Abstract
Purpose
Pro-inflammatory cytokines such as Interleukin-17A (IL17A) and Interleukin-32 (IL32), known to enhance natural killer and T cell responses, are also elevated in human malignancies and linked to poor clinical outcomes. To address this paradox, we evaluated relation between IL17A and IL32 expression and other inflammation- and T cell response-associated genes in breast tumors.
Methods
TaqMan-based gene expression analysis was carried out in seventy-eight breast tumors. The association between IL17A and IL32 transcript levels and T cell response genes, ER status as well as lymph node status was also examined in breast tumors from TCGA dataset.
Results
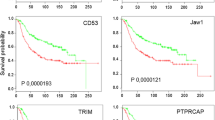
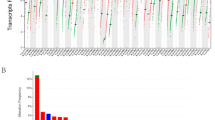
IL17A expression was detected in 32.7% ER-positive and 84.6% ER-negative tumors, with higher expression in the latter group (26.2 vs 7.1-fold, p < 0.01). ER-negative tumors also showed higher expression of IL32 as opposed to ER-positive tumors (8.7 vs 2.5-fold, p < 0.01). Expression of both IL17A and IL32 genes positively correlated with CCL5, GNLY, TBX21, IL21 and IL23 transcript levels (p < 0.01). Amongst ER-positive tumors, higher IL32 expression significantly correlated with lymph node metastases (p < 0.05). Conversely, in ER-negative subtype, high IL17A and IL32 expression was seen in patients with negative lymph node status (p < 0.05). Tumors with high IL32 and IL17A expression showed higher expression of TH1 response genes studied, an observation validated by similar analysis in the TCGA breast tumors (n=1041). Of note, these tumors were characterized by low expression of a potentially immunosuppressive isoform of IL32 (IL32γ).
Conclusion
These results suggest that high expression of both IL17A and IL32 leads to enhancement of T cell responses. Our study, thus, provides basis for the emergence of strong T cell responses in an inflammatory milieu that have been shown to be associated with better prognosis in ER-negative breast cancer.




Similar content being viewed by others
Abbreviations
- IL:
-
Interleukin
- ER:
-
Estrogen receptor
- LN:
-
Lymph node
- CRI:
-
Cancer-related inflammation
- CTLs:
-
Cytotoxic T lymphocytes
- Th:
-
T helper
- Tc:
-
T cytotoxic
- PCR:
-
Polymerase chain reaction
References
Ankathatti Munegowda M, Deng Y, Mulligan SJ, Xiang J (2011) Th17 and Th17-stimulated CD8(+) T cells play a distinct role in Th17-induced preventive and therapeutic antitumor immunity. Cancer Immunol Immunother 60(10):1473–1484. doi:10.1007/s00262-011-1054-y
Balkwill F, Charles KA, Mantovani A (2005) Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7(3):211–217
Ben-Baruch A (2006) Inflammation-associated immune suppression in cancer: the roles played by cytokines, chemokines and additional mediators. Semin Cancer Biol 16(1):38–52
Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautes-Fridman C, Fossiez F, Haicheur N, Fridman WH, Tartour E (2002) Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood 99(6):2114–2121
Benevides L, da Fonseca DM, Donate PB, Tiezzi DG, De Carvalho DD, de Andrade JM, Martins GA, Silva JS (2015) IL17 promotes mammary tumor progression by changing the behavior of tumor cells and eliciting tumorigenic neutrophils recruitment. Cancer Res 75(18):3788–3799. doi:10.1158/0008-5472.CAN-15-0054
Carter CL, Allen C, Henson DE (1989) Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 63(1):181–187
Chavey C, Bibeau F, Gourgou-Bourgade S, Burlinchon S, Boissiere F, Laune D, Roques S, Lazennec G (2007) Oestrogen receptor negative breast cancers exhibit high cytokine content. Breast Cancer Res 9(1):R15
Chen X, Wan J, Liu J, Xie W, Diao X, Xu J, Zhu B, Chen Z (2010) Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer 69(3):348–354. doi:10.1016/j.lungcan.2009.11.013
