Abstract
It has been well established that following sensory loss, cortical areas that would normally be involved in perceiving stimuli in the absent modality are recruited to subserve the remaining senses. Despite this compensatory functional reorganization, there is little evidence to date for any substantial change in the patterns of anatomical connectivity between sensory cortices. However, while many auditory areas are contracted in the deaf, the second auditory cortex (A2) of the cat undergoes a volumetric expansion following hearing loss, suggesting this cortical area may demonstrate a region-specific pattern of structural reorganization. To address this hypothesis, and to complement existing literature on connectivity within auditory cortex, we injected a retrograde neuronal tracer across the breadth and cortical thickness of A2 to provide the first comprehensive quantification of projections from cortical and thalamic auditory and non-auditory regions to the second auditory cortex, and to determine how these patterns are affected by the onset of deafness. Neural projections arising from auditory, visual, somatomotor, and limbic cortices, as well as thalamic nuclei, were compared across normal hearing, early-deaf, and late-deaf animals. The results demonstrate that, despite previously identified changes in A2 volume, the pattern of projections into this cortical region are unaffected by the onset of hearing loss. These results fail to support the idea that crossmodal plasticity reflects changes in the pattern of projections between cortical regions and provides evidence that the pattern of connectivity that supports normal hearing is retained in the deaf brain.


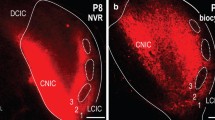
Modified from the findings of Wong et al. (2014)















Similar content being viewed by others
Abbreviations
- AAF:
-
Anterior auditory field
- AES:
-
Anterior ectosylvian sulcus
- AEV:
-
Anterior ectosylvian visual area
- ABR:
-
Auditory brainstem response
- ALLS:
-
Anterolateral lateral suprasylvian area
- AMLS:
-
Anteromedial lateral suprasylvian area
- A1:
-
Primary auditory cortex
- A2:
-
Second auditory cortex
- BDA:
-
Biotinylated dextran amine
- CGP:
-
Posterior cingulate area
- CVA:
-
Cingulate visual area
- dB:
-
Decibel
- DLS:
-
Dorsal lateral suprasylvian area
- dPE:
-
Dorsal division of the posterior ectosylvian gyrus
- DZ:
-
Dorsal zone of auditory cortex
- ED:
-
Early-deaf
- EEG:
-
Electroencephalography
- EPp:
-
Posterior aspect of the posterior ectosylvian gyrus
- fAES:
-
Auditory field of the anterior ectosylvian sulcus
- IN:
-
Insular auditory cortical area
- iPE:
-
Intermediate division of the posterior ectosylvian gyrus
- LD:
-
Late-deaf
- LP:
-
Lateral posterior nucleus
- MGBd:
-
Dorsal division of the medial geniculate body
- MGBm:
-
Medial division of the medial geniculate body
- MGBv:
-
Ventral division of the medial geniculate body
- MZ:
-
Multisensory zone
- NH:
-
Normal hearing
- nHL:
-
Normal hearing level
- PAF:
-
Posterior auditory field
- PES:
-
Posterior ectosylvian sulcus
- PLLS:
-
Posterolateral lateral suprasylvian area
- PMLS:
-
Posteromedial lateral suprasylvian area
- PO:
-
Posterior complex
- PS:
-
Posterior suprasylvian area
- Psb:
-
Presubiculum
- Rsp:
-
Posterior limb of the rostral suprasylvian sulcus
- SGN:
-
Suprageniculate nucleus
- SVA:
-
Splenial visual area
- S2:
-
Second somatosensory area
- S2m:
-
Medial division of the second somatosensory area
- S3:
-
Third somatosensory area
- S4:
-
Fourth somatosensory area
- S5:
-
Fifth somatosensory area
- T:
-
Temporal auditory cortical area
- VAF:
-
Ventral auditory field
- VLS:
-
Ventral lateral suprasylvian area
- VPAF:
-
Ventral posterior auditory field
- vPE:
-
Ventral division of the posterior ectosylvian gyrus
References
Allman BL, Keniston LP, Meredith MA (2009) Adult deafness induces somatosensory conversion of ferret auditory cortex. Proc Natl Acad Sci USA 106:5925–5930
Amedi A, Raz N, Malach R, Zohary E (2003) Early ‘visual’ cortex activation correlates with superior verbal memory performance in the blind. Nat Neurosci 6:758–766
Auer ET, Bernstein LE, Sungkarat W, Singh M (2007) Vibrotactile activation of the auditory cortices in deaf versus hearing adults. NeuroReport 18:645–658
Batardière A, Barone P, Knoblauch K, Giroud P, Berland M, Dumas AM, Kennedy H (2002) Early specification of the hierarchical organization of visual cortical areas in the macaque monkey. Cereb Cortex 12:453–565
