Abstract
Background
Intestinal schistosomiasis remains a worrying health problem, particularly in western Côte d'Ivoire, despite control efforts. It is therefore necessary to understand all the factors involved in the development of the disease, including biotic and abiotic factors. The aim of this study was to examine the factors that could support the maintenance of the intermediate host and its vectorial capacity in western Côte d'Ivoire.
Methods
Data on river physicochemical, microbiological, and climatic parameters, the presence or absence of snails with Schistosoma mansoni, and human infections were collected between January 2020 and February 2021. Spearman rank correlation tests, Mann–Whitney, analysis of variance (ANOVA), and an appropriate model selection procedure were used to analyze the data.
Results
The overall prevalence of infected snails was 56.05%, with infection reaching 100% in some collection sites and localities. Of 26 sites examined, 25 contained thermophilic coliforms and 22 contained Escherichia coli. Biomphalaria pfeifferi was observed in environments with lower land surface temperature (LST) and higher relative air humidity (RAH), and B. pfeifferi infection predominated in more acidic environments. Thermal coliforms and E. coli preferred higher pH levels. Lower maximum LST (LST_Max) and higher RAH and minimum LST (LST_Min) were favorable to E. coli, and lower LST_Max favored coliforms. The presence of B. pfeifferi was positively influenced by water temperature (T °C), LST_Min, RAH, and precipitation (Pp) (P < 0.05) and negatively influenced by pH, total dissolved solids (TDS), electrical conductivity (EC), LST_Max, and mean land surface temperature (LST). The parameters pH, TDS, EC, LST_Min, LST, and Pp had a positive impact on snail infection, while LST_Max had a negative impact on infection. Only pH had a positive effect on coliform and E. coli abundance. Of the 701 people examined for human schistosomiasis, 73.13% were positive for the point-of-care circulating cathodic antigen (POC-CCA) test and 12.01% for the Kato–Katz (KK) test. A positive correlation was established between human infections and the abundance of Biomphalaria (r2 = 0.879, P = 0.04959).
Conclusions
The results obtained reflect the environmental conditions that are conducive to the maintenance of S. mansoni infection in this part of the country. To combat this infection as effectively as possible, it will be necessary not only to redouble efforts but also to prioritize control according to the level of endemicity at the village level.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Schistosomiasis is one of the most prevalent neglected tropical diseases (NTDs) of water-borne origin and represents the second most important parasitic disease after malaria [1, 2]. Schistosomiasis is endemic in particular in areas where populations are dependent on non-sanitized and contaminated surface water [3]. Humans become infected when they are exposed to fecally contaminated water that serves as a habitat for the parasite’s intermediate host, mainly freshwater snails of the genera Biomphalaria, Bulinus, and Oncomelania [4, 5].
In Côte d'Ivoire, intestinal schistosomiasis is one of the NTDs commonly observed in the western part of the country [6]. It is caused by Schistosoma mansoni, whose known intermediate host is Biomphalaria pfeifferi [7, 8]. As in many bilharzia-affected countries, control of the disease relies on mass administration of the drug praziquantel [2]. However, to date, this strategy has not been successful in mitigating the infection burden, which is expanding in Côte d'Ivoire [9] despite the efforts of the schistosomiasis national control program. Indeed, the persistence of the disease cycle and its spread in the population are both the result of unhygienic conditions, lack and inadequacy of sanitation, cultural habits, and domestic and professional work, exacerbated by the presence of the snail B. pfeifferi in western Côte d'Ivoire [4, 10]. Interruption of schistosomiasis transmission therefore requires the complete integration of the different factors involved in the disease cycle [11]
This process requires an understanding of all the factors involved, including biotic factors (S. mansoni, B. pfeifferi, human) and abiotic factors (climate, vegetation, physicochemical parameters of fresh water, etc.). This understanding will allow us to prioritize the risk of transmission and consequently to adapt the strategy to fight and control this infection in endemic regions.
The overall objective of this study is to contribute to the elimination of schistosomiasis in Côte d'Ivoire. More specifically, we will examine the factors that could support the maintenance of the intermediate host and its vectorial capacity.
Methods
Study area
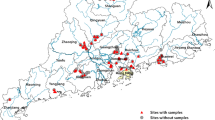
The study was carried out between January 2020 and February 2021 in 17 villages in four departments belonging to two regions in western Côte d'Ivoire that are endemic for intestinal bilharziasis (Fig. 1). Populations from these villages live mainly by agriculture, artisanal fishing, and gold panning. Some of the main environmental problems facing the population are open waste, lack of access to drinking water, inadequate latrines, and poor sanitation [10].