Chen WC, Lai YH, Chen HY, Guo HR, Su IJ, Chen HH (2013) Interleukin-17-producing cell infiltration in the breast cancer tumour microenvironment is a poor prognostic factor. Histopathology 63(2):225–233. doi:10.1111/his.12156
Choi JD, Bae SY, Hong JW, Azam T, Dinarello CA, Her E, Choi WS, Kim BK, Lee CK, Yoon DY, Kim SJ, Kim SH (2009) Identification of the most active interleukin-32 isoform. Immunology 126(4):535–542. doi:10.1111/j.1365-2567.2008.02917.x
Cochaud S, Giustiniani J, Thomas C, Laprevotte E, Garbar C, Savoye AM, Cure H, Mascaux C, Alberici G, Bonnefoy N, Eliaou JF, Bensussan A, Bastid J (2013) IL-17A is produced by breast cancer TILs and promotes chemoresistance and proliferation through ERK1/2. Sci Rep 3:3456. doi:10.1038/srep03456
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A (2009) Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 30(7):1073–1081
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420(6917):860–867
Droeser R, Zlobec I, Kilic E, Guth U, Heberer M, Spagnoli G, Oertli D, Tapia C (2012) Differential pattern and prognostic significance of CD4+, FOXP3+ and IL-17+ tumor infiltrating lymphocytes in ductal and lobular breast cancers. BMC Cancer 12:134. doi:10.1186/1471-2407-12-134
El-Far M, Kouassi P, Sylla M, Zhang Y, Fouda A, Fabre T, Goulet JP, van Grevenynghe J, Lee T, Singer J, Harris M, Baril JG, Trottier B, Ancuta P, Routy JP, Bernard N, Tremblay CL, Investigators of the Canadian HIV+ Slow Progressor Cohort (2016) Proinflammatory isoforms of IL-32 as novel and robust biomarkers for control failure in HIV-infected slow progressors. Sci Rep 15(6):22902. doi:10.1038/srep22902
Guenin S, Mouallif M, Hubert P, Jacobs N, Krusy N, Duray A, Ennaji MM, Saussez S, Delvenne P (2014) Interleukin-32 expression is associated with a poorer prognosis in head and neck squamous cell carcinoma. Mol Carcinog 53(8):667–673. doi:10.1002/mc.21996
Haibe-Kains B, Desmedt C, Rothe F, Piccart M, Sotiriou C, Bontempi G (2010) A fuzzy gene expression-based computational approach improves breast cancer prognostication. Genome Biol 11(2):R18. doi:10.1186/gb-2010-11-2-r18
Ham M, Moon A (2013) Inflammatory and microenvironmental factors involved in breast cancer progression. Arch Pharm Res 36(12):1419–1431. doi:10.1007/s12272-013-0271-7
He D, Li H, Yusuf N, Elmets CA, Li J, Mountz JD, Xu H (2010) IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J Immunol 184(5):2281–2288. doi:10.4049/jimmunol.0902574
Heinhuis B, Netea MG, van den Berg WB, Dinarello CA, Joosten LA (2012) Interleukin-32: a predominantly intracellular proinflammatory mediator that controls cell activation and cell death. Cytokine 60(2):321–327. doi:10.1016/j.cyto.2012.07.010
Hernandez-Aya LF, Chavez-Macgregor M, Lei X, Meric-Bernstam F, Buchholz TA, Hsu L, Sahin AA, Do KA, Valero V, Hortobagyi GN, Gonzalez-Angulo AM (2011) Nodal status and clinical outcomes in a large cohort of patients with triple-negative breast cancer. J Clin Oncol 29(19):2628–2634. doi:10.1200/JCO.2010.32.1877
Jatoi I, Hilsenbeck SG, Clark GM, Osborne CK (1999) Significance of axillary lymph node metastasis in primary breast cancer. J Clin Oncol 17(8):2334–2340
Joosten LA, Heinhuis B, Netea MG, Dinarello CA (2013) Novel insights into the biology of interleukin-32. Cell Mol Life Sci 70(20):3883–3892. doi:10.1007/s00018-013-1301-9
Joshi N, Kannan S, Kotian N, Bhat S, Kale M, Hake S (2014) Interleukin 6–174G>C polymorphism and cancer risk: meta-analysis reveals a site dependent differential influence in Ancestral North Indians. Hum Immunol 75(8):901–908. doi:10.1016/j.humimm.2014.06.018
Jung MY, Son MH, Kim SH, Cho D, Kim TS (2011) IL-32gamma induces the maturation of dendritic cells with Th1- and Th17-polarizing ability through enhanced IL-12 and IL-6 production. J Immunol 186(12):6848–6859. doi:10.4049/jimmunol.1003996
