Abstract
Background
Butyrate is a common short-chain fatty acids (SCFA), and it has been demonstrated to regulate the development of breast cancer (BC), while the underlying mechanism is still unreported.
Methods
Gas chromatography was used to measure the amounts of SCFA (acetate, propionate, and butyrate) in the feces. Cell viability was measured by the CCK-8 assay. The wound healing assay demonstrated cell migration, and the transwell assay demonstrated cell invasion. The levels of protein and gene were determined by western blot assay and RT-qPCR assay, respectively.
Results
The levels of SCFA were lower in the faecal samples from BC patients compared to control samples. In cellular experiments, butyrate significantly suppressed the cell viability, migration and invasion of T47D in a dose-dependent manner. In animal experiments, butyrate effectively impeded the growth of BC tumors. Toll like receptor 4 (TLR4) was highly expressed in the tumors from BC patients. Butyrate inhibited the expression of TLR4. In addition, butyrate promoted the expression of cuproptosis-related genes including PDXK (pyridoxal kinase) and SLC25A28 (solute carrier family 25 member 28), which was lowly expressed in BC tumors. Importantly, overexpression of TLR4 can reverses the promotion of butyrate to PDXK and SLC25A28 expression and the prevention of butyrate to the malignant biological behaviors of T47D cells.
Conclusion
In summary, butyrate inhibits the development of BC by facilitating the expression of PDXK and SLC25A28 through inhibition of TLR4. Our investigation first identified a connection among butyrate, TLR4 and cuproptosis-related genes in BC progression. These findings may provide novel target for the treatment of BC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is one of the leading causes of cancer-related death, accounting for 15% of female deaths in 2018 and 25% of cancers in women globally (Xu et al. 2020; Bray et al. 2018). BC is characterized by high morbidity, high mortality, and high recurrence rate. Over the past two decades, the approach to the treatment of BC have evolved, incorporating various modalities such as surgery, chemotherapy, radiation therapy, targeted therapeutics, and other new emerging technologies (Lau 2022; Kerr et al. 2022). Neoadjuvant chemotherapy has shown promise in personalized treatment of BC, but its effectiveness varies among individuals and can lead to drug resistance (Korde and Somerfield 2021; Mao et al. 2021; Sheikh et al. 2022). Therefore, a deeper understanding of the pathogenic factors and molecular mechanisms involved in the pathogenesis of BC is necessary.
Mounting evidence has demonstrated the significant role of gut microbiota in cancer development, acting as a potential preventive or risk factor for cancers through various mechanisms such as affecting immune response, drug resistance and other events (Long et al. 2023; Fernandes et al. 2022). Clinical trial data has revealed significantly differences in gut microbiota proportion in fecal sample of the patients with BC, such as Faecalibacterium prausnitzii, Bifidobacterium, Akkermansia muciniphila and Blautia (Laborda-Illanes et al. 2020; Luu et al. 2017; Fuhrman et al. 2014). The metabolites released by gut microbiota, such as bile acids, short-chain fatty acids (SCFA) and branched-chain fatty acids (BCFAs), can impact the migration, invasion, proliferation and apoptosis of cancer cells (Hou et al. 2022). Commonly, SCFAs like propionate, acetate, and butyrate have been proved to exhibit anti-cancer and anti-inflammation properties (Nakkarach and Foo 2021; González-Bosch et al. 2023). For instance, Park et al. (2021) have indicated that propionate suppressed the proliferation and contributed the apoptosis of BC cells, and impedes the growth of tumor in nude mice by regulating STAT3/MAPK signalling pathway. Butyrate has been reported to have anti-cancer effect in the development of BC, and it can induce apoptosis of BC cells by affecting the formation of reaction oxygen species and impairing mitochondria (Sharma and Tollefsbol 2022; Salimi et al. 2017), but the related mechanism is still unclear. Studies have indicated that toll like receptor 4 (TLR4) could be a downstream target of butyrate, mediating its anti-inflammatory effects (Liu et al. 2022). Activation of TLR4, especially upon binding with lipopolysaccharide, has been linked to promoting the progression of BC by inducing gene expression associated with cancer progression (Afroz et al. 2022).
Cuproptosis is a novel discovered Cu2+-dependent pathway of cell death, which is closely associated with the metabolism of mitochondria. It has been demonstrated that cuproptosis plays an important auxiliary function in the development of cancers, potentially by inhibiting metastasis and impeding cancer development (Tong et al. 2022). For instance, Ferredoxin 1, a key gene involved in cuproptosis, has been identified as a suppressor of clear cell renal cell carcinoma (Xie et al. 2022). The upregulation of cuproptosis-related gene solute carrier family 31 member 1 (SLC31A1) in BC tumors is associated with higher risk and shorter overall survival (Li et al. 2022a). While cuproptosis-related genes show promise as prognostic and therapeutic targets for BC, but the understanding of the functions of cuproptosis in the pathogenesis of BC is inadequate.
