Abstract
Introduction
An emerging hypothesis suggests that cytokines could play an important role in cancer as potential modulators of angiogenesis and leucocyte infiltration.
Methods
A novel multiplexed flow cytometry technology was used to measure the expression of 17 cytokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 [p70], IL-13, IL-17, granulocyte colony-stimulating factor [CSF], granulocyte-macrophage CSF, IFN-γ, monocyte chemoattractant protein [MCP]-1, macrophage inflammatory protein [MIP]-1β, tumour necrosis factor [TNF]-α) at the protein level in 105 breast carcinoma. B lymphocyte, T lymphocyte and macrophage levels were determined by immunohistochemistry.
Results
Fourteen of the 17 cytokines were expressed in breast carcinoma, whereas only nine cytokines could be detected in normal breast. Most cytokines were more abundant in breast carcinoma than in normal breast, with IL-6, IL-8, granulocyte CSF, IFN-γ, MCP-1 and MIP-1β being very abundant. IL-2, IL-6, IL-8, IL-10, IFN-γ, MCP-1, MIP-1β and TNF-α, and to a lesser extent IL-1β and IL-13 exhibited levels of expression that were inversely correlated to oestrogen receptor and progesterone receptor status. Most cytokines were not correlated with age at cancer diagnosis, tumour size, histological type, or lymph node status. However, IL-1β, IL-6, IL-8, IL-10, IL-12, MCP-1 and MIP-1β were more abundant in high-grade tumours than in low-grade tumours. In addition, IL-8 and MIP-1β were expressed to a greater degree in HER2-positive than in HER2-negative patients. The expression of most of the studied cytokines was correlated to levels of activator protein-1, which is known to regulate numerous cytokines. Overexpression of MCP-1 and MIP-1β were linked to B lymphocyte, T lymphocyte and macrophage infiltration, whereas high levels of IL-8 were correlated with high macrophage content in tumour. Moreover, IL-8 positive tumours exhibited increased vascularization.
Conclusion
We found that multiple cytokines were overexpressed in oestrogen receptor negative breast carcinoma, and that the three major cytokines – MCP-1, MIP-1β and IL-8 – were correlated with inflammatory cell component, which could account for the aggressiveness of these tumours.
Similar content being viewed by others
Introduction
Breast cancer represents the leading cause of cancer death among women in developed countries [1]. Among the various prognostic factors, lack of oestrogen receptor (ER) has consistently been associated with poorer prognosis [2]. Most human breast cancers express ER-α, and the presence of this receptor is generally considered an indication of hormone dependence [3]. In addition to ER-α, cytokines are now emerging as factors that are potentially involved in breast carcinogenesis [4, 5]. Cytokines constitute a diverse group of proteins that include haematopoietic growth factors, interferons, lymphokines and chemokines [6].
Cytokines are produced by many cell populations, but the predominant suppliers are T-helper (Th) cells and macrophages. Th cells have two important functions: to stimulate cellular immunity and inflammation, and to stimulate B cells to produce antibody. Two functionally distinct subsets of Th cells (Th1 and Th2) secrete cytokines that promote these different activities. Th1 cells produce IL-2 and IFN-γ, which activate cytotoxic lymphocytes and macrophages to stimulate cellular immunity and inflammation [7]. Th2 cells secrete IL-4 and IL-5, which stimulate antibody production by B cells.
It has become evident that cancer tissues also produce cytokines [4, 8]. Numerous studies have analyzed the expression of diverse cytokines independently in breast cancer, but only a few have examined the profile of a group of cytokines at the tumour site. In the present study we screened 17 members of the cytokine family (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 [p70], IL-13, IL-17, granulocyte colony-stimulating factor [G-CSF], granulocyte-macrophage colony-stimulating factor [GM-CSF], IFN-γ, monocyte chemoattractant protein [MCP]-1, macrophage inflammatory protein [MIP]-1β and tumour necrosis factor [TNF]-α) in breast cancer tissues. We found that most cytokines were overexpressed in breast cancer compared with normal tissues. IL-2, IL-6, IL-8, IL-10, INF-γ, MCP-1, MIP-1β, TNF-α, IL-1β and IL-13 were inversely correlated with ER and progesterone receptor (PR) status. Most of the cytokines were not correlated with patient age, tumour size, histological type, or lymph node status. IL-1β, IL-6, IL-8, IL-10, IL-12, MCP-1 and MIP-1β were abundant in high-grade tumours. Moreover, IL-8 and MIP-1β were linked to HER2 expression. In addition, IL-8 positive tumours exhibited greater vascularization. Robust expression of IL-8, MCP-1 and MIP-1β was correlated with a strong inflammatory cell component.
