Abstract
Purpose
This study was attempted to investigate the safety and clinical efficacy of percutaneous irreversible electroporation combined with allogeneic natural killer cell therapy for treating stage III/IV pancreatic cancer, evaluate median progression-free survival (PFS), and overall survival (OS).
Methods
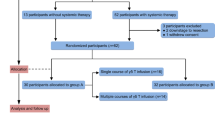
Between March 2016 and February 2017, we enrolled 67 patients who met the enrollment criteria. According to the latest NCCN Guidelines, the patients were divided into stage III (35 patients, 16 patients received only irreversible electroporation (IRE) and 19 patients received IRE-NK: 8 patients underwent one course NK and 11 patients underwent ≥3 courses) and stage IV (32 patients, 14 patients received only IRE and 18 patients received IRE-NK: 8 patients underwent one course NK and 10 patients underwent ≥3 courses). The safety and short-term effects were evaluated first, then the median PFS, median OS, response rate (RR) and disease control rate (DCR) were assessed.
Results
Adverse events of all patients were limited to grades A and B, included local (mainly cough 12.7%, nausea and emesis 6.8%, pain of puncture point 25.3% and duodenum and gastric retention 5.9%) and systemic (mainly fatigue 21.5, fever 33.5%, and blood pressure intraoperative transient reduction 27.4% and white cell count reduction 22.6%) reactions, fever was most frequent. The serum amylase level at 24 h and 7 d after IRE was not significantly changed compared to those before IRE (P > 0.05). CA19-9 value was lower in IRE-NK group than in IRE at 1 month after treatment (P < 0.05). After a median follow-up of 7.9 months (3.8–12.1 months): in stage III group, median PFS was higher in IRE-NK group (9.1 months) than in IRE group (7.9 months, P = 0.0432), median OS was higher in IRE-NK (13.6 months) than in IRE (12.2 months; P = 0.0327), and median PFS was higher in who received multiple NK than single NK (9.9 vs. 8.2 months; P = 0.0387, respectively), median OS who received multiple NK was higher than single NK (13.7 vs. 12.1 months; P = 0.0451, respectively), the RR in IRE-NK (63.2%) was higher than in IRE (50.0%; P < 0.05); in stage IV group, median OS was higher in IRE-NK (10.2 months) than in IRE (9.1 months; P = 0.0367), the DCR in IRE-NK (66.7%) was higher than in IRE (42.9%; P < 0.05).
Conclusion
Percutaneous irreversible electroporation combined with allogeneic natural killer cell immunotherapy significantly increased median PFS and median OS in stage III pancreatic cancer and extended the median OS of stage IV pancreatic cancer. Multiple allogeneic natural killer cells infusion was associated with better prognosis to stage III pancreatic cancer.






Similar content being viewed by others
Abbreviations
- PC:
-
Pancreatic cancer
- NK:
-
Natural killer
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- RR:
-
Response rate
- DCR:
-
Disease control rate
- MHC:
-
Major histocompatibility complex
- KIRs:
-
Killer cell immunoglobulin-like receptors
References
Aerts JG, Hegmans JP (2013) Tumor-specific cytotoxic T cells are crucial for efficacy of immunomodulatory antibodies in patients with lung cancer. Can Res 73:2381–2388. doi:10.1158/0008-5472.CAN-12-3932
Chen AP, Setser A, Anadkat MJ, Cotliar J, Olsen EA, Garden BC et al (2012) Grading dermatologic adverse events of cancer treatments: the common terminology criteria for adverse events version 4.0. J Am Acad Dermatol 67:1025–1039. doi:10.1016/j.jaad.2012.02.010
Cheng M, Chen Y, Xiao W, Sun R, Tian Z (2013) NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol 10:230–252. doi:10.1038/cmi.2013.10
Drake CG, Jaffee E, Pardoll DM (2006) Mechanisms of immune evasion by tumors. Adv Immunol 90:51–81. doi:10.1016/S0065-2776(06)90002-9
