Abstract
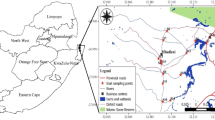
Schistosomiasis is one of the most important parasitic diseases in tropical and subtropical areas. Its prevalence is associated with the distribution of freshwater snails, which are their intermediate hosts. Thus, control of freshwater snails is the solution to reduce the transmission of this disease. This will be achieved by understanding the relationship between the snails and their habitats including natural enemies and associated aquatic plants as well as the factors affecting their distribution. In this study, Maximum Entropy model (MaxEnt) was used for mapping and predicting the possible geographic distribution of Bulinus truncatus snail (the intermediate host of Schistosoma haematobium), Odonata nymph (predatory aquatic insect), and Ceratophyllum demersum (the associated aquatic plant) in Egypt based on topographic and climatic factors. The models of the investigated species were evaluated using the area under receiver operating characteristic curve. The results showed that the potential risk areas were along the banks of the Nile River and its irrigation canals. In addition, the MaxEnt models revealed some similarities in the distribution pattern of the vector, the predator, and the aquatic plant. It is obvious that the predictive distribution range of B. truncatus was affected by altitude, precipitation seasonality, isothermality, and mean temperature of warmest quarter. The presence of B. truncatus decreases with the increase of altitude and precipitation seasonality values. It could be concluded that the MaxEnt model could help introducing a predictive risk map for Schistosoma haematobium prevalence and performing better management strategies for schistosomiasis.
Similar content being viewed by others
References
Abd El-Wakeil KF, Obuid-Allah AH, Mohamed AH, Abd El-Aziz FA (2013) Community structure of molluscs in River Nile and its branches in Assiut governorate. Egypt Egypt J Aqua Res 39:193–198
Arostegui MC, Wood CL, Jones IJ, Chamberlin AJ, Jouanard N, Faye DS, Kuris AM, Riveau G, De Leo GA, Sokolow SH (2019) Potential biological control of schistosomiasis by fishes in the Lower Senegal River Basin. Am J Trop Med Hyg 100:117–126
Badawy RM, El Hoseny I, Talal M (2013) Biodiversity and seasonal fluctuation of aquatic and semiaquatic insects in Rashid stream, Kafr El Zayat (Gharbyia governorate). Egypt Acad J Biol Sci 6:47–66
Bouchard RW (2004) Guide to Aquatic macroinvertebrates of the Upper Midwest. University of Minnesota, St Pau, Water Resources Center
Brown DS (1994) Freshwater Snails of Africa and their Medical Importance. Taylor & Francis, London
Butler RG, DeMaynadier PG (2008) The significance of littoral and shoreline habitat integrity to the conservation of lacustrine damselflies (Odonata). J Insect Conserv 12:23–36
Byeon DH, Jung J, Jung S, Lee W (2017) Prediction of global geographic distribution of Metcalfa pruinosa using CLIMEX. Entomol Res 48:99–107
Ciss M, Biteye B, Fall AG, Fall M, Gahn MCB, Leroux L, Apolloni A (2019) Ecological niche modelling to estimate the distribution of Culicoides, potential vectors of bluetongue virus in Senegal. BMC Ecol 19:1–12
Colley DG, Bustinduy AL, Secor WE, King CH (2014) Human schistosomiasis. Lancet 383:2253–2264
Collins SD, McIntyre NE (2015) Modeling the distribution of odonates: A review. Freshw Sci 34:1144–1158
Du Z, He Y, Wang H, Wang C, Duan Y (2021) Potential geographical distribution and habitat shift of the genus Ammopiptanthus in China under current and future climate change based on the MaxEnt model. J Arid Environ 184:104328–104336
