Abstract
Aromatic L-amino acid decarboxylase (AADC) deficiency is a rare inherited neurometabolic disorder that can lead to severe physical and developmental impairment. This report includes 16 patients from the Middle East and is the largest series of patients with confirmed AADC deficiency from this region reported to date. The patients displayed a range of signs and symptoms at presentation and almost all failed to reach major motor milestones. Missed and delayed diagnoses were common leading to the late introduction of targeted treatments. Eight unique variants were identified in the DDC gene, including six missense and two intronic variants. A previously undescribed variant was identified: an intronic variant between exons 13 and 14 (c.1243-10A>G). The patients were mostly treated with currently recommended medications, including dopamine agonists, vitamin B6, and monoamine oxidase inhibitors. One patient responded well, but treatment outcomes were otherwise mostly limited to mild symptomatic improvements. Five patients had died by the time of data collection, confirming that the condition is associated with premature mortality. There is an urgent need for earlier diagnosis, particularly given the potential for gene therapy as a transformative treatment for AADC deficiency when provided at an early age.
Conclusions: Delays in the diagnosis of AADC deficiency are common. There is an urgent need for earlier diagnosis, particularly given the potential for gene therapy as a transformative treatment for AADC deficiency when provided at an early age.
What is Known: • Aromatic L-amino acid decarboxylase deficiency is a rare neurometabolic disorder that can lead to severe physical and developmental impairment. • Currently recommended medications provide mostly mild symptomatic improvements. | |
What is New: • The clinical presentation of sixteen patients with confirmed AADC deficiency varied considerably and almost all failed to reach major motor milestones. • There is an urgent need for earlier diagnosis, given the potential for gene therapy as a transformative treatment for AADC deficiency when provided at an early age. |
Similar content being viewed by others
Introduction
First described in 1990, aromatic L-amino acid decarboxylase (AADC) deficiency is an ultrarare, autosomal recessive, neurotransmitter metabolic disorder resulting from pathogenic variants within the dopa decarboxylase (DDC) gene [1, 2]. To date, at least 261 cases have been reported in the medical literature [3] and, as of June 2022, there are currently 420 variants listed in the Pediatric Neurotransmitter Disease database (PNDdb; available at: http://biopku.org/pnddb/home.asp), including 370 that are associated with a neurotransmitter deficiency phenotype. Although the global prevalence of AADC deficiency is unknown, it is believed to be higher in Asian populations owing to the presence of the founder variant c.714+4A>T [4]. In Taiwan, a pilot newborn screening project indicated that the incidence of AADC deficiency was 1:32,000 [5], whereas in the USA, Europe, and Japan, birth rates of 1:42:000–1:90,000, 1:116,000, and 1:162,000, respectively, were estimated [6,7,8].
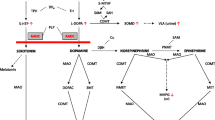
The AADC enzyme is required for the final step in the synthesis of monoamine neurotransmitters. Deficiency of this enzyme results in a loss of dopamine, serotonin, epinephrine, and norepinephrine production leading to severe physical and intellectual disabilities and risk of early death [1, 9, 10]. The symptom spectrum can be highly variable including motor delay, hypotonia, dystonia, oculogyric crises, autonomic symptoms, and behavioral problems [10]. Most reported cases have no or very limited attainment of motor or developmental milestones [9].
There is a significant heterogeneous phenotypic spectrum and symptoms can be non-specific, and the diagnosis of AADC deficiency is frequently delayed, with patients often misdiagnosed with other more common conditions, such as cerebral palsy or seizure disorders [11]. Lack of awareness of this rare condition among primary care physicians may also mean that clinical suspicion of this disease is low, further contributing to diagnostic delays [12]. Key diagnostic tests for AADC deficiency include the analysis of neurotransmitter metabolites in cerebrospinal fluid (CSF), measurement of AADC activity in plasma, and genetic testing to identify pathogenic variants in the DDC gene. Routine brain magnetic resonance imaging (MRI) is not required for the diagnosis of AADC deficiency as no specific pattern of changes has been observed; however, it can be beneficial for ruling out cerebral palsy as a differential diagnosis. Similarly, electroencephalography (EEG) is not required for diagnosis, although it can be useful to differentiate oculogyric crises from epileptic events [9]. AADC deficiency can also be identified by measurement of 3-O-methyldopa (3-OMD) in dried blood spots, providing the potential for newborn screening for this disease [5].