In the current study, we focused on the effects of butyrate on the cell viability, migration and invasion of BC cell and the growth of BC tumor. Meantime, we detected the regulation of butyrate to its downstream target TLR4 and the cuproptosis-related genes [pyridoxal kinase (PDXK) and solute carrier family 25 member 28 (SLC25A28)], so as to investigate the role and underlying mechanism of butyrate in BC.
Materials and methods
Analysis of SCFA concentration
Faecal samples were obtained from 12 patients with BC, whose underwent operative treatment in our hospital from June 2020 to October 2021. At the same time, 12 healthy volunteers were recruited for the collection of control faecal samples. The histological classifications of BC patients were listed in Supplementary Table 1. Every participant was provided with sterile plastic containers for the collection of faecal samples. Faecal SCFA (acetate, propionate and butyrate) level was measured by using gas chromatograph (Shimadzu Corporation, Kyoto, Japan) according to previous studies (Birkeland et al. 2020; Ubachs et al. 2021). Frozen faeces (500 mg) were dried in a vacuum dryer, and the level of SCFA was corrected for dry weight. The levels of acetate, propionate, and butyrate were presented as mM/g.
Collection of clinical and cell samples
12 pairs of tumor tissue and adjacent paracancerous tissue were obtained from the female patients with BC. This study was approved by the ethical committee of The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University. Written informed consents were received from all patients. Tumors were stored in − 80 °C until to use. The mRNA and protein levels of TLR4, PDXK and SLC25A28 in tumors and non-tumors were examined.
BC cell lines (T47D and MCF-7) and HEK293 (a human embryonic kidney cell line) were obtained from American Type Culture Collection (ATCC; USA). Cells were cultured in RPMI-1640 medium (Hyclone, Logan, UT, USA) supplemented with 1% penicillin–streptomycin (Invitrogen, Carlsbad, USA) and 10% fetal bovine serum (FBS; Invitrogen) in an incubator with 5% CO2. The ambient temperature during cell culture was 37 °C. T47D cells were treated with 0, 2.0 and 5.0 mM concentration of butyrate (Selleck Chemicals, Houston, USA) for 48 h. The plasmid expressing TLR4 and empty plasmid were purchased from Hanbio Biotechnology Co., Ltd. (Shanghai, China). Cell transfection was carried out by using Lipofectamine 3000 (Invitrogen) according the instruction.
CCK-8 assay
The viability of T47D cells was assessed using a Cell Counting Kit-8 (Solarbio, Beijing, China). T47D cells were seeded into a 96-well plate a density of 2 × 103 cells/well, then either treated with butyrate or transfected with the plasmid expressing TLR4. Then, 10 μL of CCK-8 solution was added to each well, and the cells were incubated for 1 h at 37 °C. The absorbance at 450 nm was measured using a microplate reader (BioTek ELx800, USA).
Wound healing assay
T47D cells were seeded to 6-well plate at a density of 1 × 105 cells/well. After 12 h of cell culture, the suspended cells were washed with 0.01 M PBS and the cell medium was changed. To draw a horizontal line in the bottom of the cell culture plate, a 200 μl pipette tip was held perpendicularly to the plate. The time was written down as 0 h at this point. After 48 h of butyrate treatment and the transfection of plasmid expressing TLR4, cell migration was measured using Image J software.
Transwell assay
Serum-free medium was added into the upper chamber of Transwell (Corning Costar, USA), which was per-coated with matrigel (Matrigel GFR Membrane Matrix, Corning Costar). T47D cells were planted at the upper chamber at a density of 5 × 104 cells/well. The cell culture medium supplemented with 20% FBS was added into the lower chamber. After 48 h of butyrate treatment and cell transfection, the cells attached to the bottom of upper chamber were fixed with 4% paraformaldehyde, and were then stained with crystal violet. The invasive cells were captured by using a microscope and the number of the cells was measured by Image J software.
Western blot assay
The protein levels of TLR4, PDXK and SLC25A28 in tumors or BC cells were determined by western blot assay. Total protein was extracted from the experimental samples by using RIPA lysis buffer (Solarbio). After determining the concentration of protein by using a BCA Protein Assay Kit (Beijing Dingguochangsheng Biotechnology Company, LTD), 25 μg protein per sample was loaded onto 12% SDS-PAGE gels and separated. Then, proteins were transferred onto PVDF membrane (0.45 μm; Millipore, Carrigtwohill, Ireland), which was maintained with 5% non-fat milk for 1 h at room temperature. The membranes were incubated with primary antibodies including TLR4 (1:2000; Cell Signaling Technology, USA), PDXK (1:2000; Cell Signaling Technology) and SLC25A28 (1:2000; Cell Signaling Technology) overnight at 4 °C. After that, the membranes were incubated with HRP-conjugated goat anti-mouse or goat anti-rabbit secondary antibodies (Proteintech, Wuhan, China) for 1 h at room temperature. At last, protein bands were analyzed by using an ECL kit (Solarbio) and Image J software. GAPDH served as internal control.