Collection of abiotic parameters: physicochemical and climatic parameters
Data on the physicochemical parameters of water quality were collected at the sampling sites (Fig. 1). Using a multiparameter probe (Hanna Instruments, Møllevången, Sweden), data on the water temperature (T °C), hydrogen potential (pH), total dissolved solids (TDS), and electrical conductivity of the water (EC) were collected at each contact point (CP). Climatic data, including the minimum land surface temperature (LST_Min), maximum land surface temperature (LST_Max), mean land surface temperature (LST), relative air humidity (RAH), and precipitation (Pp), were obtained from the National Aeronautics and Space Administration (NASA) website (https://power.larc.nasa.gov/data-access-viewer/) (consulted 29/07/2022).
Malacological survey
Snails were collected with a dip net and tongs. Collection by dip net was performed by dipping the strainer in water and gently shaking the submerged plants that were difficult to access. Alternatively, the snails were collected by detaching them from the submerged plants and supports with a pair of tongs. The search for snail vectors at each CP was performed by two collectors over a period of 15 min, which could be repeated once in the case of low snail density [12]. Snails were identified by observation [13], and only schistosomiasis vectors for each CP were carefully stored in plastic boxes and transferred to the laboratory for infection testing.
Excretion of cercariae
Snails collected in the field and brought back to the laboratory were individually placed in glass tubes containing 10 ml of borehole water or mineral water [14]. They were then exposed to artificial light for 4 h [4, 15, 16]. This lighting device generates a temperature between 28 and 30 °C capable of triggering the excretion of S. mansoni cercariae [4]. The pillboxes were taken to a binocular stereomicroscope for observation of the cercariae [12, 17], and identification was performed as described previously [13, 17]. This enabled the confirmation of schistosomiasis infection and identification of schistosomiasis transmission foci. Between the excretion stimulation experiments, negative snails were kept in aquaria at room temperature, where they were fed lettuce and fish weed to allow parasites to develop. Positive snails were set aside.
Fecal contamination
Water samples of about 100 ml were collected in Stomacher bags to determine the presence of fecal contamination. Violet red bile lactose (VRBL) agar culture medium [18,19,20] was used to test for the presence of thermophilic coliforms, and RAPID’E.coli medium (Bio-Rad, France) [21, 22] was used to confirm the presence of Escherichia coli. Quantification was performed per colony-forming unit (CFU).
Parasitological and serological survey of intestinal schistosomiasis
Populations from villages with rivers containing snails of the genus Biomphalaria were surveyed for S. mansoni infection. Each individual who agreed to participate in the study was given two sterile polypropylene sampling bottles for sample collection: one for the morning fresh stool sample (about 5 g) and the second for the morning fresh urine (about 40 ml). Parasitological diagnosis was carried out using the Kato–Katz (KK) test [23, 24]. In practice, two thick KK smears were prepared from the stool samples of each participant. The smears were then examined microscopically by two technicians for the detection and quantification of S. mansoni eggs [23, 25]. Eggs were counted and expressed as eggs per gram (EPG). Infections were classified according to the World Health Organization (WHO) recommendations [25].
The serological test consisted of the point-of-care circulating cathodic antigen (POC-CCA) test (ICT Diagnostics, Cape Town, South Africa). This test detects and quantifies the circulating cathodic antigens (CCA) of S. mansoni. First, two drops of fresh urine are inoculated into the well of the cassette, and after 20 min of complete absorption and incubation, a visual (semi-quantitative) result is obtained. This result is based on the absence/presence and intensity of coloration of the test line. It is scored as follows: negative (absence of test line staining), trace, +1, +2, +3 (depending on the intensity of test line staining) [26, 27]. To assess the intensity of infection, the incubated cassette is systematically introduced into the ESEQuant LR3 reader (Qiagen, Germany) designed for quantification of target analytes on lateral flow test strips, including CCA strips. The ESEQuant LR3 reader is pre-calibrated using samples of different concentrations of CCA supplied by the University of Leiden, Netherlands, implemented in Lateral Flow Studio (LF Studio) software. Finally, a correlation is established between the amount of antigen (CCA) and the intensity of the staining on the strip, which is expressed in millivolts for each sample. The millivolt value is therefore considered to be a relative measure of the amount of CCA present in the urine sample [26, 27].
Statistical analysis
Data mining and regression modeling were performed using R software (version 4.2.2).
Each climatic and physicochemical variable was analyzed using a descriptive statistics test. Spearman’s rank correlation test was performed to evaluate the relationships between variables [28, 29]. In addition, the Mann–Whitney U-test was used to test the heterogeneity of climatic and physicochemical characteristics for study sites with and without the biotic factors (total B. pfeifferi collected, infected B. pfeifferi, thermophilic coliforms, and E. coli). A Shapiro–Wilk normality test was performed on the numerical data (shellfish abundance, CCA amount, S. mansoni egg number) to determine the type of distribution. Parametric (Student’s t-test) and non-parametric (Mann–Whitney and Kruskal–Wallis U-test) analysis of variance (ANOVA) tests were used to compare the different groups. All statistical tests were performed at the 0.05 significance level.