Materials and methods
Patients and samples
Breast tumour surgical specimens were selected from the files of the Pathology Department (CRLC Val d'Aurelle) tumour bank. Breast biopsies were from surgical waste; they were not formal samples taken for diagnosis and they did not undergo genetic evaluation. The samples were selected by a pathologist from fresh surgical specimens immediately after resection. For each sample, a twin sample from the same tissue was taken at the same time, embedded in paraffin and evaluated to ensure that the tissue was indeed tumourous. When possible, a sample of normal breast tissue in the vicinity of the tumour was also taken. All of the patients had primary unilateral, nonmetastatic breast carcinoma, and complete clinical, histological and biological information was available for all of them. The clinical and pathological characteristics of the patients are given in Additional file 1. The tumours included invasive carcinoma, mainly ductal and lobular, and other rare subtypes (mixed ductal and lobular carcinoma, medullary carcinoma and apocrine carcinoma). The tumours were graded according to the modified Nottingham SBR system [9] and categorized according to the sixth edition of the AJCC (American Joint Committee on Cancer) Cancer Staging Manual for pTNM staging. ER-α, progesterone receptor (PR) and HER2 status were determined at the protein level by immunohistochemistry. When equivocal, HER2 results were confirmed by fluorescence in situ hybridization.
Immunohistochemistry and fluorescence in situhybridization analysis
Representative tissue sections from cases of invasive breast carcinoma were fixed in 10% formalin or in alcohol-formalin-acetic acid and embedded in paraffin. Sections from each case were deparaffinized, rehydrated and subsequently subjected to heat induced antigen retrieval by immersing them, depending on the antibody, either in a water bath with a sodium citrate buffer (pH 6) or an EDTA buffer (pH 8). Immunohistochemistry was performed using a Dako autostainer (Dako, Glostrup, Denmark). Then, the sections were incubated with the following primary antibodies: ER-α (clone 6F11, monoclonal; Novocastra, Newcastle-Upon-Tyne, UK), PR (clone PgR636, monoclonal; DakoCytomation, Glostrup, Denmark) and HER 2 (A0485, polyclonal; DakoCytomation). They were respectively used at 1:50, 1:100 and 1:1500 dilutions with an incubation time of 30 min. Antibody was localized using the LSAB® 2 Detection System (Dako). Diaminobenzidine (Dako) was used as the chromogen and the sections were lightly counterstained with haematoxylin. ER and PR expression was considered positive when at least 10% of invasive tumoural cells exhibited nuclear staining, regardless of intensity. For HER2, the immunohistochemical score was assigned according to the Herceptest® scoring system (0 = absence of membranous staining or fewer than 10% positive cells; 1+ = more than 10% stained cells with weak and incomplete membranous staining; 2+ = more than 10% stained cells with weak or moderate complete membranous staining; and 3+ = more than 10% stained cells with strong and complete membranous staining). A case was considered to be HER2 overexpressing if it scored 3+. A case scoring 2+ was considered to be HER2 overexpressing only if fluorescence in situ hybridization analysis using the PathVysion HER-2 Probe Kit (Vysis, Downer's Grove, IL, USA) revealed HER2 gene amplification.
The inflammatory cell component was evaluated in a subset of 10 tumours exhibiting high levels of IL-8 and 10 tumours with low IL-8 content using CD3 (T-lymphocyte lineage), CD20 (B-lymphocyte lineage) and CD68 (macrophages) antibodies. The intensity of each pattern of inflammatory infiltrate was graded as absent (0), minimal (1), moderate (2), or marked (3). The staining result for each antibody was scored by two investigators who were blinded as to the IL-8 status of the patients. Vessels were assessed within the invasive carcinoma in CD31-stained sections. The most vascular areas were identified by examination at low power (× 40 magnification). The number of clusters or single cells stained for CD31 was recorded from the three most vascular × 400 fields (0.18 mm2 each) and the vascular density (number of vessels/mm2) was calculated.
Protein extract preparation
Biopsies were first crushed in liquid nitrogen. The powder was then resuspended in TEG (10 mmol/l Tris-HCl [pH 7.4], 1.5 mmol/l EDTA and 10% glycerol) containing protease inhibitors (5 μg/ml aprotinin, leupeptin and pepstatin A, and 0.1 mmol/l phenylmethylsulfonyl fluoride). The mixture was then sonicated and the cellular debris was pelleted by centrifugation at 13,000 g for 20 minutes at 4°C in microfuge tubes.