RT-qPCR assay
TRIzol reagent (Invitrogen) was utilized for extraction of total RNA, which was then reverse transcripted into cDNA by using HiScript III RT SuperMix kit (Vazyme Biotech, Nanjing, China). After that, qPCR was carried out to determine the expression levels of TLR4, PDXK and SLC25A28 mRNAs by using SYBR Green PCR Master Mix (TAKARA, Dalian, China). Gene expression was analyzed using the 2−ΔΔCt method. GAPDH served as the reference gene. Primer sequences used in our study are listed as follows: GAPDH: forward, 5′-TGACTTCAACAGCGACACCCA-3′ and reverse, 5′-C ACCCTGTTGCTGTAGCCAAA-3′; TLR4: forward, 5′-GAGCCGGAAGGTTATTT GGT-3′ and reverse, 5′-CCTCTGCTGTTTGCTCAGGAT-3′; PDXK: forward, 5′-GTG TGGCTGGACTGTACTCT-3′ and reverse, 5′-GCACATAACCTGCTCTGCTC-3′; SLC25A28: forward, 5′-CTGCGTGATGTACCCCATCG-3′ and reverse, 5′-CCTGTT GCTGTGACGTTCAG-3′.
Animal experiments
All animal experiments were supported by the Animal Care Committee of The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University. Eighteen female BALC/c nude mice (weight at 15 ± 2 g, aged 4 ± 1 weeks) were purchased from the Charies river (Beijing, China). Mice were housed in SPF-grade animal room, and all of them have free access to water and food. Mice were subcutaneously implanted with slow-release 17β-estradiol pellets (0.25 mg). The following day, T47D cells were suspended in sterilized saline (1 × 106 cells/100 μl) and injected subcutaneously into the left flank of nude mice. Then, tumor volume was determined every week for 4 weeks according to the method that volume (mm3) = width2 (mm2) × length (mm)/2. The next day after injection of T47D cells, experimental animals were randomly divided into three groups (n = 6 per group): 0 mg/kg group, 100 mg/kg group, and 200 mg/kg group. The mice in 100 mg/kg or 200 mg/kg group were administered intraperitoneally with 100 mg/kg or 200 mg/kg of butyrate (Selleck Chemicals, Houston, TX, USA) weekly for 4 weeks. The mice in 0 mg/kg were administrated with an equal volume of sterilized saline. At last, tumor was carefully removed from mice and frozen in liquid nitrogen immediately.
Statistical analysis
Statistical analysis was accomplished using Graphpad Prism 8.0 (USA), and data were presented as mean ± standard deviation (SD). The difference between independent groups was determined by Student’s t-test, one-way analysis of variance followed by Tukey's post-hoc method was utilized for multiple groups. P < 0.05 was recognized as statistically significantly. * represents P < 0.05, ** means P < 0.01, and *** means P < 0.001.
Results
SCFA level was downregulated but TLR4 expression level was upregulated in BC
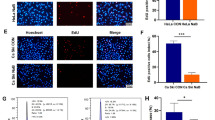
Studies have indicated that SCFA generated by gut microbiota is important for maintaining cell homeostasis and involves in the progression of BC(Mirzaei et al. 2021; Gonçalves et al. 2011). In the current study, the amounts of acetate (Fig. 1A), propionate (Fig. 1B) and butyrate (Fig. 1C) were lower in the feces samples of patients with BC when contrasted to healthy individuals. It has been demonstrated TLR4-mediated signalling pathways are crucial downstream targets of SCFA (Lu et al. 2022a). Here, higher TLR4 mRNA level was found in BC tumor when compared with paracancerous tissue (Fig. 1D). The expression of TLR4 protein was also more in BC tumor than that in paracancerous tissue (Fig. 1E). The expression levels of TLR4 mRNA in T47D and MCF-7 cell lines were higher than that in HEK293 cell line (Fig. 1F). Because of the above findings, we hypothesized that there may be some links between low SCFA level and high TLR4 level in the development of BC.