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. doi:10.1016/j.ejca.2008.10.026
Felip E, Martinez-Marti A, Martinez P, Cedres S, Navarro A (2013) Adjuvant treatment of resected nonsmall cell lung cancer: state of the art and new potential developments. Curr Opin Oncol 25:115–120. doi:10.1097/CCO.0b013e32835ca1b0
Forte P, Baumann BC, Schneider MK, Seebach JD (2009) HLA-Cw4 expression on porcine endothelial cells reduces cytotoxicity and adhesion mediated by CD158a + human NK cells. Xenotransplantation 16:19–26. doi:10.1111/j.1399-3089.2009.00510.x
Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD 3rd, Dupuy DE et al (2005a) Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Intervent Radiol JVIR 16:765–778. doi:10.1097/01.RVI.0000170858.46668.65
Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD 3rd, Dupuy DE et al (2005b) Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology 235:728–739. doi:10.1148/radiol.2353042205
Hadden JW (1999) The immunology and immunotherapy of breast cancer: an update. Int J Immunopharmacol 21:79–101
Han RX, Liu X, Pan P, Jia YJ, Yu JC (2014) Effectiveness and safety of chemotherapy combined with dendritic cells co-cultured with cytokine-induced killer cells in the treatment of advanced non-small-cell lung cancer: a systematic review and meta-analysis. PLoS ONE 9:e108958. doi:10.1371/journal.pone.0108958
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. doi:10.1016/j.cell.2011.02.013
Hasegawa T, Suzuki H, Yamaura T, Muto S, Okabe N, Osugi J et al (2014) Prognostic value of peripheral and local forkhead box P3+ regulatory T cells in patients with non-small-cell lung cancer. Mol Clin Oncol 2:685–694. doi:10.3892/mco.2014.299
Imai C, Iwamoto S, Campana D (2005) Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 106:376–383. doi:10.1182/blood-2004-12-4797
Izumi Y, Oyama T, Ikeda E, Kawamura M, Kobayashi K (2005) The acute effects of transthoracic cryoablation on normal lung evaluated in a porcine model. Ann Thorac Surg 79:318–322. doi:10.1016/j.athoracsur.2003.09.082 (discussion 22)
Keane MG, Bramis K, Pereira SP, Fusai GK (2014) Systematic review of novel ablative methods in locally advanced pancreatic cancer. World J Gastroenterol 20:2267–2278. doi:10.3748/wjg.v20.i9.2267
Keller SM, Adak S, Wagner H, Herskovic A, Komaki R, Brooks BJ et al (2000) A randomized trial of postoperative adjuvant therapy in patients with completely resected stage II or IIIA non-small-cell lung cancer. Eastern Cooperative Oncology Group. N Engl J Med 343:1217–1222. doi:10.1056/NEJM200010263431703
Kluger MD, Epelboym I, Schrope BA, Mahendraraj K, Hecht EM, Susman J et al (2016) Single-Institution experience with irreversible electroporation for T4 pancreatic cancer: first 50 patients. Ann Surg Oncol 23:1736–1743. doi:10.1245/s10434-015-5034-x
Kubo M, Morisaki T, Kuroki H, Tasaki A, Yamanaka N, Matsumoto K et al (2003) Combination of adoptive immunotherapy with Herceptin for patients with HER2-expressing breast cancer. Anticancer Res 23:4443–4449
Kunert K, Seiler M, Mashreghi MF, Klippert K, Schonemann C, Neumann K et al (2007) KIR/HLA ligand incompatibility in kidney transplantation. Transplantation 84:1527–1533. doi:10.1097/01.tp.0000290681.41859.41
Li JJ, Gu MF, Pan K, Liu LZ, Zhang H, Shen WX et al (2012a) Autologous cytokine-induced killer cell transfusion in combination with gemcitabine plus cisplatin regimen chemotherapy for metastatic nasopharyngeal carcinoma. J Immunother 35:189–195. doi:10.1097/CJI.0b013e318241d9de
Li R, Wang C, Liu L, Du C, Cao S, Yu J et al (2012b) Autologous cytokine-induced killer cell immunotherapy in lung cancer: a phase II clinical study. Cancer Immunol Immunother CII 61:2125–2133. doi:10.1007/s00262-012-1260-2