Ejotre I, Makanga B, Nachuha S, Mpezamihigo M (2015) Environmental parameters and Biomphalaria snail distribution along River Kochi, West Nile Region, Uganda. Adv Res 3:244–250
El-Hawey AM, Amer MM, Abdel Rahman AH, El-liary SA, Agina AM (2000) The epidemiology of schistosomiasis in Egypt: Gharbia Governorate. Am J Trop Med Hyg 62:42–48
Elith J, Graham CH, Anderson RP, Dudík M, Ferrier S, Guisan A, Hijmans RJ, Huettmann F, Leathwick JR, Lehmann A, Li J, Lohmann LG, Loiselle BA, Manion G, Moritz C, Nakamura M, Nakazawa Y, Overton J, Peterson AT, Phillips SJ, Richardson KS, Scachetti-Pereira R, Schapire RE, Soberón J, Williams S, Wisz MS, Zimmermann NE (2006) Novel methods improve prediction of species’ distributions from occurrence data. Ecography 29:129–151
Elith J, Phillips SJ, Hastie T, Dudík M, Chee YE, Yates CJ (2011) A statistical explanation of MaxEnt for ecologists. Divers Distrib 17:43–57
Fan KW (2012) Schistosomiasis control and snail elimination in China. Am J Public Health 102:2231–2232
Fenwick A (2019) Egypt’s schistosomiasis control programme in the 1980s prepared the ground for the global elimination of schistosomiasis by 2030. Trans R Soc Trop Med Hyg 113:1–3
Gomes VHF, Ijff SD, Raes N, Amaral IL, Salomão RP, Coelho LDS, Matos FDDA, Castilho CV, Filho DDAL, López DC, Guevara JE, Magnusson WE, Phillips OL, Wittmann F, Carim MDJV, Martins MP, Irume MV, Sabatier D, Molino JF, Bánki OS, Guimarães JRDS, Pitman NCA, Piedade MTF, Mendoza AM, Luize BG, Venticinque EM, Novo EMMDL, Vargas PN, Silva TSF, Manzatto AG, Terborgh J, Reis NFC, Montero JC, Casula KR, Marimon BS, Marimon BH, Coronado ENH, Feldpausch TR, Duque A, Zartman CE, Arboleda NC, Killeen TJ, Mostacedo B, Vasquez R, Schöngart J, Assis RL, Medeiros MB, Simon MF, Andrade A, Laurance WF, Camargo JL, Demarchi LO, Laurance SGW, Farias EDS, Nascimento HEM, Revilla JDC, Quaresma A, Costa FRC, Vieira ICG, Cintra BBL, Castellanos H, Brienen R, Stevenson PR, Feitosa Y, Duivenvoorden JF, Aymard GAC, Mogollón HF, Targhetta N, Comiskey JA, Vicentini A, Lopes A, Damasco G, Dávila N, García-Villacorta R, Levis C, Schietti J, Souza P, Emilio T, Alonso A, Neill D, Dallmeier F, Ferreira LV, Araujo-Murakami A, Praia D, Amaral DD, Carvalho FA, Souza FC, Feeley K, Arroyo L, Pansonato MP, Gribel R, Villa B, Licona JC, Fine PVA, Cerón C, Baraloto C, Jimenez EM, Stropp J, Engel J, Silveira M, Mora MCP, Petronelli P, Maas P, Thomas-Caesar R, Henkel TW, Daly D, Paredes MR, Baker TR, Fuentes A, Peres CA, Chave J, Pena JLM, Dexter KG, Silman MR, Jørgensen PM, Pennington T, Fiore AD, Valverde FC, Phillips JF, Rivas-Torres G, Hildebrand P, Andel TR, Ruschel AR, Prieto A, Rudas A, Hoffman B, Vela CIA, Barbosa EM, Zent EL, Gonzales GPG, Doza HPD, Miranda IPDA, Guillaumet JL, Pinto LFM, Bonates LCDM, Silva N, Gómez RZ, Zent S, Gonzales T, Vos VA, Malhi Y, Oliveira AA, Cano A, Albuquerque BW, Vriesendorp C, Correa DF, Torre EV, Heijden VDG, Ramirez-Angulo H, Ramos JF, Young KR, Rocha M, Nascimento MT, Medina MNU, Tirado M, Wang O, Sierra R, Torres-Lezama A, Mendoza C, Ferreira C, Baider C, Villarroel D, Balslev H, Mesones I, Giraldo LEU, Casas LF, Reategui MAA, Linares-Palomino R, Zagt R, Cárdenas S, Farfan-Rios W, Sampaio AF, Pauletto D, Sandoval EHV, Arevalo FR, Huamantupa-Chuquimaco I, Garcia-Cabrera K, Hernandez L, Gamarra LV, Alexiades MN, Pansini S, Cuenca WP, Milliken W, Ricardo J, Lopez-Gonzalez G, Pos E, Steege H (2018) Species Distribution Modelling: contrasting presence-only models with plot abundance data. Sci Rep 8:1–12
Graham CH, Hijmans RJ (2006) A comparison of methods for mapping species ranges and species richness. Global Ecol Biogeogr 15:578–587
Guo S, Ge X, Zou Y, Zhou Y, Wang T, Zong S (2019) Projecting the potential global distribution of Carpomya vesuviana (Diptera: Tephritidae), considering climate change and irrigation patterns. Forests 10:355–373