The levels of acetate, propionate and butyrate are decreased but the expression of TLR4 is increased in BC. A, C Faecal samples were obtained from the patients with BC or healthy individuals, and were used to measure the levels of acetate, propionate and butyrate by gas chromatography. D 12 cases of paracancerous tissue and 12 cases of BC tumor specimen were obtained and used for detecting the expression of TLR4 mRNA. E The expression levels of TLR protein in tumors and paracancerous tissues were determined by western blot assay, and three representative results were shown. F RT-qPCR revealed TLR4 mRNA expression in BC cell lines (T47D and MCF-7) and control cell line HEK293. *P < 0.05, **P < 0.01, and ***P < 0.001. N = 3
Butyrate suppressed the malignant biological behaviors of T47D cells
In order to investigate the influence of SCFA on the malignant biological behaviors of BC cells, butyrate was selected as a representative SCFA in the present study. T47D cells were incubated with 0, 2.0 and 5.0 mM doses of butyrate. Then, cell viability was determined by using CCK-8 assay, and the results showed that butyrate significantly attenuated the cell viability of T47D in a dose-dependent manner (Fig. 2A). The migratory distance of T47D cells was narrowed by butyrate treatment in a dose-dependent manner (Fig. 2B, C). The number of invasive T47D cell was reduced after butyrate treatment at the 2.0 and 5.0 mM doses (Fig. 2D). In summary, butyrate effectively suppresses the cell viability, migration and invasion of T47D cells in a dose-dependent manner.
Butyrate inhibits BC cell viability, migration and invasion. T47D cells were treated with 0, 2.0 and 5.0 mM doses of butyrate. A Cell viability was determined by CCK-8 assay. B Wound healing assay was utilized for analyzing cell migration. (C) Transwell assay was used for detecting cell invasion. *P < 0.05, **P < 0.01, and ***P < 0.001. N = 3
Butyrate inhibited BC tumor growth and promoted cuproptosis protein expression
The results of cellular experiments indicated that butyrate inhibited the malignant biological behaviors of T47D cells. Subsequently, we explored the effects of butyrate on the growth of BC tumors. Tumor mice were accepted with different doses (0, 100, and 200 mg/kg) of butyrate. Butyrate effectively inhibited BC tumor growth in a dose-dependent manner (Fig. 3A, B). The weight of tumors in the mice accepted with 100 and 200 mg/kg butyrate was lower than that in the mice accepted without butyrate (Fig. 3C). The expression of TLR4 mRNA (Fig. 3D) in tumors was downregulated by butyrate treatment in a dose-dependent manner. It has been reported that the serum level of copper in patients with BC is increased when compared with controls (Pavithra et al. 2015). As a mode of cell death caused by Cu accumulation, cuproptosis has been proved to inhibit cancer cell proliferation and tumor growth (Tong et al. 2022). Here, the expression levels of PDXK and SLC25A28 in tumors were detected. The expression of PDXK mRNA (Fig. 3E) and SLC25A28 mRNA (Fig. 3F) in tumors was upregulated after butyrate treatment at 100 and 200 mg/kg doses. When compared with the mice accepted without butyrate, the expression of PDXK and SLC25A28 proteins was increasing in the tumors which from the mice accepted with butyrate (Fig. 3G). Oppositely, the expression of TLR4 protein was downregulated in the tumors isolated from the mice accepted with butyrate (Fig. 3G). In summary, butyrate significantly inhibits the growth of BC tumor and facilitates the expression of PDXK and SLC25A28 in a dose-dependent manner.
Butyrate impedes the growth of BC tumor. Tumor-bearing mice accepted with butyrate treatment at 0, 100 and 200 mg/kg doses. A The images of three tumors from each group were displayed. B Tumor volume was measured every week for 4 weeks. C Tumor weight was determined after four weeks of butyrate treatment. D The expression level of TLR4 mRNA in tumors was determined by RT-qPCR. E RT-qPCR revealed the expression level of PDXK mRNA. F SLC25A28 mRNA level in tumors was also measured by RT-qPCR assay. G The expression levels of TLR4, PDXK and SLC25A28 were measured by western blot assay. *P < 0.05, **P < 0.01, and ***P < 0.001. N = 3
In cellular experiments, butyrate dose-dependently inhibited TLR4 mRNA (Fig. 4A) and TLR4 protein (Fig. 4B) expression in T47D cells. In addition, the expression of PDXK mRNA (Fig. 4C) and SLC25A28 mRNA (Fig. 4D) in T47D cells was increased by butyrate treatment in a dose-dependent manner. The results of western blot demonstrated that PDXK protein and SLC25A18 protein expression was also increased after the treatment of 2.0 and 5.0 mM butyrate (Fig. 4E). Moreover, decreased PDXK protein expression and decreased SLC25A28 protein expression were found in clinical BC tumor specimens (Fig. 4F). Based on the above data, butyrate may inhibit tumor growth through TLR4 and cuproptosis-associated proteins.
