Abstract
Bacillus anthracis (BA), the etiological agent of anthrax, secretes protective antigen (PA), lethal factor (LF), and edema factor (EF) as major virulence mediators. Amongst these, PA-based vaccines are most effective for providing immunity against BA, but their low shelf life limits their usage. Previous studies showed that B-cell epitopes, ID II and ID III present in PA domain IV possess higher toxin neutralization activity and elicit higher antibody titer than ID I. Moreover, N-terminal region of both LF and EF harbors PA-binding sites which share 100% identity with each other. Here, in this study, we have developed an epitope-based chimeric vaccine (ID–LFn) comprising ID II–ID III region of PA and N-terminal region of LF. We have also evaluated its protective efficacy as well as stability and found it to be more stable than PA-based vaccine. Binding reactivities of ID–LFn with anti-PA/LF/EF antibodies were determined by ELISA. The stability of chimeric vaccine was assessed using circular dichroism spectroscopy. ID–LFn response was characterized by toxin neutralization, lymphocyte proliferation isotyping and cytokine profiling. The protective efficacy was analyzed by challenging ID–LFn-immunized mice with B. anthracis (pXO1+ and pXO2+). ID–LFn was found to be significantly stable as compared to PA. Anti-ID–LFn antibodies recognized PA, LF as well as EF. The T-cell response and the protective efficacy of ID–LFn were found to be almost similar to PA. ID–LFn exhibits equal protective efficacy in mice and possesses more stability as compared to PA along with the capability of recognizing PA, LF and EF at the same time. Thus, it can be considered as an improved vaccine against anthrax with better shelf life.
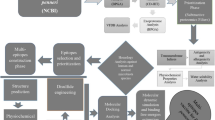
Graphical abstract

ID-LFn, a novel multiepitope chimeric anthrax vaccine: ID-LFn comprises of immunodominant epitopes of domain 4 of PA and N-terminal homologous stretch of LF and EF. The administration of this protein as a vaccine provides protection against anthrax.








Similar content being viewed by others
Abbreviations
- BA:
-
Bacillus anthracis
- PA:
-
Protective antigen
- LF:
-
Lethal factor
- EF:
-
Edema factor
- ID:
-
Immunodominant
- LFn:
-
N-terminal of LF
References
Lamps LW, Havens JM, Sjostedt A, Page DL, Scott MA (2004) Histologic and molecular diagnosis of tularemia: a potential bioterrorism agent endemic to North America. Mod Pathol 17(5):489
Riedel S (2005) Anthrax: a continuing concern in the era of bioterrorism. Proc Baylor Univ Med Center. 18(3):234
Grundmann O (2014) The current state of bioterrorist attack surveillance and preparedness in the US. Risk Manage Healthcare Pol 7:177
Cogliati S, Costa J, Ayala F, Donato V, Grau R (2016) Bacterial spores and its relatives as agents of mass destruction. J Bioterror Biodef 7:141
Cybulski RJ Jr, Sanz P, O’Brien AD (2009) Anthrax vaccination strategies. Mol Asp Med 30(6):490–502
Kaur M, Bhatnagar R (2011) Recent progress in the development of anthrax vaccines. Recent Patents Biotechnol 5(3):148–159
Lu S, Wang S (2009) Technical transformation of biodefense vaccines. Vaccine 27:D8–D15
Merkel TJ, Perera P-Y, Lee GM, Verma A, Hiroi T, Yokote H et al (2013) Protective-antigen (PA) based anthrax vaccines confer protection against inhalation anthrax by precluding the establishment of a systemic infection. Hum Vaccines Immunother 9(9):1841–1848
Kaur M, Chug H, Singh H, Chandra S, Mishra M, Sharma M et al (2009) Identification and characterization of immunodominant B-cell epitope of the C-terminus of protective antigen of Bacillus anthracis. Mol Immunol 46(10):2107–2115
Petosa C, Collier RJ, Klimpel KR, Leppla SH, Liddington RC (1997) Crystal structure of the anthrax toxin protective antigen. Nature 385(6619):833
Williams AS, Lovell S, Anbanandam A, El-Chami R, Bann JG (2009) Domain 4 of the anthrax protective antigen maintains structure and binding to the host receptor CMG2 at low pH. Protein Sci 18(11):2277–2286
Flick-Smith HC, Walker NJ, Gibson P, Bullifent H, Hayward S, Miller J et al (2002) A recombinant carboxy-terminal domain of the protective antigen of Bacillus anthracis protects mice against anthrax infection. Infect Immun 70(3):1653–1656
Price BM, Liner AL, Park S, Leppla SH, Mateczun A, Galloway DR (2001) Protection against anthrax lethal toxin challenge by genetic immunization with a plasmid encoding the lethal factor protein. Infect Immun 69(7):4509–4515
