Abstract
Background
FGFR2 is a therapy-relevant target in tumors of the upper gastrointestinal tract (GIT), and clinical trials are currently underway to test the efficacy of FGFR2 inhibitors. Tumor heterogeneity is one of the relevant causes of treatment failure. Almost nothing is known about the heterogeneous distribution of FGFR2-amplified clones in adenocarcinomas of the upper GIT.
Patients and methods
To assess FGFR2 gene copy number alteration and intratumoral heterogeneity of upper GIT adenocarcinomas, we analyzed 893 patient-derived formalin-fixed paraffin-embedded tumor specimens, including primary operated and neoadjuvant-treated tumors (462 gastric carcinomas and 429 esophageal adenocarcinomas) as well as complementary lymph node and distant metastasis by fluorescence in situ hybridization.
Results
Twenty-six gastric tumors (5.6%) and 21 esophageal adenocarcinomas (4.9%) showed FGFR2 amplification. Overall, 93% of gastric carcinomas and 83% of esophageal carcinomas showed heterogeneous amplification. FGFR2 amplification was found in different histological growth patterns, including intestinal and diffuse type according to the Lauren classification. In the primary gastric carcinoma group, FGFR2 amplification was associated with poor prognosis (p = 0.005).
Conclusion
Homogeneous FGFR2 amplification in tumors of the upper GIT is the exception. This has highly relevant implications in the nature of FGFR2 diagnostics (sufficient tumor cell number, determination of amplification at metastasis versus primary tumor, etc.) and on the response probability of appropriate inhibitors. It is relevant that the often poorly treatable and aggressive subtype of diffuse carcinomas (poorly cohesive carcinomas) also shows FGFR2 amplification and that an individualized therapy option with FGFR2 inhibitors could be an option in this group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the fourth and esophageal cancer is the sixth leading cause of mortality worldwide (Sung et al. 2021). Therapeutic options are still needed to improve the outcome of patients with these aggressive tumors of the upper gastrointestinal tract (GIT); they are often diagnosed at an advanced tumor stage, when curative surgical treatment alone is no longer possible. Fibroblast growth factor receptor 2 (FGFR2) is emerging as a promising target for personalized therapies alongside other targets such as human epidermal growth factor receptor 2 (HER2)/neu, epidermal growth factor receptor (EGFR), and vascular endothelial growth factor receptor (VEGFR). FGFR2 belongs to the family of fibroblast growth factor receptor tyrosine kinases (RTKs), which activate various downstream pathways after binding their ligands.(Lau et al. 2018) Abnormal FGFR signaling pathway activation is known to drive cancer development through increased cell proliferation, prolonged survival, and angiogenesis, and it promotes metastasis (Turner and Grose 2010).
FGFR2 amplification is the most common genetic alteration of FGFR genes in gastric cancer (Cristescu et al. 2015; Gu et al. 2021). It has also been detected in a share of gastroesophageal adenocarcinomas (Klempner et al. 2019). According to the results of larger case series, the proportion of FGFR2-amplified tumors of these entities is around 4–7% (Klempner et al. 2019; Hur et al. 2020; Matsumoto et al. 2012; O’Sullivan et al. 2014) Two splice variants of FGFR2 have been described: FGFR2b and FGFR2c (Miki et al. 1992).
FGFR2 amplification leads to specific overexpression of the FGFR2b isoform of the receptor in GIT carcinomas (Gemo et al. 2014; Pierce et al. 2014; Ahn et al. 2016). FGFR2c overexpression has only been described in a small proportion (0.7%) of FGFR2-amplified and FGFR2b-overexpressing GIT carcinomas, and thus its role as an independent prognostic factor is questionable (Yashiro et al. 2021). Bemarituzumab, an afucosylated monoclonal antibody against FGFR2b, has shown promising results in clinical trials as a targeted therapeutic for patients with FGFR2-amplified and/or FGFR2b-overexpressing and HER2-negative metastatic and locally advanced upper gastrointestinal adenocarcinoma (Catenacci et al. 2019; Wainberg et al. 2021).
Biomarkers with high sensitivity and specificity as well as a precise knowledge of the tumor composition are essential for the success of such specific therapy. Intratumoral heterogeneity is frequently observed in adenocarcinomas of the upper GIT (Grillo et al. 2016; Zubarayev et al. 2019). Thus, the failure of the Gatsby study is most likely due to the intratumoral heterogeneity of Her2/neu in gastric cancer (Thuss-Patience et al. 2017). The intratumoral heterogeneity of FGFR2-amplified adenocarcinomas of the upper GIT has been investigated in only a few studies using different methods (Ye et al. 2015; Pectasides et al. 2018; Schrumpf et al. 2022; Tokunaga et al. 2016; Kuboki et al. 2018). Fluorescence in situ hybridization (FISH) is considered the gold standard to detect copy-number alterations (CNA) in tumor cells despite technical alternatives. (F)ISH is the only technique that can reliably detect potential CNA heterogeneity within the tumor.
The previously published results on the distribution of FGFR2b overexpression/FGFR2 amplification in the different histological types according to the Lauren classification have been inconsistent. Recently, researchers have suggested an association between FGFR2b overexpression/FGFR2 amplification and intestinal phenotype and lower tumor grade (Schrumpf et al. 2022), while others have described a significant correlation with the diffuse histological type (Matsumoto et al. 2012; Ahn et al. 2016) or even no association with the histological subtype (O'Sullivan et al. 2014; Shoji et al. 2015).