Cytokine multiplexed bioplex assay
A panel of 17 cytokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 [p70], IL-13, IL-17, G-CSF, GM-CSF, IFN-γ, MCP-1, MIP-1β, TNF-α) was measured in duplicate using the bioplex cytokine multiplexed assay (BioRad, Hercules, CA, USA), in accordance with the manufacturer's instructions (Additional file 2). This novel multiplexed, particle-based, flow cytometric assay uses anticytokine monoclonal antibodies linked to microspheres incorporating distinct proportions of two fluorescent dyes. Five hundred micrograms of total protein extracts of breast biopsies was used to measure cytokine concentration. For each cytokine, eight standards ranging from 2 to 32,000 pg/ml were used, and the minimum detectable dose was 10 pg/ml. The assay was validated by comparing the results obtained by bioplex measure of IL-8 with a conventional enzyme-linked immunosorbent assay measure of IL-8 (Additional file 3).
Gel shift assays
Gel shift assays were performed as described previously [10]. Briefly, 30,000 count per minute of the [32P]-labelled double-strand oligonucleotides were combined with 1 μg poly(dI-dC) and 5 μg total cell extract. The reaction buffer contained 20 mmol/l Hepes (pH 7.9), 1 mmol/l DTT, 50 mmol/l KCl, 10% glycerol and 2.5 mmol/l MgCl2. Protein-DNA complexes were separated from the free probe by nondenaturating gel electrophoresis with 4% polyacrylamide gels in 0.5 × TBE. We used the activator protein (AP)-1 consensus oligonucleotide: 3'-CGCTTGATGAGTCAGCCGGAA-5'. The levels of shifted complexes were detected and quantified by counting using a Fujix-Bas 1000 (Fuji Photo-Film, Tokyo, Japan).
Statistical analysis
Data were summarized by frequency for categorical variables and by median and range for continuous variables. Correlation between variables (cytokines) was evaluated using the Spearman correlation coefficient after log transformation. Associations between categorical variables were examined using χ2 analyses.
The median levels of expression of analyzed interleukins were compared using Kruskal-Wallis test according to clinical data (age, ER, PR, SBR status, pT and pN status) or analysis of variance if possible.
For all statistical analysis, P < 0.05 was considered statistically significant.
Results
Breast tumours overexpress cytokines
A total of 105 samples of primary unilateral, nonmetastatic breast carcinomas (Additional file 1) and 13 healthy breast biopsies were analyzed for cytokine expression at the protein level (Table 1). This was made possible by the use of a novel technology that couples enzyme-linked immunosorbent assay to clourescence-activated cell sorting analysis, which allows detection of multiple proteins in a single well.
We observed that healthy breast samples expressed very low levels of cytokines except for IFN-γ and MIP-1β (Table 1). In contrast, breast tumours exhibited high levels of IL-6, G-CSF and IFN-γ, and extremely high levels of IL-8, MCP-1 and MIP-1β. The cytokines IL-5, IL-17 and GM-CSF were not detected in either normal or tumour sample, whereas IL-1β, IL-2, IL-4, G-CSF and IL-10 were only detected in breast carcinoma. IL-6, IL-8, IL-12, IL-13, IFN-γ, MCP-1, MIP-1β and TNF-α were significantly more abundant in carcinoma than in normal breast. The greatest differences in expression between normal breast tissue and carcinoma occurred in IL-8, MCP-1 and MIP-1β, which were 60-fold more abundant in carcinoma than in healthy breast, followed by IL-6, which was about 14-fold overexpressed in carcinoma. In summary, we found that breast tumours express higher levels of multiple cytokines than do normal tissues.
Correlation between cytokines and ER and PR
We next evaluated whether there was any correlation between cytokine expression and ER levels (Table 2). We observed that IL-4 and G-CSF were not correlated with ER status. On the other hand, IL-2, IL-6, IL-8, IL-10, IFN-γ, MCP-1, MIP-1β, TNF-α and, to a lesser extent, IL-1β and IL-13 were significantly overexpressed in ER-negative tumours compared with ER-positive ones, with the greatest differences observed for IL-8 and MCP-1.
We performed the same type of analysis to identify whether there was any correlation between cytokine levels and PR expression (Table 2). IL-2 and IL-13 were not correlated with PR status, whereas IL-1β, IL-6, IL-8, IL-10, IFN-γ, MCP-1, MIP-1β, TNF-α and, to a lesser extent, IL-4, IL-12 and G-CSF were more abundant in PR-negative tumours than in PR-positive ones. The greatest differences were obtained for IL-8, IL-6 and MCP-1.