Kang YH, Park MY, Yoon DY, Han SR, Lee CI, Ji NY, Myung PK, Lee HG, Kim JW, Yeom YI, Jang YJ, Ahn DK, Kim JW (2012) Song EY (2012) Dysregulation of overexpressed IL-32α in hepatocellular carcinoma suppresses cell growth and induces apoptosis through inactivation of NF-κB and Bcl-2. Cancer Lett 318(2):226–233. doi:10.1016/j.canlet.2011.12.023
Kang JW, Park YS, Lee DH, Kim MS, Bak Y, Park SH, Ham SY, Yang Y, Hong JT, Yoon DY (2013) Interleukin-32delta interacts with IL-32beta and inhibits IL-32beta-mediated IL-10 production. FEBS Lett. doi:10.1016/j.febslet.2013.10.019
Kang JW, Park YS, Lee DH, Kim MS, Bak Y, Ham SY, Park SH, Kim H, Ahn JH, Hong JT, Yoon DY (2014) Interaction network mapping among IL-32 isoforms. Biochimie 101:248–251. doi:10.1016/j.biochi.2014.01.013
Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA (2005) Interleukin-32: a cytokine and inducer of TNFalpha. Immunity 22(1):131–142
Lee S, Kim JH, Kim H, Kang JW, Kim SH, Yang Y, Kim J, Park J, Park S, Hong J, Yoon DY (2011) Activation of the interleukin-32 pro-inflammatory pathway in response to human papillomavirus infection and over-expression of interleukin-32 controls the expression of the human papillomavirus oncogene. Immunology 132(3):410–420. doi:10.1111/j.1365-2567.2010.03377.x
Lee HJ, Liang ZL, Huang SM, Lim JS, Yoon DY, Kim JM (2012) Overexpression of IL-32 is a novel prognostic factor in patients with localized clear cell renal cell carcinoma. Oncol Lett 3(2):490–496. doi:10.3892/ol.2011.511
Liu J, Duan Y, Cheng X, Chen X, Xie W, Long H, Lin Z, Zhu B (2011) IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun 407(2):348–354. doi:10.1016/j.bbrc.2011.03.021
Majid S, Dar AA, Saini S, Yamamura S, Hirata H, Tanaka Y, Deng G, Dahiya R (2010) MicroRNA-205-directed transcriptional activation of tumor suppressor genes in prostate cancer. Cancer 116(24):5637–5649. doi:10.1002/cncr.25488
Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C (2009) T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 31(5):787–798
McNeill RE, Miller N, Kerin MJ (2007) Evaluation and validation of candidate endogenous control genes for real-time quantitative PCR studies of breast cancer. BMC Mol Biol 8:107
Montoya D, Inkeles MS, Liu PT, Realegeno S, Teles RM, Vaidya P, Munoz MA, Schenk M, Swindell WR, Chun R, Zavala K, Hewison M, Adams JS, Horvath S, Pellegrini M, Bloom BR, Modlin RL (2014) IL-32 is a molecular marker of a host defense network in human tuberculosis. Sci Transl Med 6(250):250ra114. doi:10.1126/scitranslmed.3009546
Murugaiyan G, Saha B (2009) Protumor vs. antitumor functions of IL-17. J Immunol 183(7):4169–4175
Nold-Petry CA, Rudloff I, Baumer Y, Ruvo M, Marasco D, Botti P, Farkas L, Cho SX, Zepp JA, Azam T, Dinkel H, Palmer BE, Boisvert WA, Cool CD, Taraseviciene-Stewart L, Heinhuis B, Joosten LA, Dinarello CA, Voelkel NF, Nold MF (2014) IL-32 promotes angiogenesis. J Immunol 192(2):589–602. doi:10.4049/jimmunol.1202802
Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT (2003) Interleukin-17 promotes angiogenesis and tumor growth. Blood 101(7):2620–2627
Nunez S, Saez JJ, Fernandez D, Flores-Santibanez F, Alvarez K, Tejon G, Ruiz P, Maldonado P, Hidalgo Y, Manriquez V, Bono MR, Rosemblatt M, Sauma D (2013) T helper type 17 cells contribute to anti-tumour immunity and promote the recruitment of T helper type 1 cells to the tumour. Immunology 139(1):61–71. doi:10.1111/imm.12055
Omrane I, Benammar-Elgaaied A (2015) The immune microenvironment of the colorectal tumor: involvement of immunity genes and microRNAs belonging to the TH17 pathway. Biochem Biophys Acta 1856 1:28–38. doi:10.1016/j.bbcan.2015.04.001
Park MH, Song MJ, Cho MC, Moon DC, Yoon do Y, Han SB, Hong JT (2012) Interleukin-32 enhances cytotoxic effect of natural killer cells to cancer cells via activation of death receptor 3. Immunology 135(1):63–72. doi:10.1111/j.1365-2567.2011.03513.x