The present study addresses the following questions: (a) How frequently is heterogeneous FGFR2 amplification found in adenocarcinomas of the upper GIT? (b) What are the morphological characteristics of FGFR2-amplified carcinomas? (c) What is the actual relevance of FGFR2 amplification in adenocarcinoma of the esophagus? (d) What is the impact of neoadjuvant therapy on FGFR2 amplification? To answer these questions, we used FISH to investigate 462 gastric carcinomas and adenocarcinomas of the gastroesophageal junction and 429 adenocarcinomas of the esophagus as well as complementary lymph node metastases of Caucasian patients.
Patients and methods
The gastric carcinoma cohort
The gastric carcinoma cohort consisted of 462 patients. Of these, 272 (58.9%) underwent primary surgery and 190 (41.1%) received neoadjuvant therapy before surgery (Table 1).
Standardized surgical treatment included subtotal distal or total gastrectomy with trans-hiatal resection of the distal esophagus in the case of an adenocarcinoma of the gastroesophageal junction (Siewert 2), and a systematic D2 lymphadenectomy with the goal of complete resection (R0). Roux-en-Y jejunal loop with gastrojejunostomy was considered the method of choice in the reconstruction procedures.
In the subgroup that received neoadjuvant treatment, three different regimens were used (PFL, MAGIC, FLOT, considering that the patients have been treated over the last 20 years). The majority of the patients were treated according to the MAGIC and FLOT protocols.
Molecular subtyping of gastric carcinomas into the four The Cancer Genome Atlas (TCGA)-defined subtypes (CIN, GS, MSI, and EBV) was performed as described previously (Quaas et al. 2021).
The esophageal adenocarcinoma cohort
This cohort consisted of 429 patients, also divided into (a) patients receiving primary resection (n = 177, 41.4%) and (b) patients receiving neoadjuvant treatment (n = 252, 58.6%) (Table 2).
The standard surgical procedure was laparoscopic gastrolysis and right transthoracic en bloc esophagectomy including two-field lymphadenectomy of mediastinal and abdominal lymph nodes. Reconstruction was performed by high intrathoracic esophagogastrostomy as described previously (Holscher et al. 2007). Patients with advanced esophageal cancer (cT3, cNx, M0) received preoperative chemoradiation (5-fluorouracil, cisplatin, and 40 Gy, provided to patient treated prior to the CROSS trial) or chemotherapy alone.
During the first 2 years, patients were followed up clinically in the hospital every 3 months. Subsequently, annual exams were carried out. Follow-up examinations included a detailed history, clinical evaluation, abdominal ultrasound, chest X-ray, and additional diagnostic procedures as required. Follow-up data were available for all patients. There was a preponderance of minor responders based on the tissue microarrays (TMAs), defined as histopathological residual tumor of ≥ 10% (Schneider et al. 2008).
TMA construction
For TMAs, 1 tissue core (and up to 12 tissue cores for multi-spot TMAs; see below) from each tumor was punched out and transferred to a TMA recipient block. Each TMA was constructed as described previously (Simon et al. 2004; Helbig et al. 2016). In brief, one tissue cylinder with a diameter of 1.2 mm was punched from selected tumor tissue blocks using a self-constructed, semi-automated precision instrument and embedded in empty recipient paraffin blocks.
Consecutive sections of the resulting TMA blocks were transferred to an adhesive-coated slide system (Instrumedics Inc., Hackensack, NJ, USA) for immunohistochemistry and FISH. See the following subsection for details on how we constructed the heterogeneity array (multi-spot TMA).
Heterogeneity of FGFR2 amplification
To address the question of heterogeneous FGFR2 amplification within a tumor, we studied primary resected tumors. There were 119 evaluable primary operated gastric carcinomas and 144 adenocarcinomas of the esophagus with corresponding lymph node metastases available for single-spot TMAs. This investigation could answer the following question: How often do different amplification results occur between the primary tumor and its lymph node metastases?
Out of the above-mentioned samples, we analyzed 21 different tumors using whole tumor blocks (10 paired as well as 5 unpaired primary gastric tumors and 5 paired as well as 1 unpaired primary esophageal tumors).
In an additional step, we used a multi-spot TMA considering primary resected esophageal adenocarcinomas as described previously (Gebauer et al. 2020). Briefly, we punched 12 tumor spots out of the same tumor, 4 spots each from the surface and the invasion front and 4 spots from corresponding lymph node metastases. We examined 29 tumors with multi-spot TMA. Twenty-eight of these tumors had previously been analyzed with single-spot TMA and were now being evaluate for possible heterogeneous FGFR2 amplification in the tumor using multi-spot TMA. We did not view any of the tumors analyzed with multi-spot TMA on whole tumor blocks.
In summary, we analyzed 891 carcinomas. In 263 tumors, both the primary tumor and corresponding metastases were present, and we also analyzed 50 tumors with whole tumor blocks or multi-spot TMA (Figs. 1 and 2).
