Abstract
Purpose
Hepatocyte nuclear factor 6 (HNF6) is a liver-enriched transcription factor and highly expressed in mature bile duct epithelial cells. This study sought to investigate the role of HNF6, particularly the molecular mechanisms for how HNF6 is involved in the growth and metastasis of cholangiocarcinoma (CCA) cells.
Methods
The expression of HNF6, miR-122 and key molecules was examined by Western blot analysis and real-time RT-PCR. Stable transfectants, HCCC-HNFlow and RBE-HNFhigh, were generated from human CCA HCCC-9810 and RBE cells, respectively. The regulatory effect of HNF6 on miR-122 was evaluated by luciferase reporter assay. Cell proliferation, cycle distribution, migration and invasion were analyzed. The xenograft model was used to assess the effects of HNF6 overexpression on tumorigenesis, growth, metastasis and therapeutic potentials.
Results
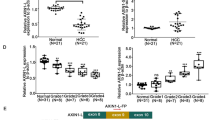
Human CCA tissues and cells expressed lower levels of HNF6, which positively correlated with miR-122. HNF6 regulated the expression of miR-122 by stimulating its promoter. HNF6 overexpression inhibited cell proliferation by inducing cell cycle arrest at G1 phase through regulating miR-122, cyclin G1 and insulin-like growth factor-1 receptor. HNF6 inhibited the migration and invasion of CCA cells by regulating matrix metalloproteinase-2 and metalloproteinase-9, reversion-inducing-cysteine-rich protein with kazal motifs, E-cadherin and N-cadherin. Co-transfection of anti-miR-122 abrogated the effects of HNF6. HNF6 overexpression inhibited the ability of cells to form tumors and to metastasize to the lungs of mice, and the growth of established tumors.
Conclusions
The results indicate that HNF6 may serve as a tumor suppressor by regulating miR-122, and its overexpression may represent a mechanism-based therapy for CCA.








Similar content being viewed by others
References
Bandiera S, Pfeffer S, Baumert TF, Zeisel MB (2015) miR-122—a key factor and therapeutic target in liver disease. J Hepatol 62:448–457. doi:10.1016/j.jhep.2014.10.004
Blechacz B, Komuta M, Roskams T, Gores GJ (2011) Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol 8:512–522. doi:10.1038/nrgastro.2011.131
Bourboulia D, Stetler-Stevenson WG (2010) Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): positive and negative regulators in tumor cell adhesion. Semin Cancer Biol 20:161–168. doi:10.1016/j.semcancer.2010.05.002
Bridgewater J et al (2014) Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 60:1268–1289. doi:10.1016/j.jhep.2014.01.021
Budhu A et al (2008) Identification of metastasis-related microRNAs in hepatocellular carcinoma. Hepatology 47:897–907. doi:10.1002/hep.22160
Coulouarn C, Factor VM, Andersen JB, Durkin ME, Thorgeirsson SS (2009) Loss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties. Oncogene 28:3526–3536. doi:10.1038/onc.2009.211
Deng XG et al (2014) Overexpression of miR-122 promotes the hepatic differentiation and maturation of mouse ESCs through a miR-122/FoxA1/HNF4a-positive feedback loop. Liver Int 34:281–295. doi:10.1111/liv.12239
Gramantieri L et al (2007) Cyclin G1 is a target of miR-122a, a microRNA frequently down-regulated in human hepatocellular carcinoma. Cancer Res 67:6092–6099. doi:10.1158/0008-5472.can-06-4607
He C et al (2015a) MiR-21 mediates sorafenib resistance of hepatocellular carcinoma cells by inhibiting autophagy via the PTEN/Akt pathway. Oncotarget 6:28867–28881. doi:10.18632/oncotarget.4814
He J et al (2015b) Upregulation of microRNA-122 by farnesoid X receptor suppresses the growth of hepatocellular carcinoma cells. Mol Cancer 14:163. doi:10.1186/s12943-015-0427-9
Hsu SH et al (2012) Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J Clin Investig 122:2871–2883. doi:10.1172/jci63539
Karakatsanis A, Papaconstantinou I, Gazouli M, Lyberopoulou A, Polymeneas G, Voros D (2013) Expression of microRNAs, miR-21, miR-31, miR-122, miR-145, miR-146a, miR-200c, miR-221, miR-222, and miR-223 in patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma and its prognostic significance. Mol Carcinog 52:297–303. doi:10.1002/mc.21864
Kyrmizi I, Hatzis P, Katrakili N, Tronche F, Gonzalez FJ, Talianidis I (2006) Plasticity and expanding complexity of the hepatic transcription factor network during liver Dev. Genes Development 20:2293–2305. doi:10.1101/gad.390906
Landgraf P et al (2007) A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 129:1401–1414. doi:10.1016/j.cell.2007.04.040
Laudadio I et al (2012) A feedback loop between the liver-enriched transcription factor network and miR-122 controls hepatocyte differentiation. Gastroenterology 142:119–129. doi:10.1053/j.gastro.2011.09.001
Lehner F, Kulik U, Klempnauer J, Borlak J (2007) The hepatocyte nuclear factor 6 (HNF6) and FOXA2 are key regulators in colorectal liver metastases. FASEB J 21:1445–1462. doi:10.1096/fj.06-6575com