The fact that some cytokines were correlated with either ER or PR led us to analyze the distribution of all cytokines by combining both factors (Additional file 4). Three categories were defined, corresponding to true ER negative (ER-/PR-) and ER positive (ER+/PR+), and intermediate (ER-/PR+ or ER+/PR-) status. This analysis revealed that IL-1β, IL-2, IL-6, IL-8, IL-10, IL-12, IFN-γ, MCP-1, MIP-1β and TNF-α were inversely correlated with true ER status. The most abundant cytokines in true ER negative breast carcinoma were IL-8, MCP-1 and MIP-1β.
Correlation with other clinical parameters
We then evaluated whether cytokine expression could be linked to clinical parameters such as tumour size, lymph node status, histological grade, or patient age. The cytokine profile was not linked to tumour size or to histological type. We did not observe any correlation between the age of patients at cancer diagnosis and cytokine expression, except for IL-1β, which was inversely correlated with the age of the patient: at age 50 years or younger the median IL-1β level was 3.14 fg/μg (range 0.2 to 194.2 fg/μg); at age 50–65 years the median IL-1β level was 2.13 fg/μg (range 0 to 42.4 fg/μg); and at age above 65 years the median IL-1β level was 1.9 fg/μg (range 0 to 260.6 fg/μg; P = 0.033). Lymph node status was not linked to any of the analyzed cytokines.
Because ER-negative tumours are generally of higher grade than ER-positive ones (which was confirmed in our samples; data not shown), we determined whether cytokine levels could be correlated with grade. Most of the cytokines that were preferentially present in ER-negative tumours (IL-1β, IL-6, IL-8, IL-10, IL-12, MCP-1 and MIP-1β) were also more abundant in high-grade tumours (Table 3).
Another factor that is frequently linked to grade and ER status is the HER2 level. We observed a correlation between HER2 levels and IL-8 and MIP-1β, which were expressed to a greater degree in HER2-positive patients; the median (range) IL-8 levels in HER2-negative patients and HER2-positive patients were 50.8 fg/μg (0 to 15,890 fg/μg) and 192.6 fg/μg (23.7 to 8,357 fg/μg), respectively (P = 0.014); and for MIP-1β the median (range) levels were 514.9 fg/μg (42.6 to 14,439 fg/μg) and 976.1 fg/μg (44.6 to 4,978 fg/μg), respectively (P = 0.021). These data suggest that high cytokine levels are associated with poor prognostic factors such as high grade, ER-negative status, and HER2-positive tumours.
AP-1 transcription factors are known to regulate the expression of many cytokines. To address this issue, we performed gel shift assays measuring global AP-1 binding to specific DNA-binding sites. As reported by others [11], AP-1 was more abundant in ER-negative tumour samples than in ER-positive ones (P < 0.001). There was also a higher level of HER2 in AP-1-positive tumours (P = 0.025). In contrast, AP-1 status was not correlated with PR expression, grade, size of the tumour and lymph node status. Interestingly, high AP-1 levels correlated with high levels of expression of several cytokines, including IL-1β, IL-2, IL-6, IL-8, IL-10, IL-12, IFN-γ, MCP-1 and TNF-α (Table 4). Among these cytokines, the greatest differences were observed for IL-8 and MCP-1, which respectively exhibited 3.9-fold and 2.7-fold higher expression in AP-1-positive tumours than in AP-1-negative ones (Table 4).
Tumors expressing high levels of IL-8 exhibit high vascularization
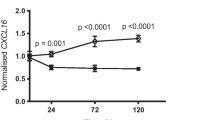
We next focused our attention on the possible association between high expression of IL-8 and vascularization. We analyzed 10 tumours exhibiting high levels of IL-8 and 10 tumours with low IL-8 content. A microscopic analysis showed that tumours with high expression of IL-8 were also more vascularized (median of 125 vessels/mm2) than were tumours with low IL-8 content (48 vessels/mm2; P = 0.0002; Table 5). Overall, these results suggest that IL-8 expression is associated with high neovascularization.
Leucocyte infiltration is correlated with high levels of cytokines
Because IL-8, MCP-1 and MIP-1β, the most highly expressed chemokines in cancer tissues, are potent chemotactic molecules, we investigated whether their expression could be correlated with tumour leucocyte infiltration. We conducted a complete immunohistochemical analysis of our collective of tumour samples using CD3 (B lymphocytes), CD20 (T lymphocytes) and CD68 (macrophages) antibodies. A representative image is shown in Figure 1. Normal breast exhibited no to low staining, whereas breast cancer tissues exhibited a complete range of leucocyte infiltration from low to high levels. Our findings indicate that high levels of IL-8 were correlated with high macrophage infiltration, whereas strong expression of MCP-1 and MIP-1β was correlated with robust presence of B lymphocytes, T lymphocytes and macrophages (Tables 6, 7, 8).