Structure of the analyzed tumor collective: A total of 891 adenocarcinomas of the upper gastrointestinal tract were analyzed by fluorescence in-situ hybridization (FISH) (462 gastric carcinomas, 429 adenocarcinomas of the esophagus). For this purpose, a tissue micro array (single-spot TMA = highlighted in yellow) was used, which considered a tumor spot from the primary tumor and an additional tumor spot from metastases where present (gastric carcinomas: 343 primary tumors without corresponding metastases and 119 tumors in which both the primary tumor and a corresponding metastasis were present. Esophageal carcinomas: 285 primary tumors without corresponding metastases and 144 tumors in which both the primary tumor and a corresponding metastasis were present). Of 462 gastric carcinomas analyzed, 26 tumors showed FGFR2 amplification (5.6%). Of 429 esophageal adenocarcinomas analyzed, 21 tumors showed FGFR2 amplification (4.9%). To better reflect tumor heterogeneity, we performed supplemental analysis of 50 tumors. 15 tumors of the stomach were analyzed on large tumor areas (= marked in green) (of which 10 were primary tumors and their corresponding metastases), 6 tumors of the esophagus were analyzed on large tumor areas (= marked in green), and 29 esophageal carcinomas were analyzed with a multi-spot TMA (= marked in red), which included 12 tumor spots from different tumor areas and corresponding metastases. 49 of these supplementary analyzed tumors were already represented on the single-spot TMA, and one additional esophageal carcinoma was considered on the multi-spot TMA
Heterogeneity of FGFR2 amplification: a Heterogeneity of FGFR2 amplification in gastric cancer (green amplified, red not amplified). Left Heterogeneity between primary tumor (PT) and lymph node/distant metastasis was analyzed on tissue micro array (TMA). Of 119 tumor/metastasis pairs 15 showed FGFR2 amplification, 67% of these were heterogenous amplified. Right: Whole tumor slides of ten tumor/metastasis pairs were analyzed for percentage of FGFR2 amplified tumor cells. Two primary tumor samples without amplification on TMA showed FGFR2 amplified tumor clones on the whole slide (*). One primary tumor showed homogenous amplification in all tumor cells (= 100%). b Heterogeneity of FGFR2 amplification in esophageal adenocarcinoma (green = amplified, red = not amplified). Left Heterogeneity between primary tumor (PT) and lymph node/distant metastasis (Met) was analyzed on single-spot tissue micro array (single-TMA). Of 144 tumor/metastasis pairs ten showed FGFR2 amplification, 70% of these were heterogenous amplified. Middle Whole tumor slides of five tumor/metastasis pairs were analyzed for percentage of FGFR2 amplified tumor cells. Only one primary tumor showed homogenous amplification in all tumor cells. Right 4/29 tumors at the multi-spot TMA showed FGFR2 amplification. Only one tumor was homogeneously amplified in different parts of the primary tumor as in the metastasis and also showed a high proportion of amplified tumor clones within the tumor
FISH
We used FISH to evaluate the FGFR2 amplification status using the Zytolight SPEC FGFR2/CEN 10 Dual Color Probe (Zytovision, Germany) according to the manufacturer’s protocol. We processed samples as described previously (Loeser et al. 2017). We scanned tumor tissue for gene copy gains including chromosomal cluster amplifications hot spots using a 63 × objective (DM5500 fluorescent microscope; Leica, Germany). If the signals were distributed homogeneously, then we used random areas to count the signals. We evaluated 20 tumor cells by counting green FGFR2 and orange centromere signals. The reading strategy for detecting amplifications followed the recommendations of an FGFR2/CEN10 ratio > 2.0 or FGFR2 extrachromosomal cluster amplification signals (O’Sullivan et al. 2014). Because there are still insufficient data on the distribution of FGFR2-amplified tumor cells in GIT adenocarcinomas, we semi-quantitatively documented all amplified tumor cells as a percentage of all tumor cells examined in a sample.
Statistical analysis
We collected patient data prospectively. We compared interdependence between staining, tumor characteristics, and clinical data using Pearson’s chi-squared test and Fisher’s exact test, illustrated by cross-tables. We evaluated overall survival from the date of surgery until death. We generated Kaplan–Meier curves and compared them using a log-rank test. We censored patient data with no events or loss to follow-up at the last known date. We performed multivariate analysis for prognostic factors using a Cox regression model. We included factors that could potentially affect survival. Specifically, we used ENTER because this method inserts all variables into the model at the same time. We considered a two-sided p < 0.05 to be statistically significant. We used SPSS Statistics Version 25 (IBM, Armonk, NY, USA) for all statistical analyses.
Results
Gastric carcinoma
Patient and tumor characteristics of the overall gastric carcinoma cohort.
The majority of patients were male (68%) and over 45 years of age (91.6%). Advanced International Union against Cancer (UICC) stages 3 and 4 were documented in 52.2% of cases. Proximal carcinomas were more frequent (42%) than distal carcinomas (22.3%). According to the four molecular subtypes defined by TCGA, the majority of tumors belonged to the CIN subgroup (76.1%), followed by GS tumors (10.8%), MSI tumors (7.8%), and EBV-positive tumors (4.8%). Overall, 41.1% of patients received neoadjuvant therapy.
Patient and tumor characteristics of the FGFR2-amplified gastric carcinoma
This cohort included 26 amplified tumors (5.6% of the total gastric carcinoma cohort). The sex ratio of FGFR2-amplified gastric tumors was comparable to the overall cohort. There was an equal number of tumors with (13/26) or without (13/26) neoadjuvant therapy. There was an equal proportion of tumors with diffuse (13/26) and intestinal (13/26) histology according to the Lauren classification. However, the proportion of FGFR2-amplified tumors in the overall cohort was slightly higher in the group of gastric carcinomas with intestinal histology (13/187, 7.0%) than that of tumors with diffuse morphology (13/225, 5.1%), possibly due to the distribution of the investigated subtypes. FGFR2-amplified tumors were more distally localized (30.8%). According to the general dominance of CIN tumors, most FGFR2-amplified tumors (22/26, 84.6%) could be assigned to this subtype. There was co-amplification of ERBB2/Her2/neu in 9.4% of FGFR2-amplified tumors. In the neoadjuvant treated, FGFR2-amplified group (13/26), as expected, young patients (< 45 years) were slightly more frequent (Table 1). Otherwise, there were no relevant differences between previously treated and treatment-naïve patients.