Haggerty CJE, Bakhoum S, Civitello DJ, De Leo GA, Jouanard N, Ndione RA, Remais JV, Riveau G, Senghor S, Sokolow SH, Sow S, Wolfe C, Wood CL, Jones I, Chamberlin AJ, Rohr JR (2020) Aquatic macrophytes and macroinvertebrate predators affect densities of snail hosts and local production of schistosome cercariae that cause human schistosomiasis. PLoS Negl Trop Dis 14:e0008417
Hernandez PA, Graham CH, Master LL, Albert DL (2006) The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 29:773–785
Ibrahim AM, Bishai H, Khalil MT (1999) Freshwater mollusks of Egypt. Department of Nature Protection, Egyptian Environmental Affairs Agency, Cairo, Egypt
Kloos H, Souza C, Gazzinelli A, Filho BSS, Temba PC, Bethony J, Page K, Grzywacz C, Lewis F, Minchella D, LoVerde P, Oliveira RC (2001) The distribution of Biomphalaria spp. in different habitats in relation to physical, biological, water contact and cognitive factors in a rural area in Minas Gerais. Brazil Mem Inst Oswaldo Cruz 96:57–66
Klumpp RK, Chu KY (1980) Importance of the aquatic weed Ceratophyllum to transmission of Schistosoma haematobium in the Volta Lake, Ghana. Bull World Health Organ 58:791–798
Manyangadze T, Chimbari MJ, Gebreslasie M, Ceccato P, Mukaratirwa S (2016) Modelling the spatial and seasonal distribution of suitable habitats of schistosomiasis intermediate host snails using Maxent in Ndumo area, KwaZulu-Natal Province. South Africa Tawanda Parasites Vectors 9:572
Mischler P, Kearney M, McCarroll JC, Scholte RGC, Vounatsou P, Malone JB (2012) Environmental and socio-economic risk modelling for Chagas disease in Bolivia. Geospat Health 6:59–66
Moser W, Greter H, Schindler C, Allan F, Ngandolo BNR, Moto DD, Utzinger J, Zinsstag J (2014) The spatial and seasonal distribution of Bulinus truncatus, Bulinus forskalii and Biomphalaria pfeifferi, the intermediate host snails of schistosomiasis, in N’Djamena. Chad Geospat Health 9:109–118
Pearson RG, Raxworthy CJ, Nakamura M, Peterson AT (2007) Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. J Biogeogr 34:102–117
Pedersen UB, Midzi N, Mduluza T, Soko W, Stensgaard A, Vennervald BJ, Mukaratirwa S, Kristensen TK (2014) Modelling spatial distribution of snails transmitting parasitic worms with importance to human and animal health and analysis of distributional changes in relation to climate. Geospat Health 8:335–343
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Model 190:231–259
Phillips SJ, Dudík M (2008) Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography 31:161–175
Pratumchart K, Suwannatraib K, Sereewonga C, Thinkhamropc K, Chaiyosa J, Boonmarsa T, Suwannatraia AT (2019) Ecological Niche Model based on Maximum Entropy for mapping distribution of Bithynia siamensis goniomphalos, first intermediate host snail of Opisthorchis viverrini in Thailand. Acta Trop 193:183–191
Prasopdee S, Kulsantiwong J, Piratae S, Khampoosa P, Thammasiri C, Suwannatrai A, Laha T, Grams R, Loukas A, Tesana S (2015) Temperature dependence of Opisthorchis viverrini infection in first intermediate host snail, Bithynia siamensis goniomphalos. Acta Trop 141:112–117
Saad A, Abu El Einin HM, Abdel-Gaber RA, Mansour SM (2016) Diversity and comparative studies on Bulinus snails collected from two localities in Egypt. J Egypt Soc Parasitol 46:509–518
Sangwan AK, Jackson B, De Glanville W, Pfeiffer DU, Stevens KB (2016) Spatial analysis and identification of environmental risk factors affecting the distribution of Indoplanorbis and Lymnaea species in semi-arid and irrigated areas of Haryana, India. Parasite Epidemiol Control 1:252–262