A stepwise top-down selection procedure was followed to build the model from the full model involving climatic, physicochemical, and biotic variables. The model with the lowest Akaike information criterion (AIC) value was selected as the optimal model [30]. The goodness of fit of the models was assessed using the relationship between the residuals and the predictor variables, and the normality of the residuals was tested using a quantile–quantile (QQ)-plot (probability plot) [31, 32]. The selected models were considered reliable only if no relationship between the residuals and the predictor variables was visually observed and the residuals were normally distributed.
Ethical considerations and survey form
This study was approved by the National Ethics Committee on Life Sciences and Health (CNESVS) under the approval number N/Ref: 040-19/MSHP/CNESVS-kp. The study also received authorization approval for publication of data from this research (N/Ref: 187-23/MSHPCMU/CNESVS-km). In addition, the study was authorized by the regional, departmental, and village health and education authorities. Informed consent and assent forms were signed by the participants and their parents/guardians. Beforehand, information about the study’s objectives was provided to participants in French or in their own language. In addition, a survey form was completed by each participant to collect sociological data.
Results
Malacological survey
Among the 17 villages visited (Fig. 1), 45 CPs in 35 streams were surveyed during this study (Fig. 1). Of the 45 CPs, 15 contained snails of the species B. pfeifferi, and a total of 306 specimens of this species were collected. Apart from B. pfeifferi, other intermediate hosts of schistosomes were collected during the different surveys. These were Bilunus globosus (n = 51) and Bilunus forskalii (n = 22), but in small quantities. Of 248 B. pfeifferi examined by the cercarial emission test, 139 were infected with S. mansoni, yielding an overall prevalence of 56.05%. The prevalence of S. mansoni infection in snails at the CPs and in the localities ranged from 0 to 100% (Fig. 2). Limnic environments with the presence of B. pfeifferi in the Tahibly, Zéaglo, and Sahibly localities showed 100% infection.
Distribution of prevalence of snails infected with Schistosoma mansoni. A1 Number of collected snails by water–human contact points; A2 prevalence of tested snails infected with S. mansoni by water–human contact points; B1 number of collected snails by village; B2 prevalence of tested snails infected with S. mansoni by village. B.p: total number of Biomphalaria pfeifferi tested; B. p_inf: number of B. pfeifferi infected with S. mansoni. AVI: aviation, BLE: Bledi, BIN: Binaho, DOK: Doke, DOM: Domobly, DOU: Doumbiadougou, FOU: Fouédougou, FRA: Francdougou, GOL: Golou, MON: Mona, PIN: Pinhou, PON: Pona, SAH: Sahibly, TAH: Tahibly, YOY: Yoya, ZEA: Zéaglo, ZIG: Ziglo
Fecal contamination
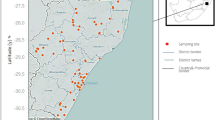
Microbial water quality analysis was performed at 26 CPs from 24 rivers. Of these 26 CPs, nine contained Biomphalaria and 17 had no Biomphalaria. Of the nine CPs containing Biomphalaria, analyses showed that only one CP was not contaminated with E. coli and thermophilic coliforms. For CPs without Biomphalaria, analyses showed that one CP was contaminated with thermophilic coliforms, while three other CPs were contaminated with E. coli. The prevalence of E. coli and thermophilic coliforms across all CPs was 84.61% and 92.31%, respectively. Bacteria counts ranged from 0 to 15 CFU for E. coli and 0 to 165 CFU for thermophilic coliforms.
Spatial distribution of biotic factors in relation to intestinal schistosomiasis
Fifteen potential points of intestinal schistosomiasis transmission (presence of B. pfeifferi) were detected, and these belonged to 13 rivers affiliated to 11 villages (Figs. 2A, 3). The rate of potential transmission points per department was 50%, 60%, 62.5%, and 100% for the departments of Guiglo, Duékoué, Bloléquin, and Toulepleu, respectively. Snails from only 7 out of 11 villages were subjected to the cercarial emission test, and all of these villages had infected snails. These seven villages were spread across all the departments visited. This represents 100% presence of infected snails at the departmental level. Contamination of fecal origin was found in all the localities analyzed (Fig. 3), although contamination was not detected in certain rivers and CPs. The distribution of the risk of infection is therefore high in localities where B. pfeifferi and E. coli are present.
Physicochemical characteristics of limnetic and climatic environments
With regard to descriptive parameters, the T °C recorded at the different CPs was between 21.70 and 32.10 °C. The pH was very acidic at some CPs and varied between 1.51 and 6.90. The climatic variables LST and Pp varied between 24.16 and 24.81 °C and between 00 and 16.97 mm, respectively. The RAH was above average (72–91.25%) (Table 1).