FGFR2 amplification had a prognostically unfavorable effect (p = 0.005) in the group of primary operated gastric carcinomas (Fig. 3). This negative prognostic effect was no longer measurable in the neoadjuvant-treated group.
Esophageal adenocarcinoma
Patient characteristics of the overall esophageal adenocarcinoma cohort
The cohort contained predominantly male patients (385/429, 90.1%). Advanced tumor stages—(y)UICC stages 3 and 4—represented more than half of the cases (66.7%, Table 2). Primary resected and neoadjuvant-treated patients showed similar distributions regarding sex, age, and UICC stage (lower proportion of UICC stages 3–4 in the primary resected subgroup).
Patient characteristics of the FGFR2-amplified esophageal adenocarcinoma cohort
There were 21 patients in this amplified cohort (4.9% of the total esophageal adenocarcinoma cohort). The sex and age distribution in FGFR2 amplified tumors was comparable to the overall esophageal adenocarcinoma cohort. As with gastric carcinoma, FGFR2-amplified esophageal adenocarcinomas showed different morphological growth patterns, like tubular, mucinous, and poorly cohesive carcinomas (Fig. 4). The proportion of FGFR2-amplified adenocarcinomas was higher in treatment-naïve patients (13/144, 7.3%) than in neoadjuvant-treated patients (8/252, 3.2%), resulting in an approximate 1/3 to 2/3 distribution of naïve to neoadjuvant-treated tumors within FGFR2-amplified esophageal adenocarcinomas.
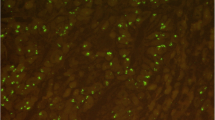
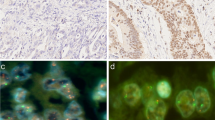
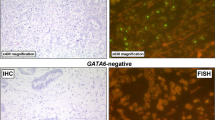
Different tumor growth patterns with FGFR2 amplification and intra-tumoral heterogeneity of FGFR2 amplification. In A1: Adenocarcinoma with DAPI signal (FISH), A2 shows cluster amplification (green signals, long arrow) and two red signals of chromosome centromeres (short arrow). In B1, hematoxylin–eosin standard (H&E) staining of a tubular and partially mucinous adenocarcinoma FGFR2-amplified (B2, cluster amplification with green signals (long arrow) and centromere signal in red (short arrow). In C1 and C2, poorly cohesive adenocarcinoma with FGFR2 amplification. In D1 and D2, solid growing carcinoma with medullary features also FGFR2 amplified. This selection of FGFR2 amplified carcinomas highlights that morphology cannot be a predictor of this gene-amplification. E1 and E2 exemplify the highly relevant intra-tumoral heterogeneity of FGFR2 amplification. E1 shows on H&E the tumor clones that are FGFR2 amplified (long arrows), and the FGFR2 non-amplified tumor portions are shown with short arrows
Primary operated patients with FGFR2-amplified esophageal adenocarcinoma showed a tendency to a worse prognosis (overall survival). However, this did not reach statistical significance (p = 0.346), in contrast to patients with gastric carcinoma (Supplemental Figure).
Heterogeneity of FGFR2 amplification in upper GIT carcinomas
Heterogeneity of the primary tumor and corresponding lymph node metastases on single-spot TMA
From 119 cases with primary gastric carcinoma and corresponding lymph node metastasis, 15 tumor pairs showed FGFR2 amplification (Figs. 2 and 4). In five cases, both the primary tumor and its lymph node metastasis showed concordant FGFR2 amplification. Ten tumors showed heterogenous amplification of FGFR2: four FGFR2-amplified primary tumors lacked FGFR2 amplification in their lymph node metastases, while six primary tumors lacked FGFR2 amplification, but the lymph node metastases did have FGFR2 amplification.
From 144 cases with primary esophageal adenocarcinoma and corresponding lymph node metastases, 10 tumor pairs showed FGFR2 amplification. Of these, three showed co-amplification in both primary tumors and lymph node metastases and seven showed amplification in either primary tumors or lymph node metastases (four FGFR2-amplified primary tumors and three FGFR2-amplified lymph node metastases).
Based on the results of single-spot TMA, we analyzed whole tumor blocks of 15 patients with gastric cancer and 6 patients with esophageal cancer as well as 29 additional patients with multi-spot TMA.
Heterogeneity of primary tumor and corresponding lymph node metastases on whole tumor blocks and multi-spot TMAs.
Figure 2A summarizes the results of the whole tumor blocks: We analyzed 10 patients with tumor pairs (primary tumor and metastasis) using whole tumor blocks (for a total of 20 tumor slides). Two of the five patients who had FGFR2 amplification in the primary tumor and metastasis on single-spot TMA were also analyzed with whole tumor blocks and, as expected, showed the same result. However, neither the two primary tumors nor their metastases were homogeneously amplified: they also showed non-amplified tumor cell clones. The percentage indicates the amplified tumor cell content relative to all tumor cells. This may indicate that the amplified tumor cell clones were biologically relevant and induced lymph node metastases. We also evaluated the four patients whose primary tumors showed FGFR2 amplification on single-spot TMA but did not show amplification in corresponding lymph node metastases by using whole tumor blocks. If the above hypothesis of the biological relevance of FGFR2-amplified tumor clones is correct, then lymph node metastases without FGFR2 amplification could not be easily explained. We confirmed the results from single-spot TMA. Indeed, a tubular adenocarcinoma showed homogeneous FGFR2 amplification in the primary tumor while the corresponding lymph node metastasis showed no FGFR2 amplification. We will discuss this aspect in more detail in the Discussion.
