Abstract
Purpose
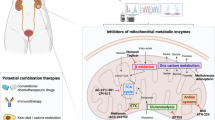
The incidence and mortality of lung cancer are continuously rising in recent years. Mitochondrial energy metabolism malfunction is found to be crucial in cancer proliferation and bioenergetic reprogramming, especially for lung cancer. In this study, we attempted to use mitochondrial-targeted drug therapy to change the energy metabolism pattern of cancer cells to inhibit the development of lung cancer, and investigated its mechanism of action and key targets through multi-omics studies.
Methods
In this study, we established the in vivo tumor mouse mode, treated mice with multiple mitochondrial-targeted drug combinations and DDP, severally. Then, we investigated the differences between the 7-drug group with the control group and the DDP treatment group by transcriptomics, proteomics and metabolomics to find the therapeutic targets.
Results
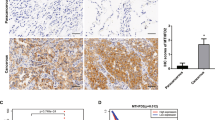
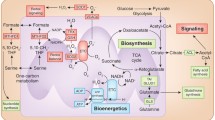
We found that mitochondria-targeting drug cocktail therapy, especially the 7-drug regimen, effectively improved mitochondrial metabolism, changed energy supply patterns in lung cancer cells, significantly increased NK cells in tumor tissues, and decreased tumor markers in plasma. Multi-omics analysis informed that the combination of 7-drug could up-regulate mitochondrial oxidative phosphorylation, ATP synthesis and autophagy related genes, and down-regulate proliferation and immune-related genes compared with the control group. By further mapping the protein interaction network, we identified a key target for 7-drug therapy to reverse tumor metabolic reprogramming and validated it in metabolomics.
Conclusions
Mitochondrial-targeted drug cocktail therapy can effectively inhibit the occurrence and development of tumors, through the reprogramming of energy metabolism and the increase in immune cells in tumor tissues. Thus, we provide a novel approach for the treatment of lung cancer and present evidence-based clues for the combined use of targeted mitochondrial drugs.







Similar content being viewed by others
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, and further inquiries can be directed to the corresponding author/s.
Abbreviations
- OXPHOS:
-
Oxidative phosphorylation
- T3:
-
Thyroxine
- MLT:
-
Melatonin
- Pre:
-
Pregnenolone
- GSH:
-
Glutathione
- LA:
-
Lipoic acid
- Q10:
-
Coenzyme Q10
- Se:
-
Selenium
- Bif:
-
Bifidobacteria
- LLC:
-
Lewis lung cancer
- DDP:
-
Cisplatin
- ROS:
-
Reactive oxygen species
- ETC:
-
Electron transport chain
- TME:
-
Tumor microenvironment
References
Ahn CS, Metallo CM (2015) Mitochondria as biosynthetic factories for cancer proliferation. Cancer Metab 3 (1):1. https://doi.org/10.1186/s40170-015-0128-2
Anderson RG, Ghiraldeli LP, Pardee TS (2018) Mitochondria in cancer metabolism, an organelle whose time has come? Biochim Biophys Acta Rev Cancer 1870 (1):96–102. https://doi.org/10.1016/j.bbcan.2018.05.005
Ashton TM, McKenna WG, Kunz-Schughart LA, Higgins GS (2018) Oxidative phosphorylation as an emerging target in cancer therapy. Clin Cancer Res 24 (11):2482–2490. https://doi.org/10.1158/1078-0432.Ccr-17-3070
Bader JE, Voss K, Rathmell JC (2020) Targeting metabolism to improve the tumor microenvironment for cancer immunotherapy. Mol Cell 78 (6):1019–1033. https://doi.org/10.1016/j.molcel.2020.05.034