Influence of abiotic parameters on the presence or absence of all B. pfeifferi, infected B. pfeifferi, thermophilic coliforms, and E. coli at contact points
Analyses indicate that LST (P = 0.02) and RAH (P = 0.02) have a significant effect on the presence or absence of B. pfeifferi (Table 2). In addition, variation in pH acidity had a significant effect (P = 0.006) on the presence of infected B. pfeifferi at the CPs studied. The pH also had a significant influence on the presence or absence of thermophilic coliforms (P = 0.0382) and E. coli (P = 0.0031) at the CPs. LST_Min values were significantly different in sites with and without coliforms and E. coli. LST_Max and RAH values were significantly different in sites with and without E. coli (Table 2).
Effects of abiotic characteristics on biotic factors
The results of the negative binomial generalized linear mixed-effects model (NB-GLMM) on abundance and presence/absence data are recorded in Table 3. Abiotic variables had no effect on the abundance of total B. pfeifferi and infected B. pfeifferi. However, the variables T °C, LST_Min, RAH, and Pp positively influenced (P < 0.05) the presence of B. pfeifferi, while pH, TDS, EC, LST_Max, and LST had a negatively influence. With regard to the presence of infected B. pfeifferi, the effects of pH, TDS, EC, LST_Min, LST, and Pp were positive, and that of LST_Max was negative. Only pH had a significant and positive effect on the abundance of both thermophilic coliforms and E. coli.
Human intestinal schistosomiasis
Epidemiological data were collected from 701 people aged 3–65 years in eight villages whose rivers were infested with Biomphalaria species. The POC-CCA test revealed S. mansoni antigens in the urine of 509 people (73.13%) (Table 4). In addition, S. mansoni eggs were observed in the feces of 81 of the 666 people examined (12.16%) using the KK test (Table 4). The prevalence varied significantly (P < 0.05) between 27% and 98.36% for the POC-CCA test and between 0 and 41.28% for the KK across all the villages studied (Table 4). Sahibly village had the highest rates of infected individuals (98.36% for POC-CCA and 41.28% for KK) and the highest CCA and KK means (454.73 ± 318.54 mV and 119.11 ± 272.97 EPG) (Table 4). Males (77.91% for POC-CCA and 13.90% for KK) and adults (96% for POC-CCA and 21% for KK) were significantly the most infected (P < 0.05); however, at the gender level, KK results were not significantly different. Adults had the highest mean CCA and EPG (289.88 ± 293.66 mV and 93.54 ± 262.66 EPG) (Table 4). Males had a higher average CCA (216.88 ± 260.78 mV), but females excreted more S. mansoni eggs (41.11 ± 196.23 EPG). However, the mean number of excreted eggs did not differ significantly between males and females (P > 0.05) (Table 4).
Spatial distribution of intestinal schistosomiasis risk
Figure 4 shows the similarities in the distribution of B. pfeifferi, infected B. pfeifferi, E. coli, and human cases (with CCA and S. mansoni eggs in the stool). Human cases of schistosomiasis were detected in all locations where B. pfeifferi was reported. Although a human diagnosis of schistosomiasis was not made in Tahibly and Doumbiadougou, the risk of infection of the populations living along the rivers surveyed was confirmed by the presence of infected B. pfeifferi. A correlation test between human infections and the abundance of Biomphalaria showed a strong positive correlation between these two variables (r2 = 0.879, t = 3.2, P = 0.04959).
Discussion
Each bilharzia endemic area has biological, ecological, social, and economic characteristics that may differ from one site to another [4]. These characteristics may therefore affect the disease spread process differently.
In order to gain a deeper understanding of the persistence of intestinal bilharzia in the western zone of Côte d’Ivoire, an environmental survey was conducted to characterize and evaluate the influence of physicochemical parameters of water and climatic factors on the presence and proliferation of the intermediate host (B. pfeifferi) and its infection by S. mansoni.
The malacological survey revealed the presence of B. globosus and B. pfeifferi and confirmed the recent presence of B. forskalii, which are intermediate hosts of bilharzia. These results corroborate those of previous studies [7, 33] which observed the same snail species. However, the presence of B. forskalii is very recent. This study is the second to note the presence of this species in this part of Côte d'Ivoire. Bulinus globosus and B. forskalii had not been found to excrete schistosomes [33]. This study also highlights the sanitary danger posed by the presence of B. pfeifferi infected with the S. mansoni parasite of intestinal bilharzia. The prevalence observed varied, and was as high as 100% in certain rivers and localities, notably those of Toulepleu. This prevalence could be the result of environmental conditions favorable to the survival and infectious capacity of the parasite. Studies have reported the impact of environmental factors such as pH and water temperature on the infection of snails [34,35,36]. Indeed, the combination of these two factors has been suggested to impact the physiology and maturation rates of S. mansoni within Biomphalaria [37, 38]. Microbiological assessment of water quality represents a critical tool for preventing water-borne diseases. In the case of bilharziasis, it allows us to highlight contamination of human fecal origin in the habitats of Bulinus and Planorbis. It is in this habitat contaminated by coliforms (aquatic environment) that the contamination of snails and humans essentially occurs [4]. Analyses of the water samples revealed contamination with total coliforms and thermophilic coliforms (E. coli). These coliforms would have reached the ponds through the dumping or drainage of human waste, as these bacteria are adapted to the human intestinal tract. This can be explained by the presence of human waste around some of the ponds surveyed, which therefore indicates a lack of sanitation and toilets. Thus, the observed infection of the snails implies that they would have been in contact with human waste containing S. mansoni eggs.
