Abstract
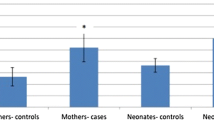
To estimate the levels of malondialdehyde (MDA) and 8-hydroxy-2-deoxyguanosine (8-OH-dG) in cord blood plasma of newborns born through meconium-stained amniotic fluid (MSAF) and also to find out the correlation between their levels with birth weight and gestation, we measured the cord blood plasma levels of MDA and 8-OH-dG in 59 newborns born through MSAF and 50 newborns born through clear liquor. The levels of cord blood plasma MDA and 8-OH-dG were significantly higher in full-term and late-preterm newborns born through MSAF. On further comparison, it was found that both full-term and late-preterm intrauterine growth restricted (IUGR) neonates had higher levels of these markers as compared to babies born as appropriate for gestational age (AGA) through MSAF. Plasma levels of MDA and 8-OH-dG were significantly correlated with birth weight even after controlling the relationship with gestational age for all cases as well as all full-term cases. These markers are also significantly correlated to each other.
Conclusions: The present study suggest that the neonates born through MSAF experience higher degrees of oxidative stress, as evidenced by increased levels of cord blood plasma MDA and 8-OH-dG.
What is known: • Aspirated meconium has been found to induce free radical generation and cellular damage in animal studies. • Its role in free radical generation and oxidative damage in human neonates is scarce. |
What is new: • Neonates born through meconium-stained amniotic fluid experience significant oxidative stress. |

Similar content being viewed by others
Abbreviations
- 8-OH-dG:
-
8-Hydroxy-2-deoxyguanosine
- AGA:
-
Appropriate for gestational age
- IUGR:
-
Intrauterine growth retardation
- MAS:
-
Meconium aspiration syndrome
- MDA:
-
Malondialdehyde
- MSAF:
-
Meconium-stained amniotic fluid
References
Aaltonen M, Soukka H, Halkola L, Jalonen J, Holopainen IE, Kaapa PO (2005) Meconium aspiration induces oxidative injury in the hippocampus of newborn piglets. Early Hum Dev 81:439–447
Ahanya S, Lakshmanan J, Morgan B, Ross M (2005) Meconium passage in utero: mechanisms, consequences and management. Obstet Gynecol Surv 60:45–56
Arjmand MH, Shah FA, Moghadam MS, Tara F, Jalili A, Bazaz MM et al (2013) Prooxidant-antioxidant balance in umbilical cord blood of infants with meconium stained of amniotic fluid. Biochemistry Research International 2013:1–4
Balchin I, Whittaker JC, Lamont RF, Philip J (2011) Maternal and fetal characteristics associated with meconium-stained amniotic fluid. Obstet Gynecol 117:828–835
Bhatia BD, Goel A (2005) Study of free radicals in neonates born through meconium stained amniotic fluid deliveries. Indian Pediatr 42:956–957
Biri A, Bozkurt N, Turp A, Kavutcu M, Himmetoglu O, Durak I (2007) Role of oxidative stress in intrauterine growth restriction. Gynecol Obstet Investig 64:187–192
Bonassi S, Au WW (2002) Biomarkers in molecular epidemiology studies for health risk prediction. Mutat Res 511:73–86
Buonocore G, Perrone S, Longini M, Terzuoli L, Bracci R (2000) Total hydroperoxide and advanced oxidation protein products in preterm hypoxic babies. Pediatr Res 47:221–224
Buonocore G, Perrone S, Longini M, Vezzosi P, Marzocchi B, Paffetti P et al (2002) Oxidative stress in preterm neonates at birth and on the seventh day of life. Pediatr Res 52:46–49