Currently recommended first-line pharmacological treatments include dopamine agonists, monoamine oxidase (MAO) inhibitors, and pyridoxine/pyridoxal phosphate (vitamin B6). However, treatment response is often disappointing, offering, at best, mild symptomatic improvements and do not treat the underlying enzyme deficiency [9]. Gene therapy will expand treatment options in the future, with recent studies demonstrating that replacement with a functional copy of the DDC gene in the basal ganglia region of the brain using an adeno-associated viral vector leads to clinical improvements [10, 13,14,15]. These improvements correlated with younger age in gene therapy trials, indicating that there is a need for earlier diagnosis of AADC deficiency [13, 14].
The objective of this study was to describe the clinical presentation and diagnostic workup for 16 patients with confirmed AADC deficiency from the Gulf Cooperation Council countries; the largest number of AADC deficiency patients included in a single case series from the Middle East.
Materials and methods
Sixteen patients with confirmed AADC deficiency were included in this case series. Clinical, laboratory, and treatment data were retrospectively collected from patients’ electronic medical records. Consent for inclusion in this study was provided by all patients and/or their families.
Results
Patient demographics
Patient demographics for this case series are shown in Table 1. Sixteen patients were included, six females and ten males. Age at diagnosis of AADC deficiency ranged from 10 months to 18 years. All patients had consanguineous parents and the majority of patients failed to reach motor or developmental milestones. Two patients were able to walk (patient 2 and patient 11). Five patients had a positive family history of AADC deficiency, with three patients having at least one affected sibling and two patients having one affected first cousin. A further four patients had a potentially relevant family history; two had a sibling diagnosed with cerebral palsy (deceased), one patient had a cousin with developmental delay, and one had parents who had previously experienced recurrent, unexplained abortions. The remaining seven patients had no relevant family history. Five patients died prior to data collection. Patients’ age at death was confirmed as 3, 9, and 10 years of age for the three patients with available data (Table 1).
Presenting signs and symptoms
Most of the patients included in this case series presented with symptoms of AADC deficiency prior to 12 months of age. The range and number of symptoms and signs were highly variable between patients (Fig. 1), with the most commonly reported symptoms being developmental delay (94%), hypotonia (75%), and insomnia/disturbed sleep and oculogyric crises (both 56%) (Fig. 2). Most patients had symptoms related to muscle tone (n=13), including hypotonia, no or poor head control, and hypertonia. In addition, the majority of the patients experienced gastrointestinal symptoms (n=11), including reflux/gastroesophageal reflux disease (GERD), diarrhea, constipation, and feeding/swallowing problems. Failure to thrive was also common (n=6).
Diagnosis
Although most patients had severe motor dysfunction and developmental delay, diagnosis was generally delayed, with only seven patients receiving a diagnosis within 1 year of symptom onset (Fig. 3). For four patients, diagnosis was markedly delayed: occurring 5, 6, 10, and 18 years after symptom onset for patients 2, 6, 12, and 9, respectively. Most patients (n=14) had been misdiagnosed prior to receiving a diagnosis of AADC deficiency, with cerebral palsy and seizures being common misdiagnoses. The two patients who did not receive a misdiagnosis had a positive family history for AADC deficiency. For many patients (n=11), the misdiagnosis resulted in delayed access to targeted treatments for AADC deficiency.
As part of the diagnostic workup, patients underwent a range of tests, including brain MRI (n=15), EEG (n=13), and biochemical analyses (n=10) (Table 2). Abnormal MRI findings included signs of brain atrophy in three patients and minor abnormalities that may represent metabolic derangement in one patient. Some minor EEG changes were reported for five patients, but no epileptic activity was seen. In most cases, MRI and EEG results were normal or there were no specific findings. Six patients had a CSF analysis performed, with all patients displaying a profile characteristic of AADC deficiency, i.e., low levels of 5-hydroxyindoleacetic acid and homovanillic acid and elevated 3-OMD, L-dopa, and 5-hydroxytryptophan. Seven patients had plasma AADC enzyme activity measured, all of which were significantly lower than normal (reference value 47–119 pmol/mL/min [16]) and close to the detection limit of the assay. Only one patient had dried blood spot 3-OMD measured, which was elevated, indicative of AADC deficiency.