The temperature range (21–32 °C) recorded during this study is characteristic of the water temperature in tropical zones [7, 39]. The waters in the study area were acidic (pH 1 to 6.9), which could be due to the use of nitrogen fertilizers [40], CO2 from the decomposition of aquatic organic matter [41], and the type of rocks present, especially acidic rocks [42]. The EC and TDS values remained within the range of values for fresh water. They are also similar to those reported by previous studies conducted in the same area [33, 39]. These values are thought to be due to low sodium and chloride ion levels and the size of the catchment [43]. The values recorded for climatic factors remained within the range of values recorded in previous studies [33], which are characteristic of tropical zones.
Regression between physicochemical parameters and the abundance of B. pfeifferi revealed no significant associations. However, significant associations with the presence of snails were detected—in particular, a positive association was found with water temperature. Indeed, high water temperatures favor the increase in aquatic plants, which allows the development and thus the availability of food, as noted in previous studies [44, 45]. A negative association was observed between pH and the presence of B. pfeifferi. This could be explained by the fact that the pH range found in this study would slow the defense system [46], reproduction, and maturation of Biomphalaria [7]. Manyangadze et al. and Rowel et al. [13, 38] found similar results supporting a negative association between pH and Biomphalaria along Lake Albert and Lake Victoria.
TDS and EC demonstrated negative associations with the presence and abundance of snails. Indeed, the presence and number of Biomphalaria decreased with the TDS and EC values of the surveyed water bodies. Other studies have also reported negative associations between Biomphalaria, EC, and TDS [38, 47]. Climatic factors LST and LST_Max) were negatively associated, and Pp, RAH, and LST_Min were positively associated with the presence of Biomphalaria. These results indicate that some of these factors are inhibitors and others are activators of mollusk reproduction [13, 48]. A close relationship exists between climatic factors and water temperature [28]. The high temperatures at the surface of the earth cause a decrease in the surface water temperatures, which leads to a decrease in the snail population [49]. The pH, TDS, EC, LST_Min, LST, high RAH, and high Pp would favor shellfish infection. Indeed, pH and water temperature affect the physiology and maturation rates of the parasite within the vector but also influence the development and survival of the cercarial stages [37]. Similar results were reported by Rowel et al. [38].
Furthermore, Biomphalaria infection was influenced by the abundance of thermophilic coliforms in the surveyed water bodies. The human infection rate recorded in this study indicates the hyperendemicity of S. mansoni infection. This rate is further representative of the level of risk represented by the environmental factors examined in addition to the positive correlation between shellfish and human infection in this study. Thus, the locations with the highest rates of infection and the highest infection intensity were characterized by the simultaneous presence of B. pfeifferi and fecal contamination. The risk of infection is therefore very high in the villages of Sahibly, Zéaglo, and Fouédougou, whereas this risk is not as high in the other localities.
Conclusions
This study revealed that almost all the physicochemical and climatic factors considered in this study (apart from the T °C and RAH of the area for infected Biomphalaria) influenced the Biomphalaria population and its infection. Thermophilic coliforms, meanwhile, were influenced only by pH. This reflects the environmental conditions that are conducive to the maintenance of infection in this part of the country. The high rates of human infection are a direct consequence of this. In order to control schistosomiasis most effectively, greater efforts are needed to clean up the environment, treat people living in the area according to the risk level of the locality, and raise awareness of the disease. The results of this study show that despite control efforts, intestinal schistosomiasis remains endemic or hyperendemic, depending on the locality in the western zone studied. This calls for urgent and assiduous control measures that are more specific and based on the level of endemicity of the disease, taking into account the entire population and not just a subset of it. In addition, the presence of B. forskalii, previously absent from the area, represents a risk of spreading urinary bilharziasis, which was previously endemic to areas outside the western part of the country. This could make the control effort more difficult.
Data availability
All relevant data are contained within the manuscript.
References
Inobaya MT, Olveda RM, Chau TN, Olveda DU, Ross AG. Prevention and control of schistosomiasis: a current perspective. Res Rep Trop Med. 2014;5:65–75.
