168. https://doi.org/10.1172/JCI34438 (PMID: 18451999; PMCID: PMC2350432)
Weeke LC, Brilstra E, Braun KP, Zonneveld-Huijssoon E, Salomons GS, Koeleman BP, van Gassen KL, van Straaten HL, Craiu D, de Vries LS (2017) Punctate white matter lesions in full-term infants with neonatal seizures associated with SLC13A5 mutations. Eur J Paediatr Neurol 21(2):396–403. https://doi.org/10.1016/j.ejpn.2016.11.002 (Epub 2016 Nov 19. PMID: 27913086)
Wormald R, Viani L, Lynch SA, Green AJ (2010) Sensorineural hearing loss in children. Ir Med J 103(2):51–54 (PMID: 20666057)
Xin B, Wang H (2011) Multiple sequence variations in SLC5A1 gene are associated with glucose-galactose malabsorption in a large cohort of Old Order Amish. Clin Genet 79(1):86–91. https://doi.org/10.1111/j.1399-0004.2010.01440.x (PMID: 20486940)
Yao Y, Yonezawa A, Yoshimatsu H, Masuda S, Katsura T, Inui K (2010) Identification and comparative functional characterization of a new human riboflavin transporter hRFT3 expressed in the brain. J Nutr 140(7):1220–1226. https://doi.org/10.3945/jn.110.122911 (Epub 2010 May 12. PMID: 20463145)
Yıldız Y, Göçmen R, Yaramış A, Coşkun T, Haliloğlu G (2020) Creatine transporter deficiency presenting as autism spectrum disorder. Pediatrics 146(5):e20193460. https://doi.org/10.1542/peds.2019-3460 (PMID: 33093139)
Zanni G, Barresi S, Cohen R, Specchio N, Basel-Vanagaite L, Valente EM, Shuper A, Vigevano F, Bertini E (2014) A novel mutation in the endosomal Na+/H+ exchanger NHE6 (SLC9A6) causes Christianson syndrome with electrical status epilepticus during slow-wave sleep (ESES). Epilepsy Res 108(4):811–815. https://doi.org/10.1016/j.eplepsyres.2014.02.009 (Epub 2014 Feb 19. PMID: 24630051)
Zhang Y, Dong H, Duan L, Yuan G, Liang W, Li Q, Zhang X, Pan Y (2019) SLC1A2 mediates refractory temporal lobe epilepsy with an initial precipitating injury by targeting the glutamatergic synapse pathway. IUBMB Life 71(2):213–222. https://doi.org/10.1002/iub.1956 (Epub 2018 Oct 25. PMID: 30360015)
Zhao R, Min SH, Qiu A, Sakaris A, Goldberg GL, Sandoval C, Malatack JJ, Rosenblatt DS, Goldman ID (2007) The spectrum of mutations in the PCFT gene, coding for an intestinal folate transporter, that are the basis for hereditary folate malabsorption. Blood 110(4):1147–1152. https://doi.org/10.1182/blood-2007-02-077099 (Epub 2007 Apr 19. PMID: 17446347; PMCID: PMC1939898)
Acknowledgements
The authors would like to thank our coordinator, Ms. Mary Joseph and our librarian, Mr. Hassan Ammar for their help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Neither of the authors has any conflict of interest to disclose.
Ethical statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mir, A., Almudhry, M., Alghamdi, F. et al. SLC gene mutations and pediatric neurological disorders: diverse clinical phenotypes in a Saudi Arabian population. Hum Genet 141, 81–99 (2022). https://doi.org/10.1007/s00439-021-02404-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-021-02404-x