Abstract
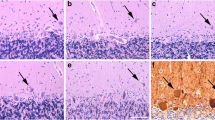
Lipofuscin pigment accumulation is among the most prominent markers of cellular aging in postmitotic cells. The formation of lipofuscin is related to oxidative enzymatic activity and free radical-induced lipid peroxidation. In various mammals such as rat, dog, macaque as well as in cheirogaleid primates, most of the large neurons, such as cerebellar Purkinje cells and neocortical pyramidal cells, show heavy lipofuscin accumulation in adulthood. In contrast, a well-known yet poorly studied feature of the aging human brain is that although lipofuscin accumulation is most marked in large neurons of the cerebral cortex, the large neurons of the cerebellar cortex—the Purkinje cells—appear to remain free of lipofuscin accumulation. It is however, not known whether this characteristic of human Purkinje cells is shared with other primates or other mammals. This study reports results from histological observation of Purkinje cells in humans, non-human primates, and other mammals. Procedures include histochemistry, immunocytochemistry, and fluorescence microscopy. Abundant lipofuscin deposition was observed in Purkinje cells of all the species we examined except Homo sapiens (including Alzheimer’s disease cases) and Pan troglodytes. In contrast, lipofuscin deposition was observed in neurons of the dentate nucleus. Our findings suggest that when compared with other primates, Purkinje cells in chimpanzees and humans might share a common aging pattern that involves mechanisms for neuroprotection. This observation is important when considering animal models of aging.










Similar content being viewed by others
References
Andersen BB, Gundersen HJG, Pakkenberg B (2003) Aging of the human cerebellum: a stereological study. J Comp Neurol 466:356–365
Apps R, Garwicz M (2005) Anatomical and physiological foundations of cerebellar information processing. Nat Rev Neurosci 6:297–311
Barden H, Brizzee KR (1987) The histochemistry of lipofuscin and neuromelanin. Adv Biosci 64:339–392
Bell CC, Han V, Sawtell NB (2008) Cerebellum-like structures and their implications for cerebellar function. Ann Rev Neurosci 31:1–24
Benavides SH, Monserrat AJ, Fariña S, Porta EA (2002) Sequential histochemical studies of neuronal lipofuscin in human cerebral cortex from the first to the ninth decade of life. Arch Gerontol Geriatr 34:219–231
Braak H, Braak E (1995) Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging 16:271–278
Bredberg L-L, Matousek M, Steen B (2003) Ninety-seven-year-old people general presentation, and some general and medical characteristics from a Swedish population study. Ach Gerontol Geriatr 36:37–47
Brizzee KR, Kaack B, Klara P (1975) Lipofuscin: intra- and extraneuronal accumulation and regional distribution. In: Ordy JM, Brizzee KR (eds) Neurobiology of aging: an interdisciplinary life-span approach. Plenum Press, New York, pp 463–484
Brunk UT, Terman A (2002a) The mitochondrial-lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur J Biochem 269:1996–2002
Brunk UT, Terman A (2002b) Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic Biol Med 33:611–619
Butti C, Santos M, Uppal N, Hof PR (2013) Von Economo neurons: clinical and evolutionary perspectives. Cortex 49:312–326
Cáceres M, Suwyn C, Maddox M, Thomas JW, Preuss TM (2007) Increased cortical expression of two synaptogenic thrombospondins in human brain evolution. Cereb Cortex 17:2312–2321
Cataldo AM, Hamilton DJ, Nixon RA (1994) Lysosomal abnormalities in degenerating neurons link neuronal compromise to senile plaque development in Alzheimer disease. Brain Res 640:68–80
Cataldo AM, Hamilton DJ, Barnett JL, Paskevich PA, Nixon RA (1996) Properties of the endosomal-lysosomal system in the human central nervous system: disturbances mark most neurons in populations at risk to degenerate in Alzheimer’s disease. J Neurosci 16:186–199