Craig S, Lopez A, Hoskin D, Markham F (2005) Meconium inhibits phagocytosis and stimulates respiratory burst in alveolar macrophages. Pediatr Res 57:813–818
Dargaville PA, Copnell B (2006) The epidemiology of meconium aspiration syndrome: incidence, risk factors, therapies, and outcome. Pediatrics 117:1712–1721
de Beaufort AJ, Bakker AC, Van Tol MJ, Poorthuis BJ, Schrama AJ, Berger RM (2003) Meconium is a source of pro-inflammatory substances and can induce cytokine production in cultured A549 epithelial cells. Pediatr Res 54:491–495
Gupta P, Narang M, Banerjee BD, Basu S (2004) Oxidative stress in term small for gestational age neonates born to undernourished mothers: a case control study. BMC Pediatr 4:14
Hackey WE (1999) Meconium Aspiration. In: Gomella TL (ed) Neonatology, 4th edn. Lange Medical Books, New york, p 507
Halliwell B, Gutteridge JMC (1989) Free radicals in biology and medicine, 2nd edn. Clarendon Press, Oxford
Halliwell B, Arouma OI (1991) DNA damage by oxygen-derived species its mechanism and measurement in mammalian systems. Elsevier Science 281:9–20
Ishikawa T, Fujioka H, Ishimura T, Takenaka A, Fujisawa M (2007) Increased testicular 8-hydroxy-2′-deoxyguanosine in patients with varicocele. BJU Int 100:863–866
Karowicz-Bilinska A, Kedziora-Kornatowska K, Bartosz G (2007) Indices of oxidative stress in pregnancy with fetal growth restriction. Free Radic Res 41:870–873
Kimmick GG, Bell RA, Bostick RM (1997) Vitamin E and breast cancer: a review. Nutr Cancer 27:109–117
Liu BY, Wang CC, Lau TK, Chu CY, Pang CP, Rogers MS (2005) Meconium-stained liquor during labor is associated with raised neonatal cord blood 8-iso-prostaglandin F2훼 concentration. Am J Obstet Gynecol 192(1):289–294
Maymon E, Chaim W, Furman B, Ghezzi F, Shoham Vardi I, Mazor M (1998) Meconium stained amniotic fluid in very low risk pregnancies at term gestation. Eur J Obstet Gynecol Reprod Biol 80:169–173
Mokra D, Drgova A, Mokry J, Antosova M, Durdik P, Calkovska A (2015) N-acetylcysteine effectively diminished meconium-induced oxidative stress in adult rabbits. J Physiol Pharmacol 66:101–110
Mundhra R, Agarwal M (2013) Fetal outcome in meconium stained deliveries. J Clin Diagn Res 7:2874–2876
National Neonatology Forum NNPD Network. (2005) National Neonatal-Perinatal Database. report 2002–2003. New Delhi
Negi R, Pande D, Kumar A, Basu S, Khanna RS, Khanna HD (2011) In-vivo oxidative DNA damage, protein oxidation and lipid peroxidation as a biomarker of oxidative stress in preterm low birth weight infants. J Med Sci 11:77–83
Negi R, Pande D, Kumar A, Khanna RS, Khanna HD (2012a) In vivo oxidative DNA damage and lipid peroxidation as a biomarker of oxidative stress in preterm low birth weight infants. J Trop Pediatr 58:326–328
Negi R, Pande D, Kumar A, Khanna RS, Khanna HD (2012b) Evaluation of biomarkers of oxidative stress and antioxidant capacity in the cord blood of preterm low birth neonates. The Journal of Maternal-Fetal and Neonatal Medicine 25:1338–1341
Negi R, Pande D, Karki K, Kumar A, Khanna RS, Khanna HD (2014) Association of oxidative DNA damage, protein oxidation and antioxidant function with oxidative stress induced cellular injury in pre-eclamptic/eclamptic mothers during fetal circulation. Chem Biol Interact 208:77–83
Negi R, Pande D, Karki K, Kumar A, Khanna RS, Khanna HD (2015) A novel approach to study oxidative stress in neonatal respiratory distress syndrome. BBA Clinical 3:65–69