Kulshreshtha P, Bhatnagar R (2011) Inhibition of anthrax toxins with a bispecific monoclonal antibody that cross reacts with edema factor as well as lethal factor of Bacillus anthracis. Mol Immunol 48(15–16):1958–1965
Lacy DB, Mourez M, Fouassier A, Collier RJ (2002) Mapping the anthrax protective antigen binding site on the lethal and edema factors. J Biol Chem 277(4):3006–3010
Nguyen ML, Terzyan S, Ballard JD, James JA, Farris AD (2009) The major neutralizing antibody responses to recombinant anthrax lethal and edema factors are directed to non-cross-reactive epitopes. Infect Immun 77(11):4714–4723
Robinson A, Farrar GH, Wiblin CN (2003) Vaccine protocols. Springer, New York
Singh D, Somani VK, Aggarwal S, Bhatnagar R (2015) PLGA (85: 15) nanoparticle based delivery of rL7/L12 ribosomal protein in mice protects against Brucella abortus 544 infection: a promising alternate to traditional adjuvants. Mol Immunol 68(2):272–279
Somani VK, Aggarwal S, Singh D, Prasad T, Bhatnagar R (2016) Identification of novel raft marker protein, FlotP in Bacillus anthracis. Front Microbiol 7:169
Aggarwal S, Somani VK, Gupta V, Kaur J, Singh D, Grover A et al. Functional characterization of PhoPR two component system and its implication in regulating phosphate homeostasis in Bacillus anthracis. Biochimica et Biophysica Acta (BBA)-general subjects. 2017;1861(1):2956–2970
Quinn CP, Sabourin CL, Niemuth NA, Li H, Semenova VA, Rudge TL et al (2012) A three-dose intramuscular injection schedule of anthrax vaccine adsorbed generates sustained humoral and cellular immune responses to protective antigen and provides long-term protection against inhalation anthrax in rhesus macaques. Clin Vaccine Immunol 19(11):1730–1745
Manish M, Rahi A, Kaur M, Bhatnagar R, Singh S (2013) A single-dose PLGA encapsulated protective antigen domain 4 nanoformulation protects mice against Bacillus anthracis spore challenge. PLoS One 8(4):e61885
Organizaion WH (2009) Guidelines on stability evaluation of vaccines. Biologicals 37(6):424–434
Friedlander AM, Little SF (2009) Advances in the development of next-generation anthrax vaccines. Vaccine 27:D28–D32
Kaur M, Singh S, Bhatnagar R (2013) Anthrax vaccines: present status and future prospects. Expert Rev vaccines 12(8):955–970
Little SF, Leppla SH, Cora E (1988) Production and characterization of monoclonal antibodies to the protective antigen component of Bacillus anthracis toxin. Infect Immun 56(7):1807–1813
Sinha K, Bhatnagar R (2010) GroEL provides protection against Bacillus anthracis infection in BALB/c mice. Mol Immunol 48(1–3):264–271
Aggarwal S, Somani VK, Bhatnagar R (2015) Phosphate starvation enhances the pathogenesis of Bacillus anthracis. Int J Med Microbiol 305(6):523–531
Pitt M, Little S, Ivins B, Fellows P, Boles J, Barth J et al (1999) In vitro correlate of immunity in an animal model of inhalational anthrax. J Appl Microbiol 87(2):304-
Baillie LW, Huwar TB, Moore S, Mellado-Sanchez G, Rodriguez L, Neeson BN et al (2010) An anthrax subunit vaccine candidate based on protective regions of Bacillus anthracis protective antigen and lethal factor. Vaccine 28(41):6740–6748
Suryanarayana N, Verma M, Thavachelvam K, Saxena N, Mankere B, Tuteja U et al (2016) Generation of a novel chimeric PALFn antigen of Bacillus anthracis and its immunological characterization in mouse model. Appl Microbiol Biotechnol 100(19):8439–8451
Varshney A, Puranik N, Kumar M, Goel A (2016) Immunogenecity of a chimeric protein of Bacillus anthracis protective antigen and lethal factor in murine model. Int J Infect Dis 45:426
Acknowledgements
SA thanks DHR for Young Scientist Fellowship, VS acknowledges DST-SERB National Post-Doctoral Fellowship, SG thanks CSIR for Senior Research Fellowship and RG acknowledges DST for Inspire Faculty Award. RB acknowledges DBT for funds BSLIII facility. The authors thank JNU for infrastructural support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by: V. A. J. Kempf.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aggarwal, S., Somani, V.K., Gupta, S. et al. Development of a novel multiepitope chimeric vaccine against anthrax. Med Microbiol Immunol 208, 185–195 (2019). https://doi.org/10.1007/s00430-019-00577-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-019-00577-x