Chakrabarti L, Eng J, Ivanov N, Garden GA, La Spada AR (2009) Autophagy activation and enhanced mitophagy characterize the Purkinje cells of pcd mice prior to neuronal death. Molecular Brain 2:24
Chen L, Na R, Gu M, Richardson A, Ran Q (2008) Lipid peroxidation up-regulates BACE1 expression in vivo: a possible early event of amyloidogenesis in Alzheimer’s disease. J Neurochem 107:197–207
Cuervo AM, Bergamini E, Brunk UT, Dröge W, French M, Terman A (2005) Autophagy and aging. The importance of maintaining “clean” cells. Autophagy 1:131–140
D’Andrea MR, Nagele RG, Gumula NA et al (2002) Lipofuscin and Abeta42 exhibit distinct distribution patterns in normal and Alzheimer’s disease brains. Neurosci Lett 323:45–49
Dapson RW, Feldman AT, Pane G (1980) Differential rates of aging in natural populations of old-field mice (Peromyscus polionotus). J Gerontol 35:39–44
De Duve C, Wattiaux R (1966) Functions of lysosomes. Ann Rev Physiol 28:435–492
Demuth JP, De Bie T, Stajich JE, Cristianini N, Hahn MW (2006) The evolution of mammalian gene families. PLoS One 1(1):e85
Doehner J, Genoud C, Imhof C, Krstic D, Knuesel I (2012) Extrusion of misfolded and aggregated proteins—a protective strategy of aging neurons? Eur J Neurosci 35:1938–1950
Double KL, Reyes S, Werry EL, Halliday GM (2010) Selective cell death in neurodegeneration: why are some neurons spared in vulnerable regions? Progr Neurobiol 92:316–329
Dowson JH (1987) Quantitative studies of the effects of aging, meclofenoxate and dihydroergotoxine on intraneuronal lipopigment accumulation in the rat. In: Totaro EA, Glees P, Pisanti FA (eds) Advances in age pigments research. Pergamon Press, Oxford, pp 93–100
Dowson JH, Mountjoy CQ, Cairns MR, Wilton-Cox H, Bondareff W (1998) Lipopigment changes in Purkinje cells in Alzheimer’s disease. J Alz Disease 1:71–79
Evrard HC, Forro T, Logothetis NK (2012) Von Economo neurons in the anterior insula of the macaque monkey. Neuron 74:482–489
Fonseca DB, Sheehy MRJ, Blackman N, Shelton PMJ, Prior AE (2005) Reversal of a hall mark of brain ageing: lipofuscin accumulation. Neurobiol Aging 26:69–76
Fraser HB, Khaitovich P, Plotkin JB, Pääbo S, Eisen MB (2005) Aging and gene expression in the primate brain. PLoS Biol 3(9):e274
Gerrits PO, Kortekaas R, de Weerd H, Veening JG, van der Want JJL (2012) Regional differences in age-related lipofuscin accumulation in the female hamster brainstem. Neurobiol Aging 33:625.e1–625.e9
Geurts FJ, De Schutter E, Dieudonné S (2003) Unraveling the cerebellar cortex: cytology and cellular physiology of large-sized interneurons in the granular layer. Cerebellum 2:290–299
Giaccone G, Orsi L, Cupidi C, Tagliavini F (2011) Lipofuscin hypothesis of Alzheimer’s disease. Dement Geriatr Cogn Disord Extra 1:292–296
Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI et al (2000) Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science 290:985–989
Gilissen E, Staneva-Dobrovski L (2013) Distinct types of lipofuscin pigment in the hippocampus and cerebellum of aged cheirogaleid primates. Anat Rec 296A:1895–1906
Gilissen E, Ghosh P, Jacobs RE, Allman JM (1998a) Topographical localization of iron in brains of the aged fat-tailed dwarf lemur (Cheirogaleus medius) and grey lesser mouse lemur (Microcebus murinus). Am J Primatol 45:291–299
Gilissen E, Jacobs RE, Allman JM (1998b) Dwarf and mouse lemurs as primate model of brain aging: distribution of age pigments. Am J Primatol 45:182–183
Gilissen E, Jacobs RE, McGuinness ER, Allman JM (1999) Topographical localization of lipofuscin pigment in the brain of the aged fat-tailed dwarf lemur (Cheirogaleus medius) and grey lesser mouse lemur (Microcebus murinus). Am J Primatol 49:183–193
Gilissen E, Dhenain M, Allman JM (2001) Brain aging in strepsirrhine primates. In: Hof PR, Mobbs CV (eds) Functional neurobiology of aging. Academic Press, San Diego, pp 421–433
Glickstein M, Oberdick J, Voogd J (2009) The evolution of the cerebellum. Elsevier, New York
Goebel HH (1988) Ultrastructure of disease-related lipopigments. In: Zs–Nagy I (ed) Lipofuscin—1987: state of the art. Elsevier, Amsterdam, pp 319–340