Ogino K, Wang DH (2007) Biomarkers of oxidative/nitrosative stress: an approach to disease prevention. Acta Med Okayama 61:181–189
Patil KP, Swamy MK, Samatha K (2006) A one year cross sectional study of management practices of meconium stained amniotic fluid and perinatal outcome. J Obstet Gynecol India 56:128–130
Perrone S, Vezzosi P, Longini M, Marzocchi B, Paffetti P, Bellieni CV et al (2009) Biomarkers of oxidative stress in babies at high risk for retinopathy of prematurity. Front Biosci (Elite Ed) 1:547–552
Perrone S, Tataranno ML, Negro S, Longini M, Marzocchi B, Proietti F et al (2010) Early identification of the risk for free radical-related diseases in preterm newborns. Early Hum Dev 86:241–244
Perrone S, Tataranno ML, Negro S, Cornacchione S, Longini M, Proietti F et al (2012) May oxidative stress biomarkers in cord blood predict the occurrence of necrotizing enterocolitis in preterm infants? J Matern Fetal Neonatal Med 25(Suppl 1):128–131
Philpot JS (1963) Estimation and identification of organic peroxides. Radiat Res. Suppl 3:55–70
Sankhyan N, Sharma VK, Sarin R, Pathania K (2006) Predictors of meconium stained amniotic fluid: a possible strategy to reduce neonatal morbidity and mortality. J Obstet Gynecol India 56:514–517
Saunders K (2002) Should we worry about meconium? A controlled study of neonatal outcome Trop Doct 32:7–10
Schock BC, Sweet DG, Halliday HL, Young IS, Ennis M (2001) Oxidative stress in lavage fluid of preterm infants at risk of chronic lung disease. Am J Physiol Lung Cell Mol Physiol 281:L1386–L1391
Shaikh EM, Mehmood S, Shaikh MA (2010) Neonatal outcome in meconium stained amniotic fluid—one year experience. J Pak Med Assoc 60:711–714
Shigenaga MK, Ames BN (1991) Assays for 8-hydroxy-2′-deoxyguanosine: a biomarker of in vivo oxidative DNA damage. Free Radic Biol Med 10:211–216
Soukka HR, Ahotupa M, Ruutu M, Kaapa PO (2002) Meconium stimulates neutrophil oxidative burst. Am J Perinatol 19:279–284
Tsukahara H, Shibata R, Ohshima Y, Todoroki Y, Sato S, Ohta N et al (2003) Oxidative stress and altered antioxidant defenses in children with acute exacerbation of atopic dermatitis. Life Sci 72(22):2509–2516
Uchida K (1999) Current status of acrolein as a lipid peroxidation product. Trends in Cardiovascular Medicine 9(5):109–113
Wang CC, Rogers MS (1997) Lipid peroxidation in cord blood: the effect of amniotic fluid volume. Br J Obstet Gynaecol 104:1140–1144
Wiswell TE (2001) Advances in the treatment of the meconium aspiration syndrome. Acta Paediatr 90:28–30
Yamada T, Minakami H, Matsubara S, Yatsuda T, Kohmura Y, Sato I (2000) Meconium-stained amniotic fluid exhibits chemotactic activity for polymorphonuclear leukocytes in vitro. J Reprod Immunol 46:21–30
Yoder BA, Kirsch EA, Barth WH Jr, Gordon MC (2002) Changing obstetric practices associated with decreasing incidence of meconium aspiration syndrome. Obstet Gynecol 99:731–739
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Source of support
None
Additional information
Communicated by Patrick Van Reempts
Rights and permissions
About this article
Cite this article
Bandyopadhyay, T., Bhatia, B.D. & Khanna, H.D. A study of oxidative stress in neonates delivered through meconium-stained amniotic fluid. Eur J Pediatr 176, 317–325 (2017). https://doi.org/10.1007/s00431-016-2845-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-016-2845-0