Butyrate inhibits TLR4 but facilitates PDXK and SLC25A28 expression. 0, 2.0, and 5.0 mM butyrate maintained with T47D cells for 48 h. A RT-qPCR revealed the expression level of TLR4 mRNA. B Western blot was carried out to analyze the level of TLR4 protein. C, D The mRNA expression levels of cuproptosis-related genes (PDXK and SLC25A28) were determined by RT-qPCR. E Western blot revealed the protein levels of PDXK and SLC25A28 in T47D cell. F 12 cases of paracancerous tissue and 12 cases of BC tumor specimen were obtained and used for detecting the expression of PDXK and SLC25A28. Three representative results were shown. *P < 0.05, **P < 0.01, and ***P < 0.001. N = 3
Butyrate suppressed the malignant biological behaviors of cells via TLR4 signalling
It was demonstrated that TLR4 is an oncogene in BC (Wang et al. 2018). Here, transfection of the plasmid expressing TLR4 led to a significantly increase in TLR4 expression in T47D cells (Fig. 5A). The effects of TLR4 on BC cell viability, migration and invasion, and its effect on SLC25A28 and PDXK expression were verified. Overexpression of TLR4 could enhance T47D cell viability (Supplementary Fig. 1A), migration (Supplementary Fig. 1B) and invasion (Supplementary Fig. 1C). Meantime, overexpression of TLR4 suppressed PDXK and SLC25A28 expression (Supplementary Fig. 1D). In order to ensure whether butyrate suppresses BC tumor growth by targeting TLR4-PDXK/SLC25A28 signalling pathway, T47D cells were transfected with the plasmid expressing TLR4 or empty plasmid, and simultaneously treated with 5.0 mM butyrate. Overexpression of TLR4 reversed butyrate-induced reduction to cell viability (Fig. 5B). The inhibition of butyrate to T47D cell migration (Fig. 5C) and invasion (Fig. 5D) was also rescued by increasing TLR4. Importantly, although the expression of PDXK and SLC25A28 was increased in T47D cells after butyrate treatment, TLR4 overexpression inhibited the expression of the above proteins (Fig. 5E). Overall, butyrate inhibits TLR4 expression and the cell viability, migration and invasion of T47D cells by promoting PDXK and SLC25A28 expression.
Butyrate inhibits TLR4 and the cell viability, migration and invasion of T47D cells by targeting PDXK and SLC25A28. A We transfected the plasmid expressing TLR4 and empty plasmid into T47D cells. RT-qPCR was utilized to detect the level of TLR4 mRNA. Moreover, T47D cells were treated with butyrate alone or in combination with TLR4 overexpression plasmid. B Cell viability was determined by CCK-8 assay. C Cell migration was determined by Wound healing assay. D Cell invasion was assessed by Transwell assay. E Western blot revealed the expression levels of PDXK and SLC25A28. *P < 0.05, **P < 0.01, and ***P < 0.001. N = 3
Discussion
In the current study, we investigate the role of butyrate in development of BC, and the data indicate that butyrate inhibits the cell viability, migration and invasion of T47D and impedes the growth of BC tumors. We further discussed the underlying mechanism of butyrate. TLR4, a known downstream target of butyrate, is highly expressed in BC tumors. Cuproptosis-associated genes (PDXK and SLC25A28) are lowly expressed in BC tumors. In BC cell lines and tumors, butyrate suppresses TLR4 expression, while facilitates PDXK and SLC25A28 expression.
A large number of evidence point to an important association between the risk and progression of cancers and the gut microbiota. Gut microbiota can impact circulating estrogen levels, which is associated with the development of some cancers like ovarian cancer and BC (Łaniewski 2020). In addition, gut microbiota also involves the development of cancer by interacting with the immune system and releasing various metabolites into the blood, including enterolignans, cadaverine, and SCFA (Matson et al. 2021; Li et al. 2019; Chen et al. 2021). SCFAs are characterized by an aliphatic tail ranging from one to six carbons, which common types being acetate, butyrate, isobutyrate, propionate, hexanoate, and valerate. Among these SCFAs, acetate, propionate, and butyrate have been shown to inhibit cancer development (Liu et al. 2021). Abnormal levels of acetate, propionate and butyrate in faecal samples have been associated with inflammatory parameters in patients with pancreatic cancer, lung cancer, ovarian cancer or BC (Ubachs et al. 2021). In the current study, abnormal production of acetate, propionate and butyrate was identified in the faecal samples of BC patients. The levels of acetate, propionate and butyrate are lower in the faecal samples of BC patients than that in control samples. In addition, quite a few recent studies have demonstrated the anti-cancer effects of SCFAs in BC. For instance, propionate and butyrate could inhibit the proliferation of BC cell line MCF7 and arrest cell cycle in the G1 phase (Semaan et al. 2020). However, there is limited research on the role and regulatory mechanisms of butyrate in BC development. Our findings prove that butyrate effectively suppresses the cell viability, migration and invasion of BC cell line T47D, as well as inhibiting experimental tumor growth.