Of six patients whose metastases showed FGFR2-amplified tumor clones on single-spot TMA but not in the primary tumor, we evaluated four patients with whole tumor blocks. Two primary tumors now had small amplified tumor clusters (< 5% of the total tumor cell number) that were not detected with single-spot TMA and apparently induced the metastases. One gastric carcinoma sample had signs of an early invasive carcinoma and was amplified on TMA. Furthermore, four TMA spots showed few amplified tumor cells. We analyzed these five unpaired gastric carcinoma sample as whole tumor blocks. The early invasive carcinoma and two of four samples with intratumoral heterogeneity on TMA showed FGFR2 amplification.
Esophageal adenocarcinoma
Figure 2B summarizes the results of the whole tumor block analysis of esophageal adenocarcinoma: we considered five patients with corresponding tumor pairs using whole tumor blocks (for a total of ten tumor slides). Two of the four patients whose primary tumors showed FGFR2 amplification on single-spot TMA but had non-corresponding FGFR2-amplified clones in their metastases showed identical results even on whole tumor blocks, and vice versa for the tumors that had only FGFR2-amplified clones in the metastases.
We also analyzed one unpaired esophageal carcinoma sample with a whole tumor slide, because it showed only a few amplified tumor cells on single-spot TMA. We confirmed this intratumoral heterogeneity on the larger tissue sample, which showed small clusters of amplified tumor cells.
Multi-spot TMAs
We also examined 29 primary operated esophageal adenocarcinomas using a multi-spot TMA that considered eight tumor spots from the primary tumor (4 from the central tumor area and four from the periphery) and 4 spots from corresponding lymph node metastases. None of these tumors were represented on the whole tumor blocks described above. We detected FGFR2-amplified tumor cells in 4 of 29 primary tumors. These samples had shown FGFR2 amplification on single-spot TMA. While FGFR2-amplified tumor cells accounted for 10–20% of cells in 3/4 primary tumors and were mainly found in the central tumor area, up to 90% of the infiltrating tumor cells counted were evenly distributed in the center and periphery in 1/4 primary tumors (75% intratumoral heterogeneity). Comparison of 19 primary tumors and their lymph node metastases revealed 100% intrasample heterogeneity (2/2), with a lower proportion of FGFR2-amplified tumors in lymph node metastases (90% in primary, 60% in metastases, and 20% primary versus 10% in metastases) (Fig. 2b). We did not find FGFR2-amplified tumor cells in lymph node metastases of non-amplified primary tumors.
Discussion
We analyzed a large number of adenocarcinomas of the stomach (n = 462) and esophagus (n = 429) from a Caucasian patient cohort (N = 891). Our results support pronounced intratumoral heterogeneity of FGFR2 amplification. Amplification of this gene has been reported in 4–7% of upper GIT carcinomas, with homogeneous FGFR2 amplification expected in < 20% of these cases (Klempner et al. 2019; Hur et al. 2020; Matsumoto et al. 2012; O'Sullivan et al. 2014). Consistent with these results, we found that 5.3% of patients (47/891) had FGFR2-amplified cells. FGFR2-amplified gastric carcinomas in our cohort (5.6%) did not show an association with histological subtype or a difference in whether neoadjuvant therapy was used. Our cohort even showed a slightly, although not significantly, higher percentage of FGFR2-amplified tumors within the group with diffuse histology compared with the ratio in intestinal tumors. FGFR2-amplified esophageal adenocarcinomas (4.9%) showed no specific clinical or histomorphological characteristics. While FGFR2 amplification in gastric carcinomas had a prognostically unfavorable effect in the primary gastric cancer group (p = 0.005), which was not detectable in the neoadjuvant treated group, this effect was not measurable in any subgroup of esophageal adenocarcinomas. This fact may be due to molecular aspects; adenocarcinomas of the esophagus belong molecularly almost exclusively to the group of chromosomally instable carcinomas (CIN), while gastric carcinomas are molecularly more heterogeneous (including a higher proportion of so-called genomically stable tumors (GS)).
To our knowledge, three papers to date has considered tumor heterogeneity of FGFR2 in gastric cancer (Ye et al. 2015; Pectasides et al. 2018; Schrumpf et al. 2022). Ye et al. (2015) investigated how many endoscopic biopsies are necessary to obtain trustworthy data for biomarkers including HER2/neu and FGFR2. They showed that the probability of false-negative results decreases with the number of biopsies (i.e., the number of tumor cells available for analysis), especially for FGFR2. In other studies, the authors showed the same for HER2/neu and programmed death ligand 1 (PD-L1) (Grabsch et al. 2010; Lordick et al. 2017; Schoemig-Markiefka et al. 2021). Pectasides et al. (Pectasides et al. 2018) investigated the heterogeneity of FGFR2 in the context of other markers such as ERBB2, MET, and EGFR, among others, using other techniques such as next-generation sequencing (NGS) and cell-free DNA (cf-DNA). Consistent with our results, that group also found relevant tumor heterogeneity between primary tumors and metastases, but they also observed a potentially diagnostically important homogeneity between distant metastases and cf-DNA. If our results can be confirmed, liquid biopsies might be able to represent the heterogeneity of FGFR2 well.