All patients had a full DDC gene sequence performed. Eight unique variants were identified, of which one intronic variant was previously unpublished (Fig. 4). Six missense variants leading to a single amino acid substitution were reported for 12 patients across exons 2 (n=1), 3 (n=3), 5 (n=1), 11 (n=2) and 13 (n=5). Two intronic variants affecting four patients were reported. All patients had homozygous variants.
Treatment and outcomes
Many of the patients were treated with the recommended first-line pharmacological treatments, i.e., dopamine agonists (n=9), vitamin B6 (n=13), and MAO inhibitors (n=3) (Table 3). A further four patients received folinic acid and three received levodopa/carbidopa. Symptomatic treatments included benzodiazepines, anti-epileptics, anti-cholinergic drugs, and α2-adrenergic agonists.
Six patients were judged to have not responded to treatment or to have had a poor response. Five patients exhibited mild-to-moderate improvements in symptoms, including oculogyric crises, and one patient exhibited improvements in dystonic and oculogyric crises. Patient 11 received early treatment with bromocriptine, benztropine, folinic acid, and selegiline and had a very good response. He is currently able to walk, speak, and attend school. Conversely, his sibling (patient 12) with the same genotype commenced treatment later and did not have such a good response, although some improvements in movement and tone were noted. Five patients were deceased at the time of data collection (Table 1).
Discussion
This report describes the clinical characteristics, diagnostic workup, and treatment of 16 patients with AADC deficiency managed in specialist centers within the Middle East.
At a young age, the majority of patients presented with severe motor dysfunction and had no or very limited attainment of developmental milestones. As has been previously reported, sex was not found to be associated with phenotype [9]. Signs and symptoms were consistent with other case reports for AADC deficiency [17, 18], with motor issues, global developmental delay, and gastrointestinal symptoms reported frequently. The specific pattern of signs and symptoms varied considerably across the 16 cases, which is also consistent with other case series [17,18,19]. Of note, oculogyric crises have previously been described as a near-universal feature of AADC deficiency [17,18,19]; however, in our case series, only 9 out of 16 patients experienced this symptom. As one of the more specific symptoms for AADC deficiency, oculogyric crises can help spark clinical suspicion for the disease. This case series highlights that lack of oculogyric crises should not exclude patients from screening for AADC deficiency. Diagnosis of AADC deficiency was frequently delayed with almost half of the patients only receiving a definitive diagnosis more than 1 year after symptom onset; the average delay to diagnosis for the 16 patients reported in this case series was 3.2 years. For four older patients, a diagnosis was not achieved for many years. This is consistent with a large case series that demonstrated a longer latency from symptom onset to diagnosis in older vs younger children [17], perhaps reflecting an increasing awareness of this disease in recent years. Current guidelines recommend that a diagnosis of AADC deficiency should be based on the identification of compound heterozygous or homozygous pathogenic variants in the DDC gene combined with either CSF analysis or measurement of plasma AADC activity. In this study, AADC deficiency was genetically confirmed in all patients, although biochemical confirmation was not performed in six cases. These included three cases with variant c.1234C>T and one with c.1040G>C, classified as pathogenic, and one case with c.571-3C>6, reported as a warm variant of uncertain significance [20]. Two of the six cases had a family history of AADCd previously confirmed with both whole exome sequencing and plasma enzyme AADC activity assessment.
Brain MRI and EEGs were performed in the majority of cases, although findings were often normal or non-specific, indicating the limited use of these modalities in the diagnosis of AADC deficiency, other than to eliminate other more common conditions.