Breast tumours exhibit various levels of leucocyte infiltration. Normal breast and breast carcinoma were stained for B lymphocytes, T lymphocytes and macrophages using CD3, CD20 and CD68 immunostaining. Representative pictures of (a) normal breast, (b) tumour with weak leucocyte infiltration and (c) tumour with robust leucocyte infiltration are shown here.
Discussion
It is well known that the interactions of tumour cells with their microenvironment may affect tumour growth and metastasis formation. Among these, inflammatory cells and cytokines were recently suggested to play a key role in breast carcinoma (for review, see Wilson and Balkwill [4]).
A previous study of breast cancer patients [12] showed that IL-8 RNA was more abundant in the neoplastic than in the normal population, whereas no difference was observed for IL-1α, IL-1β, IL-4, or IL-6. In addition, transcripts for IL-2, IL-4, IL-5, IL-7, TNF-α and IFN-γ were not detected in either group [12, 13]. Increased serum levels of the cytokines IL-6, IL-8 and IL-10 have also been observed in patients suffering from breast cancer as compared with healthy women [14, 15]. Among the 17 cytokines we analyzed, eight were not detected in normal breast (IL-1β, IL-2, IL-4, IL-5, IL-10, IL-17, G-CSF and GM-CSF) and three were absent from breast carcinoma (IL-5, IL-17 and GM-CSF). With the exception of IL-7, all cytokines that were present in both tissues were significantly overexpressed in breast carcinoma.
To date, the correlation between cytokine expression and clinical parameters remains elusive. We observed that IL-1β, IL-6, IL-8, IL-10, IL-12, MCP-1 and MIP-1β correlated with grade. IL-8 and MIP-1β were also linked to HER2 status, which is in agreement with reports demonstrating that IL-8 is regulated by HER2 in breast cancer cell lines [16]. In addition, IL-1β was inversely correlated to age. IL-1β was previously shown to be strongly expressed in high-grade invasive breast carcinoma as compared with in situ ductal carcinoma or benign lesions [17]. In contrast to these results, Green and coworkers [12] did not observe any correlation between the cytokines IL-1α, IL-1β, IL-4, IL-6, IL-8, TNF-α, TNF-β, IL-2, IL-5, IL-7 and IFN-γ and tumour histological grade, patient age or lymph node metastasis in breast cancer patients, but that study analyzed only RNA levels.
Of particular note, in our study we found an inverse correlation between expression of ER and cytokines, which is in agreement the findings of other studies [8, 18]. An inverse correlation between IL-8 and ER or PR in breast cancer biopsies has also been demonstrated [19]. Several studies have reported that IL-1α, IL-1β, or IL-6 levels correlated inversely with ER levels [20–22]. This inverse correlation between cytokines and ER status not only may reflect the greater aggressiveness of this subtype of breast tumours but it could also be the result of a direct regulation of cytokine expression by ER. Several reports have demonstrated a direct downregulation of cytokines by ER in different organs. This is not only the case for IL-6 [23, 24] and IL-8 [8, 18] but also for IL-1β (for review, see Pfeilschifter and coworkers [24]), IL-2 [25], IL-10 [26], IL-12 (for review, see Salem [27]), GM-CSF [24], IFN-γ [28, 29], MCP-1 [30, 31] and TNF-α [24]. PR is also known to downregulate the expression of a number of cytokines, including IL-1β [32], IL-6 [30, 33], IL-8 [34], MCP-1 [32, 35] and TNF-α [32], which is in agreement with our findings.
According to our work, Th1 cytokines (IL-2 and IFN-γ) and Th2 cytokines (such as IL-4 and IL-5) are not the major cytokines produced by tumours. However, several cytokines produced by monocytes (IL-1β, IL-6 and MCP-1) or macrophages (such as IL-1β, IL-6, IL-8, MIP-1β and TNF-α) are highly expressed by the tumour. We observed that tumours displaying high levels of IL-8 exhibited significantly greater vascularization. A role for IL-8 in breast cancer angiogenesis has also been suggested by in vitro studies [18], but to our knowledge ours is the first study to report an association between IL-8 levels and angiogenesis in breast cancer samples.