Webb AJ, Allan F, Kelwick RJR, Beshah FZ, Kinung’hi SM, Templeton MR, et al. Specific nucleic acid ligation for the detection of schistosomes: SNAILS. PLoS Negl Trop Dis. 2022;16:e0010632.
Braun L, Sylivester YD, Zerefa MD, Maru M, Allan F, Zewge F, et al. Chlorination of Schistosoma mansoni cercariae. PLoS Negl Trop Dis. 2020;14:e0008665.
Calasans TAS, Souza GTR, Melo CM, Madi RR, Jeraldo VDLS. Socioenvironmental factors associated with Schistosoma mansoni infection and intermediate hosts in an urban area of northeastern Brazil. PLoS ONE. 2018;13:e0195519.
Ghazy RM, Ellakany WI, Badr MM, Taktak NEM, Elhadad H, Abdo SM, et al. Determinants of Schistosoma mansoni transmission in hotspots at the late stage of elimination in Egypt. Infect Dis Poverty. 2022;11:102.
Adoubryn KD, Kouadio-Yapo CG, Ouhon J, Aka NAD, Bintto F, Assoumou A. Parasitoses intestinales infantiles à Biankouma, région des 18 Montagnes (ouest de la Côte d’Ivoire): étude de l’efficacité et de la tolérance du praziquantel et de l’albendazole. Med Sante Trop. 2012;22:170–6.
Yapi YG, Touré M, Boka OM, Tia E, Boby OAM. Dynamique de transmission des schistosomes par Biomphalaria pfeifferi dans la région de Man. Côte d’Ivoire Bull Soc Pathol Exot. 2014;107:317–22.
Assaré RK, Hürlimann E, Ouattara M, N’Guessan NA, Tian-Bi YNT, Yapi A, et al. Sustaining the control of Schistosoma mansoni in western Côte d’Ivoire: baseline findings before the implementation of a randomized trial. Am J Trop Med Hyg. 2016;94:352–60.
Ahossi NB. Pratiques pédagogiques et lutte contre la bilharziose urinaire en Côte d’Ivoire. Akofena - Revue scientifique des Sciences du Langage, Lettres, Langues & Communication. 2019;79.
Acka CA, Raso G, N’Goran EK, Tschannen AB, Bogoch II, Séraphin E, et al. Parasitic worms: knowledge, attitudes, and practices in western Côte d’Ivoire with implications for integrated control. PLoS Negl Trop Dis. 2010;4:e910.
Elmorshedy H, Bergquist R, Fayed A, Guirguis W, Abdel-Gawwad E, Eissa S, et al. Elimination of schistosomiasis requires multifactorial diagnostics: evidence from high- and low-prevalence areas in the Nile Delta, Egypt. Infect Dis Poverty. 2020;9:31.
Rabone M, Wiethase JH, Allan F, Gouvras AN, Pennance T, Hamidou AA, et al. Freshwater snails of biomedical importance in the Niger River Valley: evidence of temporal and spatial patterns in abundance, distribution and infection with Schistosoma spp. Parasit Vectors. 2019;12:498.
Manyangadze T, Chimbari MJ, Rubaba O, Soko W, Mukaratirwa S. Spatial and seasonal distribution of Bulinus globosus and Biomphalaria pfeifferi in Ingwavuma, uMkhanyakude district, KwaZulu-Natal, South Africa: Implications for schistosomiasis transmission at micro-geographical scale. Parasit Vectors. 2021;14:222.
Sturrock RF, Karamsadkar SJ, Ouma J. Schistosome infection rates in field snails: Schistosoma mansoni in Biomphalaria pfeifferi from Kenya. Ann Trop Med Parasitol. 1979;73:369–75.
Gomes ECDS, da Silva IEP, do Nascimento WRC, Loyo RM, Domingues ALC, Barbosa CS. Urban schistosomiasis: an ecological study describing a new challenge to the control of this neglected tropical disease. Lancet Reg Health Am. 2021;8:100144.
Miranda GS, Rodrigues JGM, Resende SD, Camelo GMA, Silva JKADO, dos Santos JCR, et al. From field to laboratory: isolation, genetic assessment, and parasitological behavior of Schistosoma mansoni obtained from naturally infected wild rodent Holochilus sciureus (Rodentia, Cricetidae), collected in Northeastern Brazil. Parasitol Res. 2023;122:395–411.
Frandsen F, Christensen NO. An introductory guide to the identification of cercariae from African freshwater snails with special reference to cercariae of trematode species of medical and veterinary importance. Acta Trop. 1984;41:181–202.
Bahri M, Hamidian AH, Zhang Y, Yang M. Removal of coliform bacteria from dairy wastewater using graphene-silver nanocomposite. Int J Aquat Biol. 2020;8:447–54.