This year, Schrumpf et al. (Schrumpf et al. 2022) published a paper about their analysis of nearly 500 Caucasian gastric carcinomas, studying FGFR2 mutations and FGFR2 protein expression by immunohistochemistry. In some of the tumors, correlation with protein expression was considered by FGFR2 chromogenic in situ hybridization (CISH). The authors confirmed high heterogeneity of FGFR2 expression in gastric carcinomas, a finding that fits excellently with our results. In addition, they found FGFR2 amplification occurred in poorly cohesive carcinomas.
The heterogeneous distribution of biomarker-positive tumor cell clones in primary tumors and their metastases has both diagnostic and therapeutic important implications. If a genetic alteration, such as ERBB2/Her2/neu or FGFR2 amplification, is homogeneously distributed uniformly across the tumor and its different clones, a single tumor-cell-bearing biopsy would be sufficient to reliably document this genetic alteration. We know Her2/neu amplification can be quite homogenous in come cancer—for example, breast carcinoma (Hanna et al. 2007; Hou et al. 2017). This is not true for Her2/neu amplification in upper GIT adenocarcinomas and, as we have shown in the present study, for FGFR2 amplification in gastric and esophageal cancer. This finding is relevant in terms of the methods used to determine FGFR2 amplification. According to Ye et al. (Hur et al. 2020), at least six tumor-bearing biopsies are required to diagnose a negative result (unamplified tumor). Fewer biopsies (e.g. three) increases the risk of a false negative result.
Based on our results, we believe that the primary TMA we used, considering more than 800 samples from carcinoma patients, provided realistic results despite relevant heterogeneity of FGFR2 amplification. Nevertheless, a single-spot TMA has technical weaknesses in representing tumor heterogeneity. We have responded to these weaknesses with additional methods (whole-tumor block analyses and multi-spot TMA) and believe that we have contributed valid data on FGFR2 heterogeneity.
Using these measures, we obtained results on our surgical specimens that are in good agreement with the literature. Specifically, 93% of gastric carcinoma and 83% of esophageal adenocarcinoma exhibited intratumoral heterogeneity of FGFR2 amplification. Furthermore, we demonstrated that there are discrepancies between the amplification status of the primary tumor and the corresponding lymph node metastases, which can be explained by intratumoral heterogeneity of FGFR2-amplified tumor clones. This suggests that sufficient sampling in the primary tumor and lymph node or organ metastases is advisable to determine the FGFR2 status.
For the medical-diagnostic routine, this means that in systemically ill patients who are to receive anti-FGFR2 therapy, FGFR2 biomarker testing should be extended to the biologically driving tumor component, which can also only become apparent in metastases. Alternatively, liquid biopsies could help to reliably determine this systemically relevant status in the future (Pectasides et al. 2018; Wallander et al. 2021). The high intratumoral heterogeneity of FGFR2 amplification also has implications for the potential efficacy of therapeutic FGFR2 blockade. Biologically, the more homogeneous a genetic alteration is in a tumor, the more important it is for maintaining tumor integrity. The more homogeneous an oncogenic driver is in a tumor, the more effective its therapeutic blockade is likely to be.
The TOGA study impressively demonstrated the heterogeneous occurrence of HER2/neu-positive tumor cell clones in gastric carcinoma. The high heterogeneity may be one of the reasons why the median survival benefit of Her2/neu blockade with trastuzumab was significant but still moderate at 2.9 months (Cutsem et al. 2015; Ruschoff et al. 2012; Bang et al. 2010). The heterogeneity of Her2/neu may also have been the key reason for the failure of the Gatsby study (Thuss-Patience et al. 2017). The FISH technique we have chosen is particularly good at demonstrating heterogeneous gene amplification, as these different clones are visualized (Fig. 2). Future studies will have to show how well alternative detection methods of an amplification help to map heterogeneity. While the majority of Her2/neu-amplified gastric carcinomas show an intestinal growth pattern (according to the Lauren classification), this is not true for FGFR2-amplified tumors. We have shown that half of our FGFR2-amplified tumors have a diffuse growth pattern (at least in proportions). Consequently, a clinical trial is currently being initiated to address the efficacy of FGFR2 blockade also in diffuse type carcinomas (Merz et al. 2020).
In the FIGHT study (NCT03343301), a global, randomized, double-blind, placebo-controlled phase 2 trial, patients with unresectable locally advanced or metastatic gastric/-esophageal junction carcinoma were enrolled if their tumor was HER2-negative and positive for FGFR2b overexpression by centrally performed immunohistochemistry or for FGFR2 amplification determined by circulating tumor DNA (ctDNA) (Catenacci et al. 2019). Generally, it is a method that can be used to detect gene amplification, but the sensitivity is highly dependent on the material used and discordance between other methods has been reported. FISH is the gold standard for the detection of gene amplification. Previously reported FGFR2 immunohistochemistry results did not correlate well with FGFR2 amplification due to intracellular epitopes and cross-reactivity with other FGFRs, like FGFR1, while a new FGFR2b immunohistochemistry assay has been proposed as an alternative, accurate screening tool (Ahn et al. 2016). Unfortunately, this study antibody is currently not (yet) commercially available. As FISH is the gold standard for amplicon detection and showed the strongest prediction of treatment response in the FIGHT study, we chose this analytical method for our study.