Bhattacharjee A, Ye AJ, Lisak JA, Vargas MG, Goldreich D (2010) Vibrotactile masking experiments reveal accelerated somatosensory processing in congenitally blind braille readers. J Neurosci 30:14288–14298
Bickford MA, Guido W, Godwin DW (1998) Neurofilament proteins in Y-cells of the cat lateral geniculate nucleus: normal expression and alteration with visual deprivation. J Neurosci 18:6549–6557
Butler BE, Chabot N, Lomber SG (2016a) Quantifying and comparing the pattern of thalamic and cortical projections to the posterior auditory field in hearing and deaf cats. J Comp Neurol 524:3042–3063
Butler BE, Chabot N, Lomber SG (2016b) A quantitative comparison of the hemispheric, areal, and laminar origins of sensory and motor cortical projections to the superior colliculus of the cat. J Comp Neurol 524:2623–2642
Butler BE, Chabot N, Kral A, Lomber SG (2017) Origins of thalamic and cortical projections to the posterior auditory field in congenitally deaf cats. Hear Res 343:118–127
Carrasco A, Lomber SG (2010) Reciprocal modulatory influences between tonotopic and nontonotopic cortical fields in the cat. J Neurosci 30:1476–1487
Chabot N, Mellott JG, Hall AJ, Tichenoff EL, Lomber SG (2013) Cerebral origins of the auditory projection to the superior colliculus of the cat. Hear Res 300:33–45
Chabot N, Butler BE, Lomber SG (2015) Differential modification of cortical and thalamic projections to cat primary auditory cortex following early- and late-onset deafness. J Comp Neurol 523:2297–2320
Charbonneau V, Laremee M-E, Boucher V, Bronchti G, Boire D (2012) Cortical and subcortical projections to visual cortex in anopthalmic, enucleated and sighted mice. Eur J Neurosci 36:2949–2963
Clascá F, Llamas A, Reinoso-Suárez F (1997) Insular cortex and neighboring fields in the cat: a redefinition based on cortical microarchitecture and connections with the thalamus. J Comp Neurol 384:456–482
Clemo HR, Sharma GK, Allman BL, Meredith MA (2008) Auditory projections to extrastriate visual cortex: connectional basis for multisensory processing in ‘unimodal’ visual neurons. Exp Brain Res 191:37–47
Clemo HR, Lomber SG, Meredith MA (2016) Synaptic basis for cross-modal plasticity: enhanced supragranular dendritic spine density in anterior ectosylvian auditory cortex of the early deaf cat. Cereb Cortex 26:1365–1376
Clemo HR, Lomber SG, Meredith MA (2017) Synaptic distribution and plasticity in primary auditory cortex (A1) exhibits laminar and cell-specific changes in the deaf. Hear Res 353:122–134
Dahmen JC, King AJ (2007) Learning to hear: plasticity of auditory cortical processing. Curr Opin Neurobiol 17:456–464
Eggermont JJ (2008) The role of sound in adult and developmental auditory cortical plasticity. Ear Hear 29:819–829
Finney EM, Fine I, Dobkins KR (2001) Visual stimuli activate auditory cortex in the deaf. Nat Neurosci 4:1171–1173
Finney EM, Clementz BA, Hickok G, Dobkins KR (2003) Visual stimuli activate auditory cortex in deaf subjects: evidence from MEG. NeuroReport 14:1425–1427
Horsley V, Clarke RH (1908) The structure and function of the cerebellum examined by a new method. Brain Behav Evol 31:45–124
Hunt DL, Yamoah EN, Krubitzer L (2006) Multisensory plasticity in congenitally deaf mice: how are cortical areas functionally specified? Neuroscience 139:1507–1524
Imig TJ, Reale RA (1980) Patterns of cortico-cortical connections related to tonotopic maps in cat auditory cortex. J Comp Neurol 192:293–332
Jones TA, Leake PA, Snyder RL, Stakhovskaya O, Bonham B (2007) Spontaneous discharge patterns in cochlear spiral ganglion cells before the onset of hearing in cats. J Neurophysiol 98:1898–1908
Karlen SJ, Kahn DM, Krubitzer L (2006) Early blindness results in abnormal corticocortical and thalamocortical connections. Neuroscience 142:843–858
Karns CM, Dow MW, Neville HJ (2012) Altered cross-modal processing in the primary auditory cortex of congenitally deaf adults: a visual-somatosensory fMRI study with a double-flash illusion. J Neurosci 32:9626–9638
Kok MA, Chabot N, Lomber SG (2014) Cross-modal reorganization of cortical afferents to dorsal auditory cortex following early- and late-onset deafness. J Comp Neurol 522:654–675
Kral A, Schröder J-H, Klinke R, Engel AK (2003) Absence of cross-modal reorganization in the primary auditory cortex of congenitally deaf cats. Exp Brain Res 153:605–613