Furthermore, our findings indicate that butyrate inhibits TLR4 expression in a dose-dependent manner, potentially impacting its biological function. TLR4, a member of the pattern recognition receptor TLRs family, plays a crucial role in cancer development by regulating the production of chemokines and pro-inflammatory cytokines (Chen et al. 2018). Wang et al. demonstrated that resistin could accelerate BC development through induction of stemness properties and mesenchymal phenotypes in BC cells by activating TLR4/NF-κB/STAT3 signalling pathway (Wang et al. 2018). Li et al. (2017) indicated that TLR4 is overexpressed in BC tumors, and activated TLR4 promotes the migration of BC cells by regulating AKT/GSK3β/β-catenin signalling pathway. Consistent with the study of Li et al., high expression of TLR4 in BC tumors is also find in our present study. The expression of TLR4 in BC cell lines is higher than that in control cell line. Importantly, our findings show the inhibition of butyrate to TLR4 expression in a dose-dependent manner. TLR4 is a downstream target of butyrate, and this point has been confirmed by several studies (Sun et al. 2021; Seth et al. 2018). Nevertheless, this study is the first to report on the role of TLR4 in the suppression of butyrate on BC.
In recent years, several lines of evidence have implicated that cuproptosis-related genes, including SLC31A1, DLAT, and ATP7B, could serve as potential diagnostic and therapeutic targets for BC (Li et al. 2022a, b). Here, our data demonstrate low expression levels of cuproptosis-related genes PDXK and SLC25A28 in BC tumors. Previous studies have linked PDXK to the progression of pancreatic ductal adenocarcinoma (PDAC). Circular RNA FOXK2 aggravates PDAC tumor growth and metastasis by affecting PDXK and NUF2 expression through interaction with RNA binding protein YBX1 and hnRNPK (Wong et al. 2020). The role of SLC25A28 in cancer development remains unexplored. Importantly, in our present study, butyrate treatment upregulates the expression of PDXK and SLC25A28 in cellular and animal experiments, suggesting a potential inhibitory effect of butyrate on BC development through the regulation of cuproptosis or cuproptosis-related genes. The relationship between TLR4 and PDXK/SLC25A28 has been previously reported. To explore whether the butyrate-induced upregulation of PDXK and SLC25A28 is associated with TLR4, we restored TLR4 expression in butyrate-treated T47D. Proof by facts, overexpression of TLR4 rescues the butyrate-induced upregulation of PDXK and SLC25A28, as well as the inhibition of butyrate to the cell viability, migration and invasion of T47D cells.
Conclusion
Butyrate, a representative SCFA, inhibits the cell viability, migration and invasion of BC cells and the growth of BC tumors by increasing PDXK and SLC25A28 via suppression of TLR4. Our research, for the first time, indicates the correlation among butyrate, TLR4 and cuproptosis-related PDXK/SLC25A28 in the development of BC. Our study may contribute to find novel therapeutic target for BC. Nevertheless, in this research there still exist some limitations. The mechanism that butyrate suppresses TLR4 still not known. SCFAs are thought to exert their functions via protein epigenetic modification like acetylation. Butyrate adopts epigenetic approaches that mediated acetylation of histones to promote the expression of target genes (Rangan and Mondino 2022; Zhu et al. 2023; Lu et al. 2022b). In following studies, the epigenetic regulation of SCFAs to TLR4 and other targets will be one of our research focuses.
Availability of data and materials
All data generated or analyzed are included in this article. Further inquiries can be directed to the corresponding author.
Abbreviations
- BC:
-
Breast cancer
- BCFAs:
-
Branched-chain fatty acids
- PDAC:
-
Pancreatic ductal adenocarcinoma
- PDXK:
-
Pyridoxal kinase
- SCFA:
-
Short-chain fatty acids
- SLC31A1:
-
Solute carrier family 31 member 1
- SLC25A28:
-
Solute carrier family 25 member 28
- TLR4:
-
Toll like receptor 4
References
Afroz R, Tanvir EM, Tania M, Fu J, Kamal MA, Khan MA (2022) LPS/TLR4 pathways in breast cancer: insights into cell signalling. Curr Med Chem 29:2274–2289
Birkeland E, Gharagozlian S, Birkeland KI, Valeur J, Måge I, Rud I et al (2020) Prebiotic effect of inulin-type fructans on faecal microbiota and short-chain fatty acids in type 2 diabetes: a randomised controlled trial. Eur J Nutr 59:3325–3338