We found that tumours expressing high levels of MCP-1 and MIP-1β had greater B lymphocyte, T lymphocyte and macrophage content than did tumours expressing low levels of these chemokines. Similarly, robust expression of IL-8 was correlated with marked macrophage infiltration. Our results are thus in agreement with previous studies that showed a correlation between the number of tumour associated macrophages and IL-6, IL-8 or MCP-1 levels in breast and cervical cancers [36–38]. On the other hand, the correlation between MIP-1β and macrophage infiltration has not been reported to date, or has the association of high levels of MCP-1 and MIP-1β with the presence of B and T lymphocytes in breast carcinoma. The precise role played by leucocytes in tumours remains to be elucidated in larger studies, which may determine whether they contribute to tumour growth.
Conclusion
The pattern of expression of multiple cytokines in breast cancer tissues that we report is the first step in elucidating the involvement of cytokines in breast cancer. Our data suggest that cytokines could be involved in the aggressiveness of ER-negative breast tumours. In addition, robust expression of IL-8, MCP-1 and MIP-1β, which are the three major chemokines present in breast tumours, is correlated with marked leucocyte infiltration. We therefore believe that our work provides justification for further studies to determine more precisely the role played by these cytokines in breast cancer.
Abbreviations
- AP:
-
activator protein
- ER:
-
oestrogen receptor
- G-CSF:
-
granulocyte colony-stimulating factor
- GM-CSF:
-
granulocyte-macrophage colony-stimulating factor
- IL:
-
interleukin
- IFN:
-
interferon
- MCP:
-
monocyte chemoattractant protein
- MIP:
-
macrophage inflammatory protein
- PR:
-
progesterone receptor
- Th:
-
T-helper (cell)
- TNF:
-
tumour necrosis factor.
References
Parkin DM, Bray F, Ferlay J, Pisani P: Global cancer statistics, 2002. CA Cancer J Clin. 2005, 55: 74-108.
Skoog L, Humla S, Axelsson M, Frost M, Norman A, Nordenskjold B, Wallgren A: Estrogen receptor levels and survival of breast cancer patients. A study on patients participating in randomized trials of adjuvant therapy. Acta Oncol. 1987, 26: 95-100.
McGuire WL: Endocrine therapy of breast cancer. Annu Rev Med. 1975, 26: 353-363. 10.1146/annurev.me.26.020175.002033.
Wilson J, Balkwill F: The role of cytokines in the epithelial cancer microenvironment. Semin Cancer Biol. 2002, 12: 113-120. 10.1006/scbi.2001.0419.
Boon T, Van den Eynde B: Tumour immunology. Curr Opin Immunol. 2003, 15: 129-130. 10.1016/S0952-7915(03)00010-4.
Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S: CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol. 2004, 25: 201-209. 10.1016/j.it.2004.02.006.
Elenkov IJ, Chrousos GP: Stress hormones, Th1/Th2 patterns, Pro/Anti-inflammatory Cytokines and Susceptibility to Disease. Trends Endocrinol Metab. 1999, 10: 359-368. 10.1016/S1043-2760(99)00188-5.
Freund A, Chauveau C, Brouillet JP, Lucas A, Lacroix M, Licznar A, Vignon F, Lazennec G: IL-8 expression and its possible relationship with estrogen-receptor-negative status of breast cancer cells. Oncogene. 2003, 22: 256-265. 10.1038/sj.onc.1206113.
Bloom HJ, Richardson WW: Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957, 11: 359-377.
Freund A, Jolivel V, Durand S, Kersual N, Chalbos D, Chavey C, Vignon F, Lazennec G: Mechanisms underlying differential expression of interleukin-8 in breast cancer cells. Oncogene. 2004, 23: 6105-6114. 10.1038/sj.onc.1207815.
Bamberger AM, Methner C, Lisboa BW, Stadtler C, Schulte HM, Loning T, Milde-Langosch K: Expression pattern of the AP-1 family in breast cancer: association of fosB expression with a well-differentiated, receptor-positive tumor phenotype. Int J Cancer. 1999, 84: 533-538. 10.1002/(SICI)1097-0215(19991022)84:5<533::AID-IJC16>3.0.CO;2-J.
Green AR, Green VL, White MC, Speirs V: Expression of cytokine messenger RNA in normal and neoplastic human breast tissue: identification of interleukin-8 as a potential regulatory factor in breast tumours. Int J Cancer. 1997, 72: 937-941. 10.1002/(SICI)1097-0215(19970917)72:6<937::AID-IJC3>3.0.CO;2-Q.
Venetsanakos E, Beckman I, Bradley J, Skinner JM: High incidence of interleukin 10 mRNA but not interleukin 2 mRNA detected in human breast tumours. Br J Cancer. 1997, 75: 1826-1830.