Lambertz N, Gizewski ER, de Greiff A, Forsting M (2005) Cross-modal plasticity in deaf subjects dependent on the extent of hearing loss. Brain Res Cogn Brain Res 25:884–890
Lapper SR, Bolam JP (1991) The anterograde and retrograde transport of neurobiotin in the central nervous system of the rat: comparison with biocytin. J Neurosci Methods 39:173–174
Lee CC, Winer JA (2008a) Connections of cat auditory cortex: I. Thalamocortical system. J Comp Neurol 507:1879–1900
Lee CC, Winer JA (2008b) Connections of cat auditory cortex: II. Commissural system. J Comp Neurol 507:1901–1919
Lee CC, Winer JA (2008c) Connections of cat auditory cortex: III. Corticocortical system. J Comp Neurol 507:1920–1943
Lee CC, Winer JA (2011) Convergence of thalamic and cortical pathways in cat auditory cortex. Hear Res 274:85–94
Lee DS, Lee JS, Oh SH, Kim SK, Kim JW, Chung JK, Lee MC, Kim CS (2001) Cross-modal plasticity and cochlear implants. Nature 409:149–150
Levänen S, Hamdorf D (2001) Feeling vibrations: enhanced tactile sensitivity in congenitally deaf humans. Neurosci Lett 301:75–77
Levänen S, Jousmäki V, Hari R (1998) Vibration-induced auditory-cortex activation in a congenitally deaf adult. Curr Biol 8:869–872
Lomber SG, Meredith MA, Kral A (2010) Cross-modal plasticity in specific auditory cortices underlies visual compensations in the deaf. Nat Neurosci 13:1421–1427
Lomber SG, Meredith MA, Kral A (2011) Adaptive crossmodal plasticity in deaf auditory cortex: areal and laminar contributions to supranormal vision in the deaf. In: Green AM, Chapman CE, Kalaska JF, Lepore F (eds) Enhancing performance for action and perception, multisensory integration, neuroplasticity and neuroprosthetics. Elsevier, Amsterdam, pp 251–270
Malhotra S, Hall AJ, Lomber SG (2004) Cortical control of sound localization in the cat: unilateral cooling deactivation of 19 cerebral areas. J Neurophysiol 92:1625–1643
Mellott JG, van der Gucht E, Lee CC, Carrasco A, Winer JA, Lomber SG (2010) Areas of the cat auditory cortex as defined by neurofilament proteins expressing SMI-32. Hear Res 267:119–136
Meredith MA, Clemo HR (1989) Auditory cortical projection from the anterior ectosylvian sulcus (field AES) to the superior colliculus in the cat: an anatomical and electrophysiological study. J Comp Neurol 289:687–707
Meredith MA, Lomber SG (2011) Somatosensory and visual crossmodal plasticity in the anterior auditory field of early-deaf cats. Hear Res 280:38–47
Meredith MA, Lomber SG (2017) Species-dependent role of crossmodal connectivity among the sensory cortices. Hear Res 343:83–91
Meredith MA, Kryklywy J, McMillan AJ, Malhotra S, Lum-Tai R, Lomber SG (2011) Crossmodal organization in the early deaf switches sensory, but not behavioral roles of auditory cortex. Proc Nat Acad Sci 108:8856–8861
Meredith MA, Clemo HR, Corley SB, Chabot N, Lomber SG (2016) Cortical and thalamic connectivity of the auditory anterior ectosylvian cortex of early-deaf cats: implications for neural mechanisms of crossmodal plasticity. Hear Res 333:25–36
Merzenich MM, Knight PL, Roth GL (1975) Representation of cochlea within primary auditory cortex in the cat. J Neurophys 38:231–249
Merzenich MM, Nelson RJ, Stryker MP, Cyander MS, Schoppmann A, Zook JM (1984) Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol 224:591–605
Mezzera C, Lopez-Bendito G (2016) Cross-modal plasticity in sensory deprived animal models: from the thalamocortical development point of view. J Chem Neuroanat 75:32–40
Middlebrooks JC, Xu L, Clock Eddins A, Green DM (1998) Codes for sound-source localization in nontonotopic auditory cortex. J Neurophysiol 80:863–881
Neville HJ, Schmidt A, Kutas M (1983) Altered visual-evoked potentials in congenitally deaf adults. Brain Res 266:127–132
O’Leary DD, Chou SJ, Sahara S (2007) Area patterning of the mammalian cortex. Neuron 2:252–269
Olfert ED, Cross BM, McWilliam AA (1993) Guide to the care and use of experimental animals. Canadian Council on Animal Care, Ottawa, Ontario, Canada
Palmer LA, Rosenquist AC, Tusa RJ (1978) The retinotopic organization of lateral suprasylvian visual areas in the cat. J Comp Neurol 177:237–256
Payne BR, Lomber SG (1996) Age dependent modification of cytochrome oxidase activity in the cat dorsal lateral geniculate nucleus following removal of primary visual cortex. Vis Neurosci 13:805–816
Pekkola J, Ojanen V, Autti T, Jaaskelainen IP, Mottonen R, Tarkiainen A, Sams M (2005) Primary auditory cortex activation by visual speech: an fMRI study at 3 T. NeuroReport 16:125–128