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Chen CY, Kao CL, Liu CM (2018) The cancer prevention, anti-inflammatory and anti-oxidation of bioactive phytochemicals targeting the TLR4 signaling pathway. Int J Mol Sci 19:2729
Chen Y, Liu B, Wei Y, Kuang DM (2021) Influence of gut and intratumoral microbiota on the immune microenvironment and anti-cancer therapy. Pharmacol Res 174:105966
Fernandes MR, Aggarwal P, Costa RGF, Cole AM (2022) Targeting the gut microbiota for cancer therapy. Nat Rev Cancer 22:703–722
Fuhrman BJ, Feigelson HS, Flores R, Gail MH, Xu X, Ravel J et al (2014) Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J Clin Endocrinol Metab 99:4632–4640
Gonçalves P, Gregório I, Martel F (2011) The short-chain fatty acid butyrate is a substrate of breast cancer resistance protein. Am J Physiol Cell Physiol 301:C984-994
González-Bosch C, Zunszain PA, Mann GE (2023) Control of redox homeostasis by short-chain fatty acids: implications for the prevention and treatment of breast cancer. Pathogens 12:486
Hou H, Chen D, Zhang K, Zhang W, Liu T, Wang S et al (2022) Gut microbiota-derived short-chain fatty acids and colorectal cancer: ready for clinical translation? Cancer Lett 526:225–235
Kerr AJ, Dodwell D, McGale P, Holt F, Duane F, Mannu G et al (2022) Adjuvant and neoadjuvant breast cancer treatments: a systematic review of their effects on mortality. Cancer Treat Rev 105:102375
Korde LA, Somerfield MR (2021) Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol 39:1485–1505
Laborda-Illanes A, Sanchez-Alcoholado L, Dominguez-Recio ME, Jimenez-Rodriguez B, Lavado R, Comino-Méndez I et al (2020) Breast and gut microbiota action mechanisms in breast cancer pathogenesis and treatment. Cancers (Basel) 12:2465
Łaniewski P, Ilhan ZE, Herbst-Kralovetz MM (2020) The microbiome and gynaecological cancer development, prevention and therapy. Nat Rev Urol 17:232–250
Lau KH, Tan AM, Shi Y (2022) New and emerging targeted therapies for advanced breast cancer. Int J Mol Sci 23:2288
Li J, Yin J, Shen W, Gao R, Liu Y, Chen Y et al (2017) TLR4 promotes breast cancer metastasis via Akt/GSK3β/β-catenin pathway upon LPS stimulation. Anat Rec (hoboken) 300:1219–1229
Li W, Deng Y, Chu Q, Zhang P (2019) Gut microbiome and cancer immunotherapy. Cancer Lett 447:41–47
Li J, Wu F, Li C, Sun S, Feng C, Wu H et al (2022a) The cuproptosis-related signature predicts prognosis and indicates immune microenvironment in breast cancer. Front Genet 13:977322
Li X, Ma Z, Mei L (2022b) Cuproptosis-related gene SLC31A1 is a potential predictor for diagnosis, prognosis and therapeutic response of breast cancer. Am J Cancer Res 12:3561–3580
Liu P, Wang Y, Yang G, Zhang Q, Meng L, Xin Y et al (2021) The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol Res 165:105420
Liu J, Chang G, Huang J, Ma N, Wang Y, Roy AC et al (2022) Sodium butyrate pretreatment mitigates lipopolysaccharide-induced inflammation through the TLR4/NF-κB signaling pathway in bovine embryo trachea cells. Anim Biotechnol 33:1571–1581
Long Y, Tang L, Zhou Y, Zhao S, Zhu H (2023) Causal relationship between gut microbiota and cancers: a two-sample Mendelian randomisation study. BMC Med 21:66
Lu H, Xu X, Fu D, Gu Y, Fan R, Yi H et al (2022a) Butyrate-producing Eubacterium rectale suppresses lymphomagenesis by alleviating the TNF-induced TLR4/MyD88/NF-κB axis. Cell Host Microbe 30:1139-1150.e1137
Lu Y, Yuan X, Wang M, He Z, Li H, Wang J et al (2022b) Gut microbiota influence immunotherapy responses: mechanisms and therapeutic strategies. J Hematol Oncol 15:47
Luu TH, Michel C, Bard JM, Dravet F, Nazih H, Bobin-Dubigeon C (2017) Intestinal proportion of Blautia sp. is associated with clinical stage and histoprognostic grade in patients with early-stage breast cancer. Nutr Cancer 69:267–275
Mao X, Xu J, Wang W, Liang C, Hua J, Liu J et al (2021) Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer 20:131
Matson V, Chervin CS, Gajewski TF (2021) Cancer and the microbiome-influence of the commensal microbiota on cancer, immune responses, and immunotherapy. Gastroenterology 160:600–613
Mirzaei R, Afaghi A, Babakhani S, Sohrabi MR, Hosseini-Fard SR, Babolhavaeji K et al (2021) Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomed Pharmacother 139:111619
Nakkarach A, Foo HL (2021) Anti-cancer and anti-inflammatory effects elicited by short chain fatty acids produced by Escherichia coli isolated from healthy human gut microbiota. Microb Cell Fact 20:36
Park HS, Han JH, Park JW, Lee DH, Jang KW, Lee M et al (2021) Sodium propionate exerts anticancer effect in mice bearing breast cancer cell xenograft by regulating JAK2/STAT3/ROS/p38 MAPK signaling. Acta Pharmacol Sin 42:1311–1323