Kozlowski L, Zakrzewska I, Tokajuk P, Wojtukiewicz MZ: Concentration of interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of breast cancer patients. Rocz Akad Med Bialymst. 2003, 48: 82-84.
Benoy I, Salgado R, Colpaert C, Weytjens R, Vermeulen PB, Dirix LY: Serum interleukin 6, plasma VEGF, serum VEGF, and VEGF platelet load in breast cancer patients. Clin Breast Cancer. 2002, 2: 311-315.
Wen XF, Yang G, Mao W, Thornton A, Liu J, Bast RC, Le XF: HER2 signaling modulates the equilibrium between pro- and antiangiogenic factors via distinct pathways: implications for HER2-targeted antibody therapy. Oncogene. 2006, 25: 6986-6996. 10.1038/sj.onc.1209685.
Jin L, Yuan RQ, Fuchs A, Yao Y, Joseph A, Schwall R, Schnitt SJ, Guida A, Hastings HM, Andres J, et al: Expression of interleukin-1beta in human breast carcinoma. Cancer. 1997, 80: 421-434. 10.1002/(SICI)1097-0142(19970801)80:3<421::AID-CNCR10>3.0.CO;2-Z.
Lin Y, Huang R, Chen L, Li S, Shi Q, Jordan C, Huang RP: Identification of interleukin-8 as estrogen receptor-regulated factor involved in breast cancer invasion and angiogenesis by protein arrays. Int J Cancer. 2004, 109: 507-515. 10.1002/ijc.11724.
Pantschenko AG, Pushkar I, Anderson KH, Wang Y, Miller LJ, Kurtzman SH, Barrows G, Kreutzer DL: The interleukin-1 family of cytokines and receptors in human breast cancer: implications for tumor progression. Int J Oncol. 2003, 23: 269-284.
Miller LJ, Kurtzman SH, Anderson K, Wang Y, Stankus M, Renna M, Lindquist R, Barrows G, Kreutzer DL: Interleukin-1 family expression in human breast cancer: interleukin-1 receptor antagonist. Cancer Invest. 2000, 18: 293-302.
Singer CF, Kronsteiner N, Hudelist G, Marton E, Walter I, Kubista M, Czerwenka K, Schreiber M, Seifert M, Kubista E: Interleukin 1 system and sex steroid receptor expression in human breast cancer: interleukin 1alpha protein secretion is correlated with malignant phenotype. Clin Cancer Res. 2003, 9: 4877-4883.
Chiu JJ, Sgagias MK, Cowan KH: Interleukin 6 acts as a paracrine growth factor in human mammary carcinoma cell lines. Clin Cancer Res. 1996, 2: 215-221.
Galien R, Garcia T: Estrogen receptor impairs interleukin-6 expression by preventing protein binding on the NF-kappaB site. Nucleic Acids Res. 1997, 25: 2424-2429. 10.1093/nar/25.12.2424.
Pfeilschifter J, Koditz R, Pfohl M, Schatz H: Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002, 23: 90-119. 10.1210/er.23.1.90.
McMurray RW, Ndebele K, Hardy KJ, Jenkins JK: 17-beta-estradiol suppresses IL-2 and IL-2 receptor. Cytokine. 2001, 14: 324-333. 10.1006/cyto.2001.0900.
Carruba G, D'Agostino P, Miele M, Calabro M, Barbera C, Bella GD, Milano S, Ferlazzo V, Caruso R, Rosa ML, et al: Estrogen regulates cytokine production and apoptosis in PMA-differentiated, macrophage-like U937 cells. J Cell Biochem. 2003, 90: 187-196. 10.1002/jcb.10607.
Salem ML: Estrogen, a double-edged sword: modulation of TH1- and TH2-mediated inflammations by differential regulation of TH1/TH2 cytokine production. Curr Drug Targets Inflamm Allergy. 2004, 3: 97-104. 10.2174/1568010043483944.
Curran EM, Judy BM, Newton LG, Lubahn DB, Rottinghaus GE, Macdonald RS, Franklin C, Estes DM: Dietary soy phytoestrogens and ERalpha signalling modulate interferon gamma production in response to bacterial infection. Clin Exp Immunol. 2004, 135: 219-225. 10.1111/j.1365-2249.2003.02368.x.
Nakaya M, Yamasaki M, Miyazaki Y, Tachibana H, Yamada K: Estrogenic compounds suppressed interferon-gamma production in mouse splenocytes through direct cell-cell interaction. In Vitro Cell Dev Biol Anim. 2003, 39: 383-387. 10.1290/1543-706X(2003)039<0383:ECSIPI>2.0.CO;2.