Lin M, Xu K, Liang S, Wang X, Liang Y, Zhang M et al (2017) Prospective study of percutaneous cryoablation combined with allogenic NK cell immunotherapy for advanced renal cell cancer. Immunol Lett 184:98–104. doi:10.1016/j.imlet.2017.03.004
Ljunggren HG, Malmberg KJ (2007) Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol 7:329–339. doi:10.1038/nri2073
Long J, Luo GP, Xiao ZW, Liu ZQ, Guo M, Liu L et al (2014) Cancer statistics: current diagnosis and treatment of pancreatic cancer in Shanghai, China. Cancer Lett 346:273–277. doi:10.1016/j.canlet.2014.01.004
Lv M, Xu Y, Tang R, Ren J, Shen S, Chen Y et al (2014) miR141-CXCL1-CXCR2 signaling-induced Treg recruitment regulates metastases and survival of non-small cell lung cancer. Mol Cancer Ther 13:3152–3162. doi:10.1158/1535-7163.MCT-14-0448
Martin RC (2013) Irreversible electroporation of locally advanced pancreatic head adenocarcinoma. J Gastrointest Surg 17:1850–1856. doi:10.1007/s11605-013-2309-z
Martin RC 2nd (2015) Irreversible electroporation of locally advanced pancreatic neck/body adenocarcinoma. J Gastrointest Oncol 6:329–335. doi:10.3978/j.issn.2078-6891.2015.012
Martin RC 2nd, McFarland K, Ellis S, Velanovich V (2013) Irreversible electroporation in locally advanced pancreatic cancer: potential improved overall survival. Ann Surg Oncol 20(Suppl 3):S443–S449. doi:10.1245/s10434-012-2736-1
Martin RC 2nd, Kwon D, Chalikonda S, Sellers M, Kotz E, Scoggins C et al (2015) Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: safety and efficacy. Ann Surg 262:486–494. doi:10.1097/SLA.0000000000001441 (discussion 92–94)
Martin RC 2nd, Durham AN, Besselink MG, Iannitti D, Weiss MJ, Wolfgang CL et al (2016) Irreversible electroporation in locally advanced pancreatic cancer: a call for standardization of energy delivery. J Surg Oncol 114:865–871. doi:10.1002/jso.24404
Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47:207–214
Moretta L, Moretta A (2004) Killer immunoglobulin-like receptors. Curr Opin Immunol 16:626–633. doi:10.1016/j.coi.2004.07.010
Narayanan G, Hosein PJ, Arora G, Barbery KJ, Froud T, Livingstone AS et al (2012) Percutaneous irreversible electroporation for downstaging and control of unresectable pancreatic adenocarcinoma. J Vasc Intervent Radiol JVIR 23:1613–1621. doi:10.1016/j.jvir.2012.09.012
Niu L, Xu K, Mu F (2012) Cryosurgery for lung cancer. J Thorac Dis 4:408–419. doi:10.3978/j.issn.2072-1439.2012.07.13
Niu L, Chen J, He L, Liao M, Yuan Y, Zeng J et al (2013a) Combination treatment with comprehensive cryoablation and immunotherapy in metastatic pancreatic cancer. Pancreas 42:1143–1149. doi:10.1097/MPA.0b013e3182965dde
Niu LZ, Li JL, Zeng JY, Mu F, Liao MT, Yao F et al (2013b) Combination treatment with comprehensive cryoablation and immunotherapy in metastatic hepatocellular cancer. World J Gastroenterol 19:3473–3480. doi:10.3748/wjg.v19.i22.3473
Paiella S, Butturini G, Frigerio I, Salvia R, Armatura G, Bacchion M et al (2015) Safety and feasibility of irreversible electroporation (IRE) in patients with locally advanced pancreatic cancer: results of a prospective study. Dig Surg 32:90–97. doi:10.1159/000375323
Pan K, Li YQ, Wang W, Xu L, Zhang YJ, Zheng HX et al (2013) The efficacy of cytokine-induced killer cell infusion as an adjuvant therapy for postoperative hepatocellular carcinoma patients. Ann Surg Oncol 20:4305–4311. doi:10.1245/s10434-013-3144-x
Pan K, Guan XX, Li YQ, Zhao JJ, Li JJ, Qiu HJ et al (2014) Clinical activity of adjuvant cytokine-induced killer cell immunotherapy in patients with post-mastectomy triple-negative breast cancer. Clin Cancer Res 20:3003–3011. doi:10.1158/1078-0432.CCR-14-0082
Pisters KM (2005) Adjuvant chemotherapy for non-small-cell lung cancer–the smoke clears. N Engl J Med 352:2640–2642. doi:10.1056/NEJMe058110