Sané N, Mbengue M, Laffite A, Stoll S, Poté J, Le Coustumer P. Development of a process for domestic wastewater treatment using Moringa oleifera for pathogens and antibiotic-resistant bacteria inhibition under tropical conditions. Water. 2022;14:2379.
Sidik DA, Issa B, Carole GV, Eric YK, Julien CK, Sabine VN, et al. Assessment of bacterial load in urban wastewater in drainage canals in the cities of Abidjan, Bouake and Yamousoukro, Côte d’Ivoire. South Asian J Res Microbiol. 2023;15:1–11.
Kouamé ND, Benie CKD, Koua A, Koffi AR, Adjéhi D. Growth of enteropathogenic Escherichia coli strains in porridge made from an industrial flour and traditional flours of millet (Pennicetum glaucum) and maize (Zea mays). Int J Appl Microbiol Biotechnol Res. 2021;9:1–9.
Kassem II, Assi A, Osman M, Mann D, Li S, Deng X. Letter to the Editor: First report of the detection of the plasmid-borne colistin resistance gene, mcr-1.26, in multidrug-resistant Escherichia coli isolated from a domesticated Pigeon. Microb Drug Resist. 2022;28:821–3.
World Health Organization. Bench aids for the diagnosis of intestinal parasites, 2nd edition. Geneva: World Health Organization; 2019. https://www.who.int/publications-detail-redirect/9789241515344. Accessed 20 May 2020.
Menezes DL, Santos CTDJ, Oliveira YLDC, Campos VTC, Negrão-Corrêa DA, Geiger SM, et al. Accuracy study of Kato-Katz and Helmintex methods for diagnosis of Schistosomiasis mansoni in a moderate endemicity area in Sergipe, Northeastern Brazil. Diagnostics. 2023;13:527.
World Health Organization. Schistosomiasis. Ending the neglect to attain the sustainable development goals: a road map for neglected tropical diseases 2021–2030. Geneva: World Health Organization; 2020. p. 50.
Mewamba EM, Tiofack AAZ, Kamdem CN, Ngassam RIK, Mbagnia MCT, Nyangiri O, et al. Field assessment in Cameroon of a reader of POC-CCA lateral flow strips for the quantification of Schistosoma mansoni circulating cathodic antigen in urine. PLoS Negl Trop Dis. 2021;15:e0009569.
Nyangiri OA, Edwige SA, Koffi M, Mewamba E, Simo G, Namulondo J, et al. Candidate gene family-based and case–control studies of susceptibility to high Schistosoma mansoni worm burden in African children: a protocol. AAS Open Res. 2021;4:36.
Yang Y, Huang SY, Pei FQ, Chen Y, Jiang QW, Deng ZH, et al. Spatial distribution and habitat suitability of Biomphalaria straminea, intermediate host of Schistosoma mansoni, in Guangdong. China Infect Dis Poverty. 2018;7:109.
Wei T, Simko V, Levy M, Xie Y, Jin Y, Zemla J, Freidank M, Cai J, Protivinsky T. Package corrplot: visualization of a correlation matrix. 2021. p. 11–18.
Portet S. A primer on model selection using the Akaike Information Criterion. Infect Dis Model. 2020;5:111–28.
Zuur AF, Leona EN, Walker NJ, Saveliev AA, Smith GM. Mixed effects models and extensions in ecology with R. New York: Springer Science and Business Media; 2009. p. 101–42.
Olkeba BK, Boets P, Mereta ST, Yeshigeta M, Akessa GM, Ambelu A, et al. Environmental and biotic factors affecting freshwater snail intermediate hosts in the Ethiopian Rift Valley region. Parasit Vectors. 2020;13:292.
Assaré RK, N’Tamon RN, Bellai LG, Koffi JA, Mathieu TBI, Ouattara M, et al. Characteristics of persistent hotspots of Schistosoma mansoni in western Côte d’Ivoire. Parasit Vectors. 2020;13:337.
Opisa S, Odiere MR, Jura WG, Karanja DM, Mwinzi PN. Malacological survey and geographical distribution of vector snails for schistosomiasis within informal settlements of Kisumu City, western Kenya. Parasit Vectors. 2011;4:226.
Woodhall DM, Wiegand RE, Wellman M, Matey E, Abudho B, Karanja DMS, et al. Use of geospatial modeling to predict Schistosoma mansoni prevalence in Nyanza province, Kenya. PLoS ONE. 2013;8:e71635.
Clark NJ, Umulisa I, Ruberanziza E, Owada K, Colley DG, Ortu G, et al. Mapping Schistosoma mansoni endemicity in Rwanda: a critical assessment of geographical disparities arising from circulating cathodic antigen versus Kato-Katz diagnostics. PLoS Negl Trop Dis. 2019;13:e0007723.