Kanda N, Watanabe S: 17Beta-estradiol inhibits MCP-1 production in human keratinocytes. J Invest Dermatol. 2003, 120: 1058-1066. 10.1046/j.1523-1747.2003.12255.x.
Fanti P, Nazareth M, Bucelli R, Mineo M, Gibbs K, Kumin M, Grzybek K, Hoeltke J, Raiber L, Poppenberg K, et al: Estrogen decreases chemokine levels in murine mammary tissue: implications for the regulatory role of MIP-1 alpha and MCP-1/JE in mammary tumor formation. Endocrine. 2003, 22: 161-168. 10.1385/ENDO:22:2:161.
Davies S, Dai D, Wolf DM, Leslie KK: Immunomodulatory and transcriptional effects of progesterone through progesterone A and B receptors in Hec50co poorly differentiated endometrial cancer cells. J Soc Gynecol Investig. 2004, 11: 494-499. 10.1016/j.jsgi.2004.04.003.
Bamberger CM, Else T, Bamberger AM, Beil FU, Schulte HM: Dissociative glucocorticoid activity of medroxyprogesterone acetate in normal human lymphocytes. J Clin Endocrinol Metab. 1999, 84: 4055-4061. 10.1210/jc.84.11.4055.
Loudon JA, Elliott CL, Hills F, Bennett PR: Progesterone represses interleukin-8 and cyclo-oxygenase-2 in human lower segment fibroblast cells and amnion epithelial cells. Biol Reprod. 2003, 69: 331-337. 10.1095/biolreprod.102.013698.
Kelly RW, Carr GG, Riley SC: The inhibition of synthesis of a beta-chemokine, monocyte chemotactic protein-1 (MCP-1) by progesterone. Biochem Biophys Res Commun. 1997, 239: 557-561. 10.1006/bbrc.1997.7502.
Fujimoto J, Sakaguchi H, Aoki I, Tamaya T: Clinical implications of expression of interleukin 8 related to angiogenesis in uterine cervical cancers. Cancer Res. 2000, 60: 2632-2601.
Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, Koike M, Inadera H, Matsushima K: Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000, 6: 3282-3289.
Saji H, Koike M, Yamori T, Saji S, Seiki M, Matsushima K, Toi M: Significant correlation of monocyte chemoattractant protein-1 expression with neovascularization and progression of breast carcinoma. Cancer. 2001, 92: 1085-1091. 10.1002/1097-0142(20010901)92:5<1085::AID-CNCR1424>3.0.CO;2-K.
Acknowledgements
This work was supported by grants from ARC (Association pour la Recherche contre le Cancer, Grant No. 3582 [GL]) and la Ligue Nationale contre le Cancer (GL). CC was also supported by the Ligue Nationale contre le cancer. S. Burlinchon was supported by ARTP. We are grateful to Dr A Tsicopoulos for helpful discussions on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
CC carried out the bioplex experiments and helped to draft the manuscript. FB interpreted the immunhistochemistry slides and revised the manuscript. SGB performed all stastical analysis and revised the manuscript. SB, SR and FB performed the immunohistochemistry experiments and FB also revised the manuscript. DL was involved in the bioplex experiments. GL conceived the study, supervised the work, drafted the manuscript, processed the biopsy samples, and performed the bioplex and gel shift experiments.
Carine Chavey, Frédéric Bibeau contributed equally to this work.
Electronic supplementary material
13058_2006_1613_MOESM1_ESM.doc
Additional file 1: A Word document containing a table that summarizes the clinical characteristics of the patients. (DOC 73 KB)
13058_2006_1613_MOESM2_ESM.doc
Additional file 2: A word document containing a summary of the manufacturer's instructions on the cytokine multiplexed bioplex assay. (DOC 25 KB)
13058_2006_1613_MOESM3_ESM.pdf
Additional file 3: A pdf file including a figure the compares IL-8 concentration measured by bioplex assay and by conventional enzyme-linked immunosorbent assay (n = 93). (PDF 14 KB)
13058_2006_1613_MOESM4_ESM.pdf
Additional file 4: A pdf file including a table that summarizes the distribution of cytokines by hormone receptor status. (PDF 21 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Chavey, C., Bibeau, F., Gourgou-Bourgade, S. et al. Oestrogen receptor negative breast cancers exhibit high cytokine content. Breast Cancer Res 9, R15 (2007). https://doi.org/10.1186/bcr1648
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/bcr1648