Gray DA, Woulfe J (2005) Lipofuscin and aging: a matter of toxic waste. Sci Aging Knowl Environ 5:re1
Gray DA, Tsirigotis M, Woulfe J (2003) Ubiquitin, proteasomes, and the aging brain. Sci Aging Knowl Environ 34:re6
Greco CM, Hagerman RJ, Tassone F, Chudley AE, Del Bigio MR, Jacquemont S, Leehey M, Hagerman PJ (2002) Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain 125:1760–1771
Grune T, Reinheckel T, Davies KJA (1997) Degradation of oxidized proteins in mammalian cells. FASEB J 11:526–534
Grune T, Merker K, Jung T, Sitte N, Davies KJA (2005) Protein oxidation and degradation during postmitotic senescence. Free Rad Biol Med 39:1208–1215
Hakeem A, Sandoval GR, Jones M, Allman J (1996) Brain and life span in primates. In: Birren JE, Schaie KW (eds) Handbook of the psychology of aging. Academic Press, New York, pp 78–104
Hansen TE, Johansen T (2011) Following autophagy step by step. BMC Biol 9:39
Head E, Milgram NW, Cotman CW (2001) Neurobiological models of aging in the dog and other vertebrate species. In: Hof PR, Mobbs CV (eds) Functional neurobiology of aging. Academic Press, San Diego, pp 457–468
Hedge ML, Jagganatha Rao KS (2003) Challenges and complexities of alpha-synuclein toxicity: new postulates in unfolding the mystery associated with Parkinson’s disease. Arch Biochem Biophys 418:169–178
Heinsen H (1979) Lipofuscin in the cerebellar cortex of albino rats: an electron microscopy study. Anat Embryol (Berl) 155:333–345
Heinsen H (1981) Regional differences in the distribution of lipofuscin in Purkinje cell perikarya. A quantitative pigment architectonic study of the cerebellar cortex of senile albino rats. Anat Embryol (Berl) 161:453–464
Heinsen H (1987) Quantitative investigations on regional differences in lipofuscin accumulation in Purkinje cells of old rats. In: Totaro EA, Glees P, Pisanti FA (eds) Advances in age pigments research. Pergamon Press, Oxford, pp 321–337
Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M (2002) Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature 419:808–814
Hishikawa N, Hashizume Y, Yoshida M (2003) Clinical and neuropathological correlates of Lewy body disease. Acta Neuropathol 105:341–350
Hof PR, Gilissen EP, Sherwood CC, Duan H, Lee PWH, Delman BN, Naidich TP, Gannon PJ, Perl DP, Erwin JM (2002) Comparative neuropathology of brain aging in primates. In: Erwin JM, Hof PR (eds) Aging in nonhuman primates. Interdisciplinary topics in gerontology, vol 31. Karger, Basel, pp 130–154
Höhn A, Jung T, Grimm S, Catalgol B, Weber D, Grune T (2011) Lipofuscin inhibits the proteasome by binding to surface motifs. Free Rad Biol Med 50:585–591
Holtzmann E (1976) Lysosomes: a survey, cell biology monographs, vol 3. Springer Verlag, Wien
Imhof A, Kövari E, von Gunten A, Gold G, Rivara C-B, Herrmann FR, Hof PR, Bouras C, Giannakopoulos P (2007) Morphological substrates of cognitive decline in nonagenarians and centenarians: a new paradigm. J Neurol Sci 257:72–79
Ivy GO (1992) Protease inhibition causes some manifestations of aging and Alzheimer’s disease in rodent and primate brain. Ann NY Acad Sci 674:89–102
Ivy GO, Gurd JW (1988) A proteinase inhibitor model of lipofuscin formation. In: Zs–Nagy I (ed) Lipofuscin—1987: state of the art. Elsevier, Amsterdam, pp 83–108
Ivy GO, Schottler F, Wenzel J, Baudry M, Lynch G (1984) Inhibitors of lysosomal enzymes: accumulation of lipofuscin-like dense bodies in the brain. Science 226:985–987
Ivy GO, Kanai S, Ohta M, Smith G, Sato Y, Kobayashi M, Kitani K (1990) Lipofuscin-like substances accumulate rapidly in brain, retina and internal organs with cysteine protease inhibition. In: Porta EA (ed) Lipofuscin and ceroid pigments. Plenum Press, New York, pp 31–47
Ivy GO, Roopsingh R, Kanai S, Ohta M, Sato Y, Kitani K (1996) Leupeptin causes an accumulation of lipofuscin-like substances and other signs of aging in kidneys of young rats: further evidence for the protease inhibitor model of aging. Ann NY Acad Sci 786:12–23