Rajakumar N, Elisevice K, Flumerfelt BA (1993) Biotinylated dextran: a versatile anterograde and retrograde neuronal tracer. Brain Res 607:47–53
Rauschecker JP, Grunau M, Poulin C (1987) Centrifugal organization of direction preferences in the cat’s lateral suprasylvian visual cortex and its relation to flow field processing. J Neurosci 7:943–958
Reale RA, Imig TJ (1980) Tonotopic organization in auditory cortex of the cat. J Comp Neurol 192:265–291
Rouiller EM, Hornung JP, De Ribaupierre F (1989) Extrathalamic ascending projections to physiologically identified fields of the cat auditory cortex. Hear Res 40:233–246
Sadato N, Okada T, Honda M, Yonekura Y (2002) Critical period for cross-modal plasticity in blind humans: a functional MRI study. NeuroImage 16:389–400
Scannell JW, Blakemore C, Young MP (1995) Analysis of connectivity in the cat cerebral cortex. J Neurosci 15:1463–1483
Scannell JW, Sengpiel F, Tovee MJ, Benson PJ, Blakemore C, Young MP (1996) Visual motion processing in the anterior ectosylvian sulcus of the cat. J Neurophys 76:895–907
Schreiner CE, Cyander MS (1984) Basic functional organization of second auditory cortical field (AII) of the cat. J Neurophys 51:1284–1305
Sternberger LA, Sternberger NH (1983) Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci USA 80:6126–6130
Stewart DL, Starr A (1970) Absence of visually influenced cells in auditory cortex of normal and congenitally deaf cats. Exp Neurol 28:525–528
Striem-Amit E, Ovadia-Caro S, Caramazza A, Margulies DS, Villringer A, Amedi A (2015) Functional connectivity of visual cortex in the blind follows retinotopic organization principles. Brain 138:1679–1695
Takesian AE, Hensch TK (2013) Balancing plasticity/stability across brain development. Prog Brain Res 207:3–34
van der Gucht E, Vandesande F, Arckens L (2001) Neurofilament protein: a selective marker for the architectonic parcellation of the visual cortex in adult cat brain. J Comp Neurol 441:345–368
Veenman CL, Reiner A, Honig MG (1992) Biotinylated dextran amine as an anterograde tracer for single- and double-labeling studies. J Neurosci Methods 41:239–254
von Grünau MW, Sumbroicj TJ, Poulin C (1987) Visual receptive field properties in the posterior suprasylvian cortex of the cat: a comparison between the areas PMLS and PLLS. Vis Res 27:343–356
Wong C, Chabot N, Kok MA, Lomber SG (2014) Modified areal cartography in auditory cortex following early- and late-onset deafness. Cereb Cortex 24:1778–1792
Wong C, Chabot N, Kok MA, Lomber SG (2015) Amplified somatosensory and visual cortical projections to a core auditory area, the anterior auditory field, following early- and late-onset deafness. J Comp Neurol 523:1925–1947
Xu SA, Shepherd RK, Chen Y, Clark GM (1993) Profound hearing loss in the cat following the single co-administration of kanamycin and ethacrynic acid. Hear Res 70:205–215
Xu L, Furukawa S, Middlebrooks JC (1998) Sensitivity to sound-source elevation in nontonotopic auditory cortex. J Neuruphysiol 80:883–894
Acknowledgements
The authors would like to thank Pam Nixon for technical and surgical assistance. Thanks also to Benson Li, Chris Lee, and Kevin Ly for assistance with tissue processing. Funding for this work was provided by the Natural Sciences and Engineering Research Council of Canada, the Canadian Institutes of Health Research, and the Canada Foundation for Innovation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Funding for this work was provided by the Natural Sciences and Engineering Research Council of Canada, the Canadian Institutes of Health Research, and the Canada Foundation for Innovation.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All surgical and experimental procedures were conducted in accordance with the Canadian Council on Animal Care’s Guide to the Care and Use of Experimental Animals (Olfert et al. 1993) and were approved by the University of Western Ontario Animal Use Subcommittee of the University Council on Animal Care.
Rights and permissions
About this article
Cite this article
Butler, B.E., de la Rua, A., Ward-Able, T. et al. Cortical and thalamic connectivity to the second auditory cortex of the cat is resilient to the onset of deafness. Brain Struct Funct 223, 819–835 (2018). https://doi.org/10.1007/s00429-017-1523-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-017-1523-y