All patients in this case series had a homozygous variant within the DDC gene. This contrasts with other case series where compound heterozygous variants have been more common [17, 18]. This may reflect the fact that all patients in this series were born to consanguineous parents [21]. A novel intronic variant between exons 13 and 14 (c.1243–10 A>G) was identified (patient 2). Given that this was a novel variant, both plasma AADC enzyme activity and dried blood spot 3-OMD analysis were performed to confirm a AADC deficiency diagnosis. This patient had no tonus symptoms or motor impairment and was able to walk, talk, and had normal cognition. The patient presented with diarrhea and failure to thrive and has shown moderate improvements in response to treatment with vitamin B6. No patients in this case series had the intronic variant c.714+4A > T that is found frequently in patients from Taiwan or with Chinese ancestry; however, five patients had the c.1234C>T variant which has previously been reported in six Chinese patients and is associated with a severe phenotype [18]. Three patients had the intronic variant c.571-3C>G; this variant had previously been reported in a case from Saudi Arabia, although no clinical phenotype was described [22]. Other previously described variants include c. 206C>T and c. 242C>T that lead to amino acid substitutions in loop 1 [23, 24], c.175G > A that leads to a substitution in the N-terminal domain [18, 25], and c.1040G > A that leads to a substitution in loop 3 and is associated with a considerable decrease in catalytic activity [26].
Most patients received treatment with recommended first-line agents, including dopamine agonists, vitamin B6 and MAO inhibitors, along with a number of other drugs used to treat symptoms of the disease, e.g., anti-epileptics and anti-spasmodics. One patient responded well to treatment and at the time of writing is walking, talking, and attending school. This patient was diagnosed at a relatively early age (10 months) as he had an elder sibling already diagnosed with the condition. In contrast, his sibling was only diagnosed at 10 years of age and has not exhibited as good a response to treatment. In general, patients either showed no response to treatment or had only mild or moderate improvements in specific symptoms.
Five patients died prior to data collection with the cause of death attributed to the natural history of the disease. The average age at death was 7 years of age for the three patients with available data, which is in line with previously reported data. Premature death in patients with AADCd is common [3, 17, 27, 28], with the average age at death for Taiwanese patients with AADCd reported to be 4.6 years of age [28]. In a systematic review of case studies, 13 deaths were reported in a sample of 261 patients with AADCd [3]. Six of these patients died within 3 years of age. Bergkvist et al. reported an average age of death of 8 years of age based on 16 deaths in a sample of 185 [27].
This indicates a high unmet need for effective treatments for patients with AADC deficiency to treat both the underlying pathology and the symptoms. Gene therapy trials have demonstrated that delivery of a functional copy of the DDC gene using adeno-associated viral vector to the basal ganglia leads to clinical improvements [10, 13, 14]. Young age correlated with the clinical improvements, emphasizing the need for earlier diagnosis of AADC deficiency [13, 14]. The European Commission has recently granted marketing authorization for PTC Therapeutics’ product Upstaza™ (eladocagene exuparvovec), an in vivo gene therapy for treating AADC deficiency through delivery of the DDC gene to the putamen. It is approved for patients aged 18 months of age and older [29]. Therefore, raising awareness of AADC deficiency among primary care physicians in the Middle East region is key to ensure early access to future gene therapy treatments.
A strength of this study is that it highlights the heterogeneity in symptom and sign presentation for patients with AADC deficiency and the diagnostic challenges faced by healthcare professionals in the Middle East who manage patients with this rare condition. Limited AADC deficiency case reports have been reported from this region [17, 30,31,32]. Limitations are that it is based solely on patient cases and, as for all case reports, it is vulnerable to selection and recall bias. An incomplete dataset was captured for 2 patients; information on treatment dose and response is unreported.