Segurado P, Araujo MB (2004) An Evaluation of Methods for Modelling Species Distributions. J Biogeogr 31:1555–1568
Suwannatrai A, Suwannatrai K, Haruay S, Piratae S, Thammasiri C, Khampoosa P, Kulsantiwong J, Prasopdee S, Tarbsripair P, Suwanwerakamtorn R, Sukchan S, Boonmars T, Malone JB, Kearney MT, Tesana S (2011) Effect of soil surface salt on the density and distribution of the snail Bithynia siamensis goniomphalos in northeast Thailand. Geospat Health 5:183–190
Tognelli MF, Roig-Junent SA, Marvaldi AE, Flores GE, Lobo JM (2009) An evaluation of methods for modelling distribution of Patagonian insects. Rev Chil Hist Nat 82:347–360
Wei B, Wang R, Hou K, Wang X, Wu W (2018) Predicting the current and future cultivation regions of Carthamus tinctorius L. using MaxEnt model under climate change in China. Glob Ecol Conserv 16:1–11
Wisz MS, Hijmans RJ, Li J, Peterson AT, Graham CH, Guisan A, Elith J, Dudík M, Ferrier S, Huettmann F, Leathwick JR, Lehmann A, Lohmann L, Loiselle BA, Manion G, Moritz C, Nakamura M, Nakazawa Y, Overton JMC, Phillips SJ, Richardson KS, Scachetti-Pereira R, Schapire RE, Sober’on J, Williams SE, Zimmermann NE (2008) Effects of sample size on the performance of species distribution models. Divers Distrib 14:763–773
Yang Y, Cheng W, Wu X, Huang S, Deng Z, Zeng X, Yuan D, Yang Y, Wu Z, Chen Y, Zhou Y, Jiang Q (2018) Prediction of the potential global distribution for Biomphalaria straminea, an intermediate host for Schistosoma mansoni. PLoS 12:1–16
Yi YJ, Zhou Y, Cai YP, Yang W, Li ZW, Zhao X (2018) The influence of climate change on an endangered riparian plant species: the root of riparian Homonoia. Ecol Indicat 92:40–50
Yigezu G, Mandefro B, Mengesha Y, Yewhalaw D, Beyene A, Ahmednur M, Abdie Y, Kloos H, Mereta ST (2018) Habitat suitability modeling for predicting potential habitats of freshwater snail intermediate hosts in Omo-Gibe river basin, Southwest Ethiopia. Ecol Inform 45:70–80
Younes A, El-Sherief H, Gawish F, Mahmoud M (2016) Experimental evaluation of Odonata nymph in the biocontrol of schistosomiasis intermediate hosts. Asian Pac J Trop Biomed 6:995–1000
Younes A, El-Sherief H, Gawish F, Mahmoud M (2017) Biological control of snail hosts transmitting schistosomiasis by the water bug, Sphaerodema urinator. Parasitol Res 116:1257–1264
Zanardi VS, Barbosa LM, Simoes FM, Thiengo SC, Blanton RE, Junior GR, Silva LK, Reis MG (2019) Prevalence of Infection of Biomphalariaglabrata by Schistosoma mansoni and the risk of urban Schistosomiasis mansoni in Salvador, Bahia. Brazil. Rev Soc Bras Med Trop 52:e20190171
Zein-Eddine R, Djuikwo-Teukeng FF, Dar Y, Dreyfuss G, Broeckd FV (2017) Population genetics of the Schistosoma snail host Bulinus truncatus in Egypt. Acta Trop 172:36–43
Zhang J, Yue M, Hu Y, Bergquist R, Su C, Gao F, Cao Z, Zhang Z (2020) Risk prediction of two types of potential snail habitats in Anhui Province of China: Model-based approaches. PLoS Negl Trop Dis 14:e0008178
Author information
Authors and Affiliations
Contributions
AY and MM conceived and designed the research. MM and MH conducted the experiments. AY, MK, and MM analyzed the data and wrote the manuscript. FG and HE made critical reviews and approved the final version. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Xing-Quan ZHU
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mahmoud, M.M., Younes, A.A., El-Sherif, H.A. et al. Predicting the habitat suitability of Schistosoma intermediate host Bulinus truncatus, its predatory aquatic insect Odonata nymph, and the associated aquatic plant Ceratophyllum demersum using MaxEnt. Parasitol Res 121, 205–216 (2022). https://doi.org/10.1007/s00436-021-07392-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-021-07392-5