Pavithra V, Sathisha TG, Kasturi K, Mallika DS, Amos SJ, Ragunatha S (2015) Serum levels of metal ions in female patients with breast cancer. J Clin Diagn Res 9:BC25-c27
Rangan P, Mondino A (2022) Microbial short-chain fatty acids: a strategy to tune adoptive T cell therapy. J Immunother Cancer 10:e004147
Salimi V, Shahsavari Z, Safizadeh B, Hosseini A, Khademian N, Tavakoli-Yaraki M (2017) Sodium butyrate promotes apoptosis in breast cancer cells through reactive oxygen species (ROS) formation and mitochondrial impairment. Lipids Health Dis 16:208
Semaan J, El-Hakim S, Ibrahim JN (2020) Comparative effect of sodium butyrate and sodium propionate on proliferation, cell cycle and apoptosis in human breast cancer cells MCF-7. Breast Cancer 27:696–705
Seth RK, Kimono D, Alhasson F, Sarkar S, Albadrani M, Lasley SK et al (2018) Increased butyrate priming in the gut stalls microbiome associated-gastrointestinal inflammation and hepatic metabolic reprogramming in a mouse model of Gulf War Illness. Toxicol Appl Pharmacol 350:64–77
Sharma M, Tollefsbol TO (2022) Combinatorial epigenetic mechanisms of sulforaphane, genistein and sodium butyrate in breast cancer inhibition. Exp Cell Res 416:113160
Sheikh A, Md S, Kesharwani P (2022) Aptamer grafted nanoparticle as targeted therapeutic tool for the treatment of breast cancer. Biomed Pharmacother 146:112530
Sun Q, Ji YC, Wang ZL, She X, He Y, Ai Q et al (2021) Sodium butyrate alleviates intestinal inflammation in mice with necrotizing enterocolitis. Mediators Inflamm 2021:6259381
Tong X, Tang R, Xiao M, Xu J, Wang W, Zhang B et al (2022) Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J Hematol Oncol 15:174
Ubachs J, Ziemons J, Soons Z, Aarnoutse R, van Dijk DPJ, Penders J et al (2021) Gut microbiota and short-chain fatty acid alterations in cachectic cancer patients. J Cachexia Sarcopenia Muscle 12:2007–2021
Wang CH, Wang PJ, Hsieh YC, Lo S, Lee YC, Chen YC et al (2018) Resistin facilitates breast cancer progression via TLR4-mediated induction of mesenchymal phenotypes and stemness properties. Oncogene 37:589–600
Wong CH, Lou UK, Li Y (2020) CircFOXK2 promotes growth and metastasis of pancreatic ductal adenocarcinoma by complexing with RNA-binding proteins and sponging MiR-942. Cancer Res 80:2138–2149
Xie M, Cheng B, Yu S, He Y, Cao Y, Zhou T et al (2022) Cuproptosis-related MiR-21-5p/FDX1 axis in clear cell renal cell carcinoma and its potential impact on tumor microenvironment. Cells 12:73
Xu X, Zhang M, Xu F, Jiang S (2020) Wnt signaling in breast cancer: biological mechanisms, challenges and opportunities. Mol Cancer 19:165
Zhu X, Li K, Liu G, Wu R, Zhang Y, Wang S et al (2023) Microbial metabolite butyrate promotes anti-PD-1 antitumor efficacy by modulating T cell receptor signaling of cytotoxic CD8 T cell. Gut Microbes 15:2249143
Acknowledgements
We would like to give our sincere gratitude to the reviewers for their constructive comments.
Funding
None.
Author information
Authors and Affiliations
Contributions
Liming Zhang and conceived and designed the research, Shan Huang and Ying Yuan performed the research and acquired the data, Liming Zhang analyzed and interpreted the data. All authors were involved in drafting and revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
This study was approved by the ethics committee of The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University. We obtained the written informed consents from all patients. All animal experiments were supported by the Animal Care Committee of The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
432_2024_5807_MOESM1_ESM.docx
Supplementary Fig. 1. TLR4 facilitated BC cell viability, migration and invasion, but inhibited PDXK and SLC25A28 expression in T47D cells. T47D cells were transfected with the vector expressing TLR4 or empty vector. Subsequently, (A) cell viability was determined by CCK-8 assay, (B) cell migration was detected by Wound healing assay, and (C) cell invasion was assessed by Transwell assay. (D) The expression of PDXK and SLC25A28 was detected using western blot. *P < 0.05, **P < 0.01, and ***P < 0.001. N = 3 (DOCX 15 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, L., Huang, S. & Yuan, Y. Butyrate inhibits the malignant biological behaviors of breast cancer cells by facilitating cuproptosis-associated gene expression. J Cancer Res Clin Oncol 150, 287 (2024). https://doi.org/10.1007/s00432-024-05807-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00432-024-05807-1