The findings from this study confirm that delays in the diagnosis of AADC deficiency are common, even among patients who had no or limited attainment of motor milestones. This cohort of patients presented with a wide spectrum of signs and symptoms and did not always have typical symptoms, such as motor issues or oculogyric crises. Diagnostic follow-up was variable with all patients undergoing genetic testing, but secondary biochemical analyses were not always performed. AADC deficiency patients are frequently misdiagnosed with other conditions such as cerebral palsy, which can lead to delays in the initiation of treatments targeting the underlying pathology. In order to decrease the time to diagnosis and to reduce misdiagnoses, increased awareness of the condition is required. This could be achieved through introducing screening programs in at-risk patients for raised 3-OMD levels and the increased use of genetic testing using next-generation sequencing early in the diagnostic workup of patients. This may be particularly important in the Middle East region, where parental consanguinity and large family size can increase the prevalence of autosomal recessive genetic disorders. Increased awareness, research, and education are needed to guarantee timely diagnosis, management of AADC deficiency, and early access to future gene therapy treatments.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
04 May 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00431-023-05012-1
References
Hyland K, Reott M (2020) Prevalence of aromatic L-amino acid decarboxylase deficiency in at-risk populations. Pediatr Neurol 106:38–42
Hyland K, Clayton PT (1990) Aromatic amino acid decarboxylase deficiency in twins. J Inherit Metab Dis 13:301–304
Rizzi S, Spagnoli C, Frattini D, Pisani F, Fusco C (2022) Clinical features in aromatic L-amino acid decarboxylase (AADC) deficiency: a systematic review. Behav Neurol 2022:2210555
Hwu WL, Chien YH, Lee NC, Li MH (2018) Natural history of aromatic L-amino acid decarboxylase deficiency in Taiwan. JIMD Rep 40:1–6
Chien YH et al (2016) 3-O-methyldopa levels in newborns: result of newborn screening for aromatic L-amino-acid decarboxylase deficiency. Mol Genet Metab 118:259–263
Himmelreich N et al (2021) Corrigendum to “Aromatic amino acid decarboxylase deficiency: molecular and metabolic basis and therapeutic outlook” [Mol Genet Metab. 2019 May;127(1):12–22]. Mol Genet Metab 134:216
Hyland K, Reott M (2018) Estimated prevalence of aromatic L-amino acid decarboxylase (AADC) deficiency in at-risk population (poster). Presented at the Annual Meeting of the American Society of Gene and Cell Therapy. Chicago, IL, USA
Whitehead N et al (2018) Estimated prevalence of aromatic L-amino acid decarboxylase (AADC) deficiency in the United States, European Union and Japan (poster). Presented at the Annual Congress of the European Society for Gene and Cell Therapy. Lausanne, Switzerland
Wassenberg T et al (2017) Consensus guideline for the diagnosis and treatment of aromatic L-amino acid decarboxylase (AADC) deficiency. Orphanet J Rare Dis 12:12
Pearson TS et al (2021) Gene therapy for aromatic L-amino acid decarboxylase deficiency by MR-guided direct delivery of AAV2-AADC to midbrain dopaminergic neurons. Nat Commun 12:4251
Opladen T et al (2016) The International Working Group on Neurotransmitter related Disorders (iNTD): a worldwide research project focused on primary and secondary neurotransmitter disorders. Mol Genet Metab Rep 9:61–66
Fusco C et al (2021) Aromatic L-amino acid decarboxylase (AADC) deficiency: results from an Italian modified Delphi consensus. Ital J Pediatr 47:13
Kojima K et al (2019) Gene therapy improves motor and mental function of aromatic L-amino acid decarboxylase deficiency. Brain 142:322–333
Tseng CH et al (2019) Gene therapy improves brain white matter in aromatic L-amino acid decarboxylase deficiency. Ann Neurol 85:644–652
Tai CH et al (2022) Long-term efficacy and safety of eladocagene exuparvovec in patients with AADC deficiency. Mol Ther 30:509–518
Brennenstuhl H et al (2020) High throughput newborn screening for aromatic L-amino acid decarboxylase deficiency by analysis of concentrations of 3-O-methyldopa from dried blood spots. J Inherit Metab Dis 43:602–610
Pearson TS et al (2020) AADC deficiency from infancy to adulthood: symptoms and developmental outcome in an international cohort of 63 patients. J Inherit Metab Dis 43:1121–1130