Singh KL, Singh DK, Singh VK. Characterization of the molluscicidal activity of Bauhinia variegata and Mimusops elengi plant extracts against the fasciola vector Lymnaea acuminata. Rev Inst Med Trop Sao Paulo. 2012;54:135–40.
Rowel C, Fred B, Betson M, Sousa-Figueiredo JC, Kabatereine NB, Stothard JR. Environmental epidemiology of intestinal schistosomiasis in Uganda: population dynamics of Biomphalaria (Gastropoda: Planorbidae) in Lake Albert and Lake Victoria with observations on natural infections with digenetic trematodes. Biomed Res Int. 2015;2015:717261.
Benoit KK, Séraphin KK, Severin AK, Félix KK, Charles BK, Martin KK. Qualitative assessment and typology of the water resource used for the production of drinking water in Duékoué, western Côte d’Ivoire. J Geosci Environ Prot. 2019;7:212–31.
Cui L, Li J, Gao X, Tian B, Zhang J, Wang X, et al. Human health ambient water quality criteria for 13 heavy metals and health risk assessment in Taihu Lake. Front Environ Sci Eng. 2022;16:41.
Ezenwaji NE, Andong FA, Nnachi IA, Aniede D, Okwuonu ES, Ndefo JC. Factors that influence the toxicity levels of metals in water and soils: a case study of Lake Nike, Enugu, Nigeria. Aquat Sci. 2022;84:18.
Barthen R, Sulonen MLK, Peräniemi S, Jain R, Lakaniemi AM. Removal and recovery of metal ions from acidic multi-metal mine water using waste digested activated sludge as biosorbent. Hydrometallurgy. 2022;207:105770.
Mainali J, Chang H. Environmental and spatial factors affecting surface water quality in a Himalayan watershed, Central Nepal. Environ Sustain Indic. 2021;9:100096.
Utzinger J, Tanner M. Microhabitat preferences of Biomphalaria pfeifferi and Lymnaea natalensis in a natural and a man-made habitat in Southeastern Tanzania. Mem Inst Oswaldo Cruz. 2000;95:287–94.
Bakhoum S, Ba CT, Haggerty CJE, Jouanard N, Riveau G, et al. Risk of human exposure to the intestinal schistosome, Schistosoma mansoni, across seasons along the Senegal River. J Gastroenterol Hepatology Res. 2021;6:038.
Le Clec’h W, Anderson TJC, Chevalier FD. Characterization of hemolymph phenoloxidase activity in two Biomphalaria snail species and impact of Schistosoma mansoni infection. Parasit Vectors. 2016;9:32.
Barbosa VS, Loyo RM, Guimarães RJDPS, Barbosa CS. The geographic information system applied to study schistosomiasis in Pernambuco. Rev Saude Publica. 2017;51:107.
Nwoko OE, Manyangadze T, Chimbari MJ. Spatial distribution, abundance, and infection rates of human schistosome-transmitting snails and related physicochemical parameters in KwaZulu-Natal (KZN) province, South Africa. Heliyon. 2023;9:e12463.
Deka MA. Predictive risk mapping of schistosomiasis in Madagascar using ecological niche modeling and precision mapping. Trop Med Infect Dis. 2022;7:15.
Acknowledgements
We thank the participants who generously donated their specimens and the field workers for their dedication in collecting and processing these specimens. We also acknowledge the TrypanoGEN+ Research group of the H3Africa Consortium https://www.trypanogen.net/.
Funding
This study constitutes output from the TrypanoGEN+ project (Grant Number H3A/18/004) funded by the African Academy of Sciences through the “Alliance for Accelerating Excellence in Science in Africa (AESA)” as part of the H3Africa Consortium (Grant No. H3A/18/004).
Author information
Authors and Affiliations
Consortia
Contributions
EM, MK, HN, and AM designed the project. MK, BA, MN, HN, and ON established the sampling and molecular analysis methodology. TK, IA, FY, and ES carried out the laboratory work. HN and ES carried out the data analysis. ES wrote the original manuscript. MK, BA, and ON revised the manuscript and contributed to the final submission. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the National Ethics Committee for Life Sciences and Health (CNESVS) under approval number N/Ref: 040-19/MSHP/CNESVS-kp. The study also received authorization approval for publication of data from this research (N/Ref: 187-23/MSHPCMU/CNESVS-km). In addition, the study was authorized by the regional, departmental, and village health and education authorities.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sokouri, E.A., Ahouty Ahouty, B., N’Djetchi, M. et al. Impact of environmental factors on Biomphalaria pfeifferi vector capacity leading to human infection by Schistosoma mansoni in two regions of western Côte d'Ivoire. Parasites Vectors 17, 179 (2024). https://doi.org/10.1186/s13071-024-06163-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-024-06163-2