Badgeley A, Anwar H, Modi K, Murphy P, Lakshmikuttyamma A (2021) Effect of probiotics and gut microbiota on anti-cancer drugs: Mechanistic perspectives. Biochim Biophys Acta Rev Cancer 1875 (1):188494. https://doi.org/10.1016/j.bbcan.2020.188494
Bartolini D, Torquato P, Piroddi M, Galli F (2019) Targeting glutathione S-transferase P and its interactome with selenium compounds in cancer therapy. Biochim Biophys Acta Gen Subj 1863 (1):130–143. https://doi.org/10.1016/j.bbagen.2018.09.023
Bhattacharya B, Mohd Omar MF, Soong R (2016) The Warburg effect and drug resistance. Br J Pharmacol 173 (6):970–979. https://doi.org/10.1111/bph.13422
Bock FJ, Tait SWG (2020) Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol 21 (2):85–100. https://doi.org/10.1038/s41580-019-0173-8
Borrelli A, Bonelli P, Tuccillo FM, Goldfine ID, Evans JL, Buonaguro FM, Mancini A (2018) Role of gut microbiota and oxidative stress in the progression of non-alcoholic fatty liver disease to hepatocarcinoma: current and innovative therapeutic approaches. Redox Biol 15:467–479. https://doi.org/10.1016/j.redox.2018.01.009
Burke PJ (2017) Mitochondria, bioenergetics and apoptosis in cancer. Trends Cancer 3 (12):857–870. https://doi.org/10.1016/j.trecan.2017.10.006
Cao Y (2019) Adipocyte and lipid metabolism in cancer drug resistance. J Clin Invest 129 (8):3006–3017. https://doi.org/10.1172/jci127201
Chen X, Hao B, Li D, Reiter RJ, Bai Y, Abay B, Chen G, Lin S, Zheng T, Ren Y, Xu X, Li M, Fan L (2021) Melatonin inhibits lung cancer development by reversing the Warburg effect via stimulating the SIRT3/PDH axis. J Pineal Res 71 (2):e12755. https://doi.org/10.1111/jpi.12755
Chi HC, Chen SL, Lin SL, Tsai CY, Chuang WY, Lin YH, Huang YH, Tsai MM, Yeh CT, Lin KH (2017) Thyroid hormone protects hepatocytes from HBx-induced carcinogenesis by enhancing mitochondrial turnover. Oncogene 36 (37):5274–5284. https://doi.org/10.1038/onc.2017.136
Dong L, Gopalan V, Holland O, Neuzil J (2020) Mitocans revisited: mitochondrial targeting as efficient anti-cancer therapy. Int J Mol Sci. https://doi.org/10.3390/ijms21217941
Ferro F, Servais S, Besson P, Roger S, Dumas JF, Brisson L (2020) Autophagy and mitophagy in cancer metabolic remodelling. Semin Cell Dev Biol 98:129–138. https://doi.org/10.1016/j.semcdb.2019.05.029
Genovese I, Vezzani B, Danese A, Modesti L, Vitto VAM, Corazzi V, Pelucchi S, Pinton P, Giorgi C (2020) Mitochondria as the decision makers for cancer cell fate: from signaling pathways to therapeutic strategies. Cell Calcium 92:102308. https://doi.org/10.1016/j.ceca.2020.102308
Gentric G, Kieffer Y, Mieulet V, Goundiam O, Bonneau C, Nemati F, Hurbain I, Raposo G, Popova T, Stern MH, Lallemand-Breitenbach V, Müller S, Cañeque T, Rodriguez R, Vincent-Salomon A, de Thé H, Rossignol R, Mechta-Grigoriou F (2019) PML-regulated mitochondrial metabolism enhances chemosensitivity in human ovarian cancers. Cell Metab 29 (1):156-173.e110. https://doi.org/10.1016/j.cmet.2018.09.002
Gottesman MM (2002) Mechanisms of cancer drug resistance. Annu Rev Med 53:615–627. https://doi.org/10.1146/annurev.med.53.082901.103929
Halbrook CJ, Lyssiotis CA (2017) Employing metabolism to improve the diagnosis and treatment of pancreatic cancer. Cancer Cell 31 (1):5–19. https://doi.org/10.1016/j.ccell.2016.12.006
Hargreaves IP (2014) Coenzyme Q10 as a therapy for mitochondrial disease. Int J Biochem Cell Biol 49:105–111. https://doi.org/10.1016/j.biocel.2014.01.020
Hatfull GF, Dedrick RM, Schooley RT (2022) Phage Therapy for Antibiotic-Resistant Bacterial Infections. Annu Rev Med 73:197–211. https://doi.org/10.1146/annurev-med-080219-122208