Taken together, FGFR2-amplified primary adenocarcinoma of the GIT shows a high rate of heterogeneously distributed tumor clones. This intratumoral heterogeneity is found in both gastric carcinoma and esophageal adenocarcinoma and leads to different expression pattern in metastases. FGFR2 testing in metastases should be performed, because a small aggressive clone of the primary tumor can drive aggressive tumor behavior and tumor spreading. We did not find any significant correlation between a specific histomorphological subtype and FGFR2 amplification in upper GIT adenocarcinoma; in particular, we found an equal proportion of diffuse and intestinal morphology in FGFR2-amplified gastric carcinomas. Therefore, FGFR2 biomarker testing should not be limited to carcinomas of a particular histomorphological subtype. The percentage of FGFR2-amplified esophageal adenocarcinoma in our cohort (4.1%) is consistent with the rate reported in the literature for gastric carcinomas. Although there is no significant association with prognosis, testing for FGFR2 amplification may provide a targeted therapeutic option for these aggressive tumors. According to our data, we could not detect a significant effect of neoadjuvant treatment on FGFR2-amplified tumor clones, as we found no significant differences in the distribution of FGFR2-amplified tumors in naïve or pretreated adenocarcinomas of the upper GIT.
Sub-analyses of currently ongoing clinical trials investigating the efficacy of FGFR2 or pan-FGFR inhibitors in solid tumors will show the impact of heterogeneity of FGFR2-amplified tumor cell clones on treatment response or failure. It is also relevant that the poorly treatable and aggressive subtype of diffuse carcinomas (poorly cohesive carcinomas) shows FGFR2 amplification. In this group, an individualized therapy with FGFR2 inhibitors might be an option.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Ahn S, Lee J, Hong M et al (2016) FGFR2 in gastric cancer: protein overexpression predicts gene amplification and high H-index predicts poor survival. Mod Pathol 29:1095–1103
Bang YJ, Van Cutsem E, Feyereislova A et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–697
Catenacci DV, Tesfaye A, Tejani M et al (2019) Bemarituzumab with modified FOLFOX6 for advanced FGFR2-positive gastroesophageal cancer: FIGHT Phase III study design. Future Oncol 15:2073–2082
Cristescu R, Lee J, Nebozhyn M et al (2015) Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 21:449–456
Gebauer F, Krämer M, Bruns C et al (2020) Lymphocyte activation gene-3 (LAG3) mRNA and protein expression on tumour infiltrating lymphocytes (TILs) in oesophageal adenocarcinoma. J Cancer Res Clin Oncol 146:2319–2327
Gemo AT, Deshpande AM, Palencia S et al (2014) Abstract 5446: FPA144: A therapeutic antibody for treating patients with gastric cancers bearing FGFR2 gene amplification. Can Res 74:5446–5446
Grabsch H, Sivakumar S, Gray S et al (2010) HER2 expression in gastric cancer: Rare, heterogeneous and of no prognostic value—conclusions from 924 cases of two independent series. Cell Oncol 32:57–65
Grillo F, Fassan M, Sarocchi F et al (2016) HER2 heterogeneity in gastric/gastroesophageal cancers: from benchside to practice. World J Gastroenterol 22:5879–5887
Gu W, Yang J, Wang Y et al (2021) Comprehensive identification of FGFR1-4 alterations in 5 557 Chinese patients with solid tumors by next-generation sequencing. Am J Cancer Res 11:3893–3906
Hanna W, Nofech-Mozes S, Kahn HJ (2007) Intratumoral heterogeneity of HER2/neu in breast cancer—a rare event. Breast J 13:122–129
Helbig D, Ihle MA, Pütz K et al (2016) Oncogene and therapeutic target analyses in atypical fibroxanthomas and pleomorphic dermal sarcomas. Oncotarget 7:21763–21774
Holscher AH, Schneider PM, Gutschow C, Schroder W (2007) Laparoscopic ischemic conditioning of the stomach for esophageal replacement. Ann Surg 245:241–246
Hou Y, Nitta H, Wei L et al (2017) HER2 intratumoral heterogeneity is independently associated with incomplete response to anti-HER2 neoadjuvant chemotherapy in HER2-positive breast carcinoma. Breast Cancer Res Treat 166:447–457
Hur JY, Chao J, Kim K et al (2020) High-level FGFR2 amplification is associated with poor prognosis and Lower response to chemotherapy in gastric cancers. Pathol Res Pract 216:152878
Klempner SJ, Madison R, Pujara V et al (2019) FGFR2-Altered gastroesophageal adenocarcinomas are an uncommon clinicopathologic entity with a distinct genomic landscape. Oncologist 24:1462–1468
Kuboki Y, Schatz CA, Koechert K et al (2018) In situ analysis of FGFR2 mRNA and comparison with FGFR2 gene copy number by dual-color in situ hybridization in a large cohort of gastric cancer patients. Gastric Cancer 21:401–412
Lau WM, Teng E, Huang KK et al (2018) Acquired resistance to FGFR inhibitor in diffuse-type gastric cancer through an AKT-independent PKC-mediated phosphorylation of GSK3β. Mol Cancer Ther 17:232–242
Loeser H, Waldschmidt D, Kuetting F et al (2017) Copy-number variation and protein expression of DOT1L in pancreatic adenocarcinoma as a potential drug target. Mol Clin Oncol 6:639–642
Lordick F, Al-Batran SE, Dietel M et al (2017) HER2 testing in gastric cancer: results of a German expert meeting. J Cancer Res Clin Oncol 143:835–841
Matsumoto K, Arao T, Hamaguchi T et al (2012) FGFR2 gene amplification and clinicopathological features in gastric cancer. Br J Cancer 106:727–732
Merz V, Zecchetto C, Simionato F et al (2020) A phase II trial of the FGFR inhibitor pemigatinib in patients with metastatic esophageal-gastric junction/gastric cancer trastuzumab resistant: the FiGhTeR trial. Ther Adv Med Oncol 12:1758835920937889