Lehner F, Kulik U, Klempnauer J, Borlak J (2010) Inhibition of the liver enriched protein FOXA2 recovers HNF6 activity in human colon carcinoma and liver hepatoma cells. PLoS ONE 5:e13344. doi:10.1371/journal.pone.0013344
Ma L et al (2010) Expression of miR-122 mediated by adenoviral vector induces apoptosis and cell cycle arrest of cancer cells. Cancer Biol Ther 9:554–561
Nelson WJ, Nusse R (2004) Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303:1483–1487. doi:10.1126/science.1094291
Oh J et al (2001) The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell 107:789–800
Pekala KR et al (2014) Loss of HNF6 expression correlates with human pancreatic cancer progression. Lab Investig 94:517–527. doi:10.1038/labinvest.2014.47
Sasaki T et al (2013) Hepatocyte nuclear factor 6 activates the transcription of CYP3A4 in hepatocyte-like cells differentiated from human induced pluripotent stem cells. Drug Metab Pharmacokinet 28:250–259
Sun XP et al (2014) Up-regulation of survivin by AKT and hypoxia-inducible factor 1alpha contributes to cisplatin resistance in gastric cancer. FEBS J 281:115–128. doi:10.1111/febs.12577
Tsai WC et al (2009) MicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinoma. Hepatology 49:1571–1582. doi:10.1002/hep.22806
Tsai WC et al (2012) MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Investig 122:2884–2897. doi:10.1172/jci63455
Valle J et al (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362:1273–1281. doi:10.1056/NEJMoa0908721
Wang K, Holterman AX (2012) Pathophysiologic role of hepatocyte nuclear factor 6. Cell Signal 24:9–16. doi:10.1016/j.cellsig.2011.08.009
Wang B, Wang H, Yang Z (2012) MiR-122 inhibits cell proliferation and tumorigenesis of breast cancer by targeting IGF1R. PLoS ONE 7:e47053. doi:10.1371/journal.pone.0047053
Wang SC et al (2014) MicroRNA-122 triggers mesenchymal-epithelial transition and suppresses hepatocellular carcinoma cell motility and invasion by targeting RhoA. PLoS ONE 9:e101330. doi:10.1371/journal.pone.0101330
Wei Z et al (2013) STAT3 interacts with Skp2/p27/p21 pathway to regulate the motility and invasion of gastric cancer cells. Cell Signal 25:931–938. doi:10.1016/j.cellsig.2013.01.011
Wu Q et al (2015) Decreased expression of hepatocyte nuclear factor 4alpha (Hnf4alpha)/microRNA-122 (miR-122) axis in hepatitis B virus-associated hepatocellular carcinoma enhances potential oncogenic GALNT10 protein activity. J Biol Chem 290:1170–1185. doi:10.1074/jbc.M114.601203
Xu H, He JH, Xiao ZD, Zhang QQ, Chen YQ, Zhou H, Qu LH (2010) Liver-enriched transcription factors regulate microRNA-122 that targets CUTL1 during liver development. Hepatology 52:1431–1442. doi:10.1002/hep.23818
Yuan XW, Wang DM, Hu Y, Tang YN, Shi WW, Guo XJ, Song JG (2013) Hepatocyte nuclear factor 6 suppresses the migration and invasive growth of lung cancer cells through p53 and the inhibition of epithelial-mesenchymal transition. J Biol Chem 288:31206–31216. doi:10.1074/jbc.M113.480285
Zeng C et al (2010) A novel GSK-3 beta-C/EBP alpha-miR-122-insulin-like growth factor 1 receptor regulatory circuitry in human hepatocellular carcinoma. Hepatology 52:1702–1712. doi:10.1002/hep.23875
Zhao X, Liu M, Li D (2015) Oleanolic acid suppresses the proliferation of lung carcinoma cells by miR-122/Cyclin G1/MEF2D axis. Mol Cell Biochem 400:1–7. doi:10.1007/s11010-014-2228-7
Acknowledgments
This study was supported by grants from the National Natural Scientific Foundation of China (81272467 and 81472321), Shandong Provincial Natural Scientific Research Foundation (BS2015YY024), Shandong Provincial Scientific and Technology Development Program (2014GGH218039) and Heilongjiang Provincial Scientific Fund for Youths (QC2011C089). Huaqiang Zhu and Yuetang Mi contributed equally to this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Ethical standards
The study has been approved by the ethic committees of Provincial Hospital Affiliated to Shandong University and Liaocheng People’s Hospital in China. Preoperative informed consent was obtained from each patient registered in this study in accordance with institutional guidance. The pathological samples were taken from the surgical resection specimens, which would not result in any disadvantages to health and prognosis of patients. We are committed to maintain the privacy of patients’ information. The animal study has been approved (permit SYXK20020009) by the Animal Ethics Committee of Harbin Medical University, in compliance with the Experimental Animal Regulations by the National Science and Technology Commission, China.
Rights and permissions
About this article
Cite this article
Zhu, H., Mi, Y., Jiang, X. et al. Hepatocyte nuclear factor 6 inhibits the growth and metastasis of cholangiocarcinoma cells by regulating miR-122. J Cancer Res Clin Oncol 142, 969–980 (2016). https://doi.org/10.1007/s00432-016-2121-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-016-2121-8