Hinshaw DC, Shevde LA (2019) The tumor microenvironment innately modulates cancer progression. Cancer Res 79 (18):4557–4566. https://doi.org/10.1158/0008-5472.Can-18-3962
Lee SH, Cho SY, Yoon Y, Park C, Sohn J, Jeong JJ, Jeon BN, Jang M, An C, Lee S, Kim YY, Kim G, Kim S, Kim Y, Lee GB, Lee EJ, Kim SG, Kim HS, Kim Y, Kim H, Yang HS, Kim S, Kim S, Chung H, Moon MH, Nam MH, Kwon JY, Won S, Park JS, Weinstock GM, Lee C, Yoon KW, Park H (2021) Bifidobacterium bifidum strains synergize with immune checkpoint inhibitors to reduce tumour burden in mice. Nat Microbiol 6 (3):277–288. https://doi.org/10.1038/s41564-020-00831-6
Li M, Hao B, Zhang M, Reiter RJ, Lin S, Zheng T, Chen X, Ren Y, Yue L, Abay B, Chen G, Xu X, Shi Y, Fan L (2021) Melatonin enhances radiofrequency-induced NK antitumor immunity, causing cancer metabolism reprogramming and inhibition of multiple pulmonary tumor development. Signal Transduct Target Ther 6 (1):330. https://doi.org/10.1038/s41392-021-00745-7
Liberti MV, Locasale JW (2016) The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci 41 (3):211–218. https://doi.org/10.1016/j.tibs.2015.12.001
Liu N, Lewis C, Zheng W, Fu ZQ (2020) Phage cocktail therapy: multiple ways to suppress pathogenicity. Trends Plant Sci 25 (4):315–317. https://doi.org/10.1016/j.tplants.2020.01.013
Liu X, Li Y, Wang K, Chen Y, Shi M, Zhang X, Pan W, Li N, Tang B (2021) GSH-responsive nanoprodrug to inhibit glycolysis and alleviate immunosuppression for cancer therapy. Nano Lett 21 (18):7862–7869. https://doi.org/10.1021/acs.nanolett.1c03089
Lopez J, Tait SW (2015) Mitochondrial apoptosis: killing cancer using the enemy within. Br J Cancer 112 (6):957–962. https://doi.org/10.1038/bjc.2015.85
Luengo A, Gui DY, Vander Heiden MG (2017) Targeting metabolism for cancer therapy. Cell Chem Biol 24 (9):1161–1180. https://doi.org/10.1016/j.chembiol.2017.08.028
Miller WL (2013) Steroid hormone synthesis in mitochondria. Mol Cell Endocrinol 379 (1–2):62–73. https://doi.org/10.1016/j.mce.2013.04.014
Papadopoulos V, Guarneri P, Kreuger KE, Guidotti A, Costa E (1992) Pregnenolone biosynthesis in C6–2B glioma cell mitochondria: regulation by a mitochondrial diazepam binding inhibitor receptor. Proc Natl Acad Sci USA 89 (11):5113–5117. https://doi.org/10.1073/pnas.89.11.5113
Pinto MC, Kihara AH, Goulart VA, Tonelli FM, Gomes KN, Ulrich H, Resende RR (2015) Calcium signaling and cell proliferation. Cell Signal 27 (11):2139–2149. https://doi.org/10.1016/j.cellsig.2015.08.006
Prasad S, Gupta SC, Tyagi AK (2017) Reactive oxygen species (ROS) and cancer: role of antioxidative nutraceuticals. Cancer Lett 387:95–105. https://doi.org/10.1016/j.canlet.2016.03.042
Quintana-Cabrera R, Bolaños JP (2013) Glutathione and γ-glutamylcysteine in the antioxidant and survival functions of mitochondria. Biochem Soc Trans 41 (1):106–110. https://doi.org/10.1042/bst20120252
Schulze A, Harris AL (2012) How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature 491 (7424):364–373. https://doi.org/10.1038/nature11706
Shen C, Ren Y, Zhang X, Xia Q, Li M, Wang C, Fan L (2019) Immunomodulatory effects of intestinal lung axis microecology and other factors on the prognosis of advanced non-small cell lung cancer. Transl Cancer Res 8 (5):2205–2210. https://doi.org/10.21037/tcr.2019.09.32