Miki T, Bottaro DP, Fleming TP et al (1992) Determination of ligand-binding specificity by alternative splicing: two distinct growth factor receptors encoded by a single gene. Proc Natl Acad Sci U S A 89:246–250
O’Sullivan CC, Moon DH, Kohn EC, Lee JM (2014) Beyond breast and ovarian cancers: PARP inhibitors for BRCA mutation-associated and BRCA-like solid Tumors. Front Oncol 4:42
Pectasides E, Stachler MD, Derks S et al (2018) Genomic heterogeneity as a barrier to precision medicine in gastroesophageal adenocarcinoma. Cancer Discov 8:37–48
Pierce KL, Deshpande AM, Stohr BA et al (2014) FPA144, a humanized monoclonal antibody for both FGFR2-amplified and nonamplified, FGFR2b-overexpressing gastric cancer patients. J Clin Oncol 32:e15074–e15074
Quaas A, Pamuk A, Klein S et al (2021) Sex-specific prognostic effect of CD66b-positive tumor-infiltrating neutrophils (TANs) in gastric and esophageal adenocarcinoma. Gastric Cancer 24:1213–1226
Ruschoff J, Hanna W, Bilous M et al (2012) HER2 testing in gastric cancer: a practical approach. Mod Pathol 25:637–650
Schneider PM, Metzger R, Schaefer H et al (2008) Response evaluation by endoscopy, rebiopsy, and endoscopic ultrasound does not accurately predict histopathologic regression after neoadjuvant chemoradiation for esophageal cancer. Ann Surg 248:902–908
Schoemig-Markiefka B, Eschbach J, Scheel AH et al (2021) Optimized PD-L1 scoring of gastric cancer. Gastric Cancer 24:1115–1122
Schrumpf T, Behrens HM, Haag J et al (2022) FGFR2 overexpression and compromised survival in diffuse-type gastric cancer in a large central European cohort. PLoS ONE 17:e0264011
Shoji H, Yamada Y, Okita N et al (2015) Amplification of FGFR2 gene in patients with advanced gastric cancer receiving chemotherapy: prevalence and prognostic significance. Anticancer Res 35:5055–5061
Simon R, Mirlacher M, Sauter G (2004) Tissue microarrays. Biotechniques 36:98–105
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Thuss-Patience PC, Shah MA, Ohtsu A et al (2017) Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol 18:640–653
Tokunaga R, Imamura Y, Nakamura K et al (2016) Fibroblast growth factor receptor 2 expression, but not its genetic amplification, is associated with tumor growth and worse survival in esophagogastric junction adenocarcinoma. Oncotarget 7:19748–19761
Turner N, Grose R (2010) Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer 10:116–129
Van Cutsem E, Bang YJ, Feng-Yi F et al (2015) HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 18:476–484
Wainberg ZA, Enzinger PC, Kang Y-K et al (2021) Randomized double-blind placebo-controlled phase 2 study of bemarituzumab combined with modified FOLFOX6 (mFOLFOX6) in first-line (1L) treatment of advanced gastric/gastroesophageal junction adenocarcinoma (FIGHT). J Clin Oncol 39:160–160
Wallander K, Eisfeldt J, Lindblad M et al (2021) Cell-free tumour DNA analysis detects copy number alterations in gastro-oesophageal cancer patients. PLoS ONE 16:e0245488
Yashiro M, Kuroda K, Masuda G et al (2021) Clinical difference between fibroblast growth factor receptor 2 subclass, type IIIb and type IIIc, in gastric cancer. Sci Rep 11:4698
Ye P, Zhang M, Fan S et al (2015) Intra-tumoral heterogeneity of HER2, FGFR2, cMET and ATM in gastric cancer: optimizing personalized healthcare through innovative pathological and statistical analysis. PLoS ONE 10:e0143207
Zubarayev M, Min EK, Son T (2019) Clinical and molecular prognostic markers of survival after surgery for gastric cancer: tumor-node-metastasis staging system and beyond. Transl Gastroenterol Hepatol 4:59
Funding
Open Access funding enabled and organized by Projekt DEAL. This research received no external funding.
Author information
Authors and Affiliations
Contributions
AQ, JA, LH, LF: made substantial contributions to conception and design. JS, FG, HA, JR, LF are responsible for analysis and interpretation of data. AQ, JA, LH: wrote the main manuscript. JS, FG, HA, JR: were reasonable for the data collection. RB, CB, WS, TZ: have reviewed the text. All authors have been involved in drafting the manuscript or revising it critically for important intellectual content. All authors give final approval of the version to be published.
Corresponding author
Ethics declarations
Conflicts of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval and consent to participate
In vivo experiments were performed in accordance with FELASA recommendations and with the approval of the local ethics committee under 81–02.04.2018.A368. Patients gave their written consent to the usage of their tumor specimens and the objective of the project is primarily in the field of diagnostics and quality assurance, approval was obtained from the University of Cologne Ethics Committee (reference number: 13–091 and 10–242; Lehmann and Grass et al.).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

432_2022_4460_MOESM1_ESM.jpg
Supplement Figure Kaplan Meier curve Primarily operated patients with FGFR2 amplified adenocarcinoma of the esophagus show a tendency to a worse prognosis. However, this does not reach statistical significance in contrast to patients with gastric carcinoma (p = 0.346) (JPG 36 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Albin, J., Fahrig, L., Siemanowski, J. et al. FGFR2-amplified tumor clones are markedly heterogeneously distributed in carcinomas of the upper gastrointestinal tract. J Cancer Res Clin Oncol 149, 5289–5300 (2023). https://doi.org/10.1007/s00432-022-04460-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04460-w