Wen Y, Wang J, Zhang Q, Chen Y, Bao X (2020) The genetic and clinical characteristics of aromatic L-amino acid decarboxylase deficiency in mainland China. J Hum Genet 65:759–769
Brun L et al (2010) Clinical and biochemical features of aromatic L-amino acid decarboxylase deficiency. Neurology 75:64–71
Himmelreich N et al (2022) Spectrum of DDC variants causing aromatic L-amino acid decarboxylase (AADC) deficiency and pathogenicity interpretation using ACMG-AMP/ACGS recommendations. Mol Genet Metab 137:359–381
Bittles AH, Black ML (2010) Evolution in health and medicine Sackler colloquium: consanguinity, human evolution, and complex diseases. Proc Natl Acad Sci U S A 107(Suppl 1):1779–1786
Alfares A et al (2017) A multicenter clinical exome study in unselected cohorts from a consanguineous population of Saudi Arabia demonstrated a high diagnostic yield. Mol Genet Metab 121:91–95
Manegold C et al (2009) Aromatic L-amino acid decarboxylase deficiency: clinical features, drug therapy and follow-up. J Inherit Metab Dis 32:371–380
Montioli R et al (2014) A comprehensive picture of the mutations associated with aromatic amino acid decarboxylase deficiency: from molecular mechanisms to therapy implications. Hum Mol Genet 23:5429–5440
Dai W, Lu D, Gu X, Yu Y (2020) Aromatic L-amino acid decarboxylase deficiency in 17 Mainland China patients: clinical phenotype, molecular spectrum, and therapy overview. Mol Genet Genomic Med 8:e1143
Montioli R, Borri Voltattorni C (2021) Aromatic amino acid decarboxylase deficiency: the added value of biochemistry. Int J Mol Sci 22:3146
Bergkvist M et al (2022) Aromatic L-amino acid decarboxylase deficiency: a systematic review. Future Neurology 17:FNL63
Lee NC, Chien YH, Hwu WL (2019) A review of aromatic L-amino acid decarboxylase (AADC) deficiency in Taiwan. Am J Med Genet C Semin Med Genet 181:226–229
PTC Therapeutics (2022) Upstaza™ granted marketing authorization be European Commission as first disease-modifying treatment for AADC deficiency. Available at: https://ir.ptcbio.com/news-releases/news-release-details/upstazatm-granted-marketing-authorization-european-commission. Accessed 15 Feb 2023
Abukhaled M, Alrakaf L, Aldhalaan H, Al Yamani S (2023) Case report: Aromatic L-amino acid decarboxylase deficiency in three patient cases from the Kingdom of Saudi Arabia. Front Pediatr 10:1016239
Alfadhel M, Kattan R (2014) Aromatic amino acid decarboxylase deficiency not responding to pyridoxine and bromocriptine therapy: case report and review of response to treatment. J Cent Nerv Syst Dis 6:1–5
Babiker MOE, Kurian MA, Suleiman J (2022) Case report: First case report of an Emirati child with a novel gene variant causing aromatic L-amino acid decarboxylase deficiency. Front Pediatr 10:964201
Acknowledgements
The authors would also like to thank Dr. Laila Alrakaf (Department of Neurosciences, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia). Writing and editorial support were provided by Steph Carter and Kirsty McCann, an employee of PharmaGenesis London, UK, for all drafts of the manuscript.
Funding
This work was funded by PTC Therapeutics.
Author information
Authors and Affiliations
Contributions
All authors provided clinical data, contributed to the article, revised all drafts, and approved the submitted version. PTC Therapeutics had no involvement in the study design, the collection, analysis and interpretation of data, or the writing of the report. The concept for the manuscript and decision to submit it for publication was agreed between PTC Therapeutics and the authors.
Corresponding author
Ethics declarations
Ethical approval
We would like to thank the patients and their families for their assent/consent for their cases to be discussed in this manuscript.
Conflict of interest
Musaad Abukhaled, Mohammed Al Muqbil, Jehan Suleiman, Tawfeg Ben-Omran, Majid Alfadhel, and Brahim Tabarki have received honoraria for advisory board meetings from PTC Therapeutics. Malak Alghamdi, Khalid Hundallah, Rehab Alsaleh, and Mohammed Almannai have no disclosures to declare.
Additional information
Communicated by Peter de Winter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to incorrect figure 4 and amendment under Diagnosis section.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abukhaled, M., Al Muqbil, M., Alghamdi, M.A. et al. Aromatic L-amino acid decarboxylase deficiency in countries in the Middle East: a case series and literature review. Eur J Pediatr 182, 2535–2545 (2023). https://doi.org/10.1007/s00431-023-04886-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-04886-5