Rombouts SJ, Vogel JA, van Santvoort HC, van Lienden KP, van Hillegersberg R, Busch OR et al (2015) Systematic review of innovative ablative therapies for the treatment of locally advanced pancreatic cancer. Br J Surg 102:182–193. doi:10.1002/bjs.9716
Silk M, Tahour D, Srimathveeravalli G, Solomon SB, Thornton RH (2014) The state of irreversible electroporation in interventional oncology. Semin Intervent Radiol 31:111–117. doi:10.1055/s-0034-1373785
Spellman A, Tang SC (2016) Immunotherapy for breast cancer: past, present, and future. Cancer Metastasis Reviews 35:525–546. doi:10.1007/s10555-016-9654-9
Thomas A, Hassan R (2012) Immunotherapies for non-small-cell lung cancer and mesothelioma. Lancet Oncol 13:e301–e310. doi:10.1016/S1470-2045(12)70126-2
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454. doi:10.1056/NEJMoa1200690
Vulfovich M, Rocha-Lima C (2008) Novel advances in pancreatic cancer treatment. Expert Rev Anticancer Ther 8:993–1002. doi:10.1586/14737140.8.6.993
Wang D, Zhang B, Gao H, Ding G, Wu Q, Zhang J et al (2014) Clinical research of genetically modified dendritic cells in combination with cytokine-induced killer cell treatment in advanced renal cancer. BMC Cancer 14:251. doi:10.1186/1471-2407-14-251
Witt CS, Christiansen FT (2006) The relevance of natural killer cell human leucocyte antigen epitopes and killer cell immunoglobulin-like receptors in bone marrow transplantation. Vox Sang 90:10–20. doi:10.1111/j.1423-0410.2005.00712.x
Yang L, Ren B, Li H, Yu J, Cao S, Hao X et al (2013) Enhanced antitumor effects of DC-activated CIKs to chemotherapy treatment in a single cohort of advanced non-small-cell lung cancer patients. Cancer Immunol Immunother CII 62:65–73. doi:10.1007/s00262-012-1311-8
Zhang M, Daniel S, Huang Y, Chancey C, Huang Q, Lei YF et al (2010) Anti-West Nile virus activity of in vitro expanded human primary natural killer cells. BMC Immunol 11:3. doi:10.1186/1471-2172-11-3
Zhang Y, Shi J, Zeng J, Alnagger M, Zhou L, Fang G et al (2017) Percutaneous irreversible electroporation for ablation of locally advanced pancreatic cancer: experience from a Chinese Institution. Pancreas 46:e12–e14. doi:10.1097/MPA.0000000000000703
Zhao Y, Hu J, Li R, Song J, Kang Y, Liu S et al (2015) Enhanced NK cell adoptive antitumor effects against breast cancer in vitro via blockade of the transforming growth factor-beta signaling pathway. Onco Targets Ther 8:1553–1559. doi:10.2147/OTT.S82616
Zhong R, Teng J, Han B, Zhong H (2011) Dendritic cells combining with cytokine-induced killer cells synergize chemotherapy in patients with late-stage non-small cell lung cancer. Cancer Immunol Immunother CII 60:1497–1502. doi:10.1007/s00262-011-1060-0
Zhong R, Han B, Zhong H (2014) A prospective study of the efficacy of a combination of autologous dendritic cells, cytokine-induced killer cells, and chemotherapy in advanced non-small cell lung cancer patients. Tumour Biol 35:987–994. doi:10.1007/s13277-013-1132-1
Acknowledgements
We would like to thank the native English speaking scientists of Elixigen Company for editing our manuscript.
Funding
This work was supported by the Science and Technology Program of Tianhe District, Guangzhou, China (Grant Number 201504KW008).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declared that they have no conflict of interest.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of Guangzhou Fuda Cancer Hospital ethics committee and with the 1964 Declaration of Helsinki.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Lin, M., Liang, S., Wang, X. et al. Percutaneous irreversible electroporation combined with allogeneic natural killer cell immunotherapy for patients with unresectable (stage III/IV) pancreatic cancer: a promising treatment. J Cancer Res Clin Oncol 143, 2607–2618 (2017). https://doi.org/10.1007/s00432-017-2513-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-017-2513-4