Shi J, Chen Q, Xu M, Xia Q, Zheng T, Teng J, Li M, Fan L (2019) Recent updates and future perspectives about amygdalin as a potential anticancer agent: a review. Cancer Med 8 (6):3004–3011. https://doi.org/10.1002/cam4.2197
Solmonson A, DeBerardinis RJ (2018) Lipoic acid metabolism and mitochondrial redox regulation. J Biol Chem 293 (20):7522–7530. https://doi.org/10.1074/jbc.TM117.000259
Tralongo P, Surbone A, Serraino D, Dal Maso L (2019) Major patterns of cancer cure: clinical implications. Eur J Cancer Care (engl) 28 (6):e13139. https://doi.org/10.1111/ecc.13139
Wallace DC (2012) Mitochondria and cancer. Nat Rev Cancer 12 (10):685–698. https://doi.org/10.1038/nrc3365
Weinberg SE, Chandel NS (2015) Targeting mitochondria metabolism for cancer therapy. Nat Chem Biol 11 (1):9–15. https://doi.org/10.1038/nchembio.1712
Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N, Yi P, Tang L, Pan Q, Rao S, Liang J, Tang Y, Su M, Luo X, Yang Y, Shi Y, Wang H, Zhou Y, Liao Q (2021) The cancer metabolic reprogramming and immune response. Mol Cancer 20 (1):28. https://doi.org/10.1186/s12943-021-01316-8
Xiao Y, Yu D (2021) Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther 221:107753. https://doi.org/10.1016/j.pharmthera.2020.107753
Yang C, Wang Z, Li L, Zhang Z, Jin X, Wu P, Sun S, Pan J, Su K, Jia F, Zhang L, Wang H, Yu X, Shao X, Wang K, Qiu F, Yan J, Huang J (2021) Aged neutrophils form mitochondria-dependent vital NETs to promote breast cancer lung metastasis. J Immunother Cancer. https://doi.org/10.1136/jitc-2021-002875
Yue L, Ren Y, Yue Q, Ding Z, Wang K, Zheng T, Chen G, Chen X, Li M, Fan L (2021) α-Lipoic acid targeting PDK1/NRF2 axis contributes to the apoptosis effect of lung cancer cells. Oxid Med Cell Longev 2021:6633419. https://doi.org/10.1155/2021/6633419
Zong WX, Rabinowitz JD, White E (2016) Mitochondria and cancer. Mol Cell 61 (5):667–676. https://doi.org/10.1016/j.molcel.2016.02.011
Funding
This work was financially supported by the National Natural Science Foundation of China (Nos. 31770131, 81473469 to L.F.), International Cooperation Project of the Belt and Road (No. 20400750600), Shanghai Municipal Commission of Health and Family Plan (201840056), Construction project of Shanghai TCM-intigrated innovative flagship hospital (ZY(2021-2023)-0205-05, ZXXT-202203) and National Administration of Traditional Chinese Medicine, Special TCM emergency response program for COVID-19 (2023ZYLCYJ02-6).
Author information
Authors and Affiliations
Contributions
LF and ML conceptualized and designed the study. TZ and LY performed animal experiment. CL, YZ, and YH contributes to omics data collation. CL, QX and BH performed the data analyses and wrote the manuscript. All authors have agreed with the content and that all gave explicit consent to submit and that they obtained consent from the responsible authorities at the institute/organization where the work has been carried out, before the work is submitted.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
Approval was obtained from Laboratory Animal Ethics Committee of Tongji University (Shanghai, China) and complied with legal requirements and national guidelines on the care and maintenance of laboratory animals. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, C., Zhang, Y., Xia, Q. et al. Multi-omics analysis revealed the mitochondrial-targeted drug combination to suppress the development of lung cancer. J Cancer Res Clin Oncol 149, 17159–17174 (2023). https://doi.org/10.1007/s00432-023-05376-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-05376-9