Park JS, Lee S, Jeong AL, Han S, Ka HI, Lim JS, Lee MS, Yoon DY, Lee JH, Yang Y (2015) Hypoxia-induced IL-32β increases glycolysis in breast cancer cells. Cancer Lett 356(2 Pt B):800–808. doi:10.1016/j.canlet.2014.10.030
Phan-Lai V, Dang Y, Gad E, Childs J, Disis ML (2016) The antitumor efficacy of IL2/IL21-cultured polyfunctional Neu-specific T cells is TNFα/IL17 dependent. Clin Cancer Res 22(9):2207–2216. doi:10.1158/1078-0432.CCR-15-2273
Player A, Oguamanam T, Okanmelu J, Burrell K, Hollomon M (2014) Preliminary characterization of IL32 in basal-like/triple negative compared to other types of breast cell lines and tissues. BMC Res Notes 7:501. doi:10.1186/1756-0500-7-501
Punt S, Houwing-Duistermaat JJ, Schulkens IA, Thijssen VL, Osse EM, de Kroon CD, Griffioen AW, Fleuren GJ, Gorter A, Jordanova ES (2015) Correlations between immune response and vascularization qRT-PCR gene expression clusters in squamous cervical cancer. Mol Cancer 14:71. doi:10.1186/s12943-015-0350-0
Reyal F, van Vliet MH, Armstrong NJ, Horlings HM, de Visser KE, Kok M, Teschendorff AE, Mook S, van’t Veer L, Caldas C, Salmon RJ, van de Vijver MJ, Wessels LF (2008) A comprehensive analysis of prognostic signatures reveals the high predictive capacity of the proliferation, immune response and RNA splicing modules in breast cancer. Breast Cancer Res 10(6):R93. doi:10.1186/bcr2192
Rody A, Holtrich U, Pusztai L, Liedtke C, Gaetje R, Ruckhaeberle E, Solbach C, Hanker L, Ahr A, Metzler D, Engels K, Karn T, Kaufmann M (2009) T-cell metagene predicts a favorable prognosis in estrogen receptor-negative and HER2-positive breast cancers. Breast Cancer Res 11(2):R15
Shoda H, Fujio K, Yamaguchi Y, Okamoto A, Sawada T, Kochi Y, Yamamoto K (2006) Interactions between IL-32 and tumor necrosis factor alpha contribute to the exacerbation of immune-inflammatory diseases. Arthritis Res Ther 8(6):R166
Sims AH, Howell A, Howell SJ, Clarke RB (2007) Origins of breast cancer subtypes and therapeutic implications. Nat Clin Pract Oncol 4(9):516–525
Smith AJ, Toledo CM, Wietgrefe SW, Duan L, Schacker TW, Reilly CS, Haase AT (2011) The immunosuppressive role of IL-32 in lymphatic tissue during HIV-1 infection. J Immunol 186(11):6576–6584. doi:10.4049/jimmunol.1100277
Sorrentino C, Di Carlo E (2009) Expression of IL-32 in human lung cancer is related to the histotype and metastatic phenotype. Am J Respir Crit Care Med 180(8):769–779
Teschendorff AE, Gomez S, Arenas A, El-Ashry D, Schmidt M, Gehrmann M, Caldas C (2010) Improved prognostic classification of breast cancer defined by antagonistic activation patterns of immune response pathway modules. BMC Cancer 10:604. doi:10.1186/1471-2407-10-604
Turner-Brannen E, Choi KY, Arsenault R, El-Gabalawy H, Napper S, Mookherjee N (2011) Inflammatory cytokines IL-32 and IL-17 have common signaling intermediates despite differential dependence on TNF-receptor 1. J Immunol 186(12):7127–7135. doi:10.4049/jimmunol.1002306
Wilke CM, Kryczek I, Wei S, Zhao E, Wu K, Wang G, Zou W (2011) Th17 cells in cancer: help or hindrance? Carcinogenesis 32(5):643–649. doi:10.1093/carcin/bgr019
Xu S, Cao X (2010) Interleukin-17 and its expanding biological functions. Cell Mol Immunol 7(3):164–174. doi:10.1038/cmi.2010.21
Yamamoto M, Kamigaki T, Yamashita K, Hori Y, Hasegawa H, Kuroda D, Moriyama H, Nagata M, Ku Y, Kuroda Y (2009) Enhancement of anti-tumor immunity by high levels of Th1 and Th17 with a combination of dendritic cell fusion hybrids and regulatory T cell depletion in pancreatic cancer. Oncol Rep 22(2):337–343
Yun HM, Oh JH, Shim JH, Ban JO, Park KR, Kim JH, Lee DH, Kang JW, Park YH, Yu D, Kim Y, Han SB, Yoon DY, Hong JT (2013) Antitumor activity of IL-32beta through the activation of lymphocytes, and the inactivation of NF-kappaB and STAT3 signals. Cell Death Dis 4:e640. doi:10.1038/cddis.2013.166
Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, Li SP, Zheng L (2009) Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol 50(5):980–989
Zhou Y, Zhu Y (2015) Important role of the IL-32 inflammatory network in the host response against viral infections. Viruses 7:3116–3299. doi:10.3390/v7062762
Zhu X, Mulcahy LA, Mohammed RA, Lee AH, Franks HA, Kilpatrick L, Yilmazer A, Paish EC, Ellis IO, Patel PM, Jackson AM (2008) IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res 10(6):R95
Acknowledgements
This work was supported by Grant from the Department of Biotechnology, Ministry of Science and Technology, Government of India. The authors would like to thank Mrs. Manisha Kulkarni and Mr. Anand Deshpande at ICMR National Tumor Tissue Repository at TMH and Dr. Kishore Amin and Mr. Madan Ludbe at tumor tissue repository at ACTREC. Help provided by Dr. Sridhar, Consultant Pathologist, ACTREC, and Mrs. Pallavi Rane, ECTU, CRC, ACTREC is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Department of Biotechnology, Government of India (Grant Number BT/PR10199/Med/30/68/2007).
Conflict of interest
All authors declares that they have no conflict of interest.
Animal study
This article does not contain any studies with animals performed by any of the authors.
Ethical approval for studies in patients
All the procedures involving human participants were performed in accordance with the ethical standards of the institutional and national research committees (guidelines) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
432_2017_2431_MOESM1_ESM.jpg
Supplementary Figure S1. Expression levels of IL17A and IL32 transcripts in tumors studied in the TCGA database (JPEG 273 kb)
432_2017_2431_MOESM2_ESM.jpg
Supplementary Figure S2. Analysis of expression of T cell response associated genes in ER positive and ER negative breast tumors from the TCGA database grouped on the basis of combinations of expression levels of IL17A and IL32 transcripts. In IL17AposIL32lo group, data for only one ER negative subject was available hence expression levels were not compared (JPEG 267 kb)
432_2017_2431_MOESM3_ESM.jpg
Supplemntary Figure S3 A. Comparison of IL-32 isoform expression levels in ER positive (S3A) tumors. Levels of IL32 isoforms, IL32α, IL32β, IL32γ and IL32ε transcripts were studied using custom designed TaqMan assays obtained from Thermofisher, for individual isoforms as follows: AII1ONV: NM_001012631 (IL-32β); AIMSI6 J:NM_001308078 (IL-32γ); AILJK0B:NM_001012636 (IL-32δ); AIN1HCR:NM_001012634 (IL-32ε). PUM1 (Hs 00472881_ml) was used as endogenous control for the studies and expression levels were normalized and computed relative to the levels of the endogenous control (JPEG 420 kb)
432_2017_2431_MOESM4_ESM.jpg
Supplemntary Figure S3 B. Comparison of IL-32 isoform expression levels in ER negative tumors (S3B). Levels of IL32 isoforms, IL32α, IL32β, IL32γ and IL32ε transcripts were studied using custom designed TaqMan assays obtained from Thermofisher, for individual isoforms as follows: AII1ONV: NM_001012631 (IL-32β); AIMSI6 J:NM_001308078 (IL-32γ); AILJK0B:NM_001012636 (IL-32δ); AIN1HCR:NM_001012634 (IL-32ε). PUM1 (Hs 00472881_ml) was used as endogenous control for the studies and expression levels were normalized and computed relative to the levels of the endogenous control (JPEG 401 kb)
Rights and permissions
About this article
Cite this article
Bhat, S., Gardi, N., Hake, S. et al. Impact of intra-tumoral IL17A and IL32 gene expression on T-cell responses and lymph node status in breast cancer patients. J Cancer Res Clin Oncol 143, 1745–1756 (2017). https://doi.org/10.1007/s00432-017-2431-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-017-2431-5