James TJ, Sharma SP (1995) Regional and lobular variation in neuronal lipofuscinosis in rat cerebellum: influence of age and protein malnourishment. Gerontology 41:213–228
Jolly RD, Palmer DN, Dakefield RR (2002) The analytical approach to the nature of lipofuscin (age pigment). Arch Gerontol Geriatr 34:205–217
Jung T, Bader N, Grune T (2007) Lipofuscin. Formation, distribution, and metabolic consequences. Ann NY Acad Sci 1119:97–111
Kanaan NM, Kordower JH, Collier TJ (2007) Age-related accumulation of Marinesco bodies and lipofuscin in rhesus monkey midbrain dopamine neurons: relevance to selective neuronal vulnerability. J Comp Neurol 502:683–700
Katz ML (2002) Potential reversibility of lipofuscin accumulation. Arch Gerontol Geriatr 34:311–317
Katz ML, Robison WG (2002) What is lipofuscin? Defining characteristics and differentiation from other autofluorescent lysosomal storage bodies. Arch Gerontol Geriatr 34:169–184
Keller JN, Dimayuga E, Chen Q, Thorpe J, Gee J, Ding Q (2004) Autophagy, proteasomes, lipofuscin, and oxidative stress in the aging brain. Int J Biochem Cell Biol 36:2376–2391
Khaitovich P, Weiss G, Lachmann M, Hellmann I, Enard W, Muetzel B, Wirkner U, Ansorge W, Pääbo S (2004) A neutral model of transcriptome evolution. PLoS Biol 2:0682–0689
Khaitovich P, Pääbo S, Weiss G (2005) Toward a neutral evolutionary model of gene expression. Genetics 170:929–939
Khan MA (1993) Histochemical and ultrastructural investigation of heterogeneous Purkinje neurons in mammalian cerebellum. Cell Mol Biol Res 39:789–795
Koeppen AH, Davis AN, Morral JA (2011) The cerebellar component of Friedreich’s ataxia. Acta Neuropathol 122:323–330
Koller WC, Glatt SL, Fox JH, Kaszniak AW, Wilson RS, Huckman MS (1981) Cerebellar atrophy: relationship to aging and cerebral atrophy. Neurology 31:1486–1488
Kumar S, Filipski A, Swarna V, Walker A, Hedges SB (2005) Placing confidence limits on the molecular age of the human-chimpanzee divergence. Proc Natl Acad Sci USA 102:18842–18847
Kurz T, Terman A, Brunk UT (2007) Autophagy, ageing and apoptosis: the role of oxidative stress and lysosomal iron. Arch Biochim Biophys 462:220–230
Kurz T, Terman A, Gustafsson B, Brunk UT (2008) Lysosomes and oxidative stress in aging and apoptosis. Biochim Biophys Acta 1780:1291–1303
Larner AJ (1997) The cerebellum in Alzheimer’s disease. Dement Geriatr Cogn Disord 8:203–209
Larsell O (1952) The morphogenesis and adult pattern of the lobules and fissures of the cerebellum of the white rat. J Comp Neurol 97:281–356
Larsen JO, Skalicky M, Viidik A (2000) Does long-term physical exercise counteract age-related Purkinje cell loss? A stereological study of rat cerebellum. J Comp Neurol 428:2013–2222
Lee JT, Miller CA, McDonald CT, Allman JM (1996) Xanthogranuloma of the choroid plexus in the fat-tailed dwarf lemur (Cheirogaleus medius). Am J Primatol 38:349–355
Leibnitz L, Wünscher W (1967) Die lebensgeschichtliche Ablagerung von intraneuralem Lipofuscin in verschiedenen Abschnitten des menschlichen Gehirns. Anat Anz 121:132–140
Lillie RD (1965) Histopathologic technic and practical histochemistry. McGraw-Hill, New York
Liu Q, Smith MA, Avilá J, DeBernardis J, Kansal M, Takeda A, Zhu X, Nunomura A, Honda K, Moreira PI, Oliveira CR, Santos MS, Shimohama S, Aliev G, de la Torre J, Ghanbari HA, Siedlak SL, Harris PLR, Sayre LM, Perry G (2005) Alzheimer-specific epitopes of tau represent lipid peroxidation-induced conformations. Free Rad Biol Med 38:746–754
Liu X, Somel M, Tang L, Yan Z, Jiang X, Guo S, Yuan Y, He L, Oleksiak A, Zhang Y, Li N, Hu Y, Chen W, Qiu Z, Pääbo S, Khaitovich P (2012) Extension of cortical synaptic development distinguishes humans from chimpanzees and macaques. Genome Res. doi:10.1101/gr.127324.111
Lockwood CA, Kimbel WH, Lynch JM (2004) Morphometrics and hominoid phylogeny: support for a chimpanzee-human clade and differentiation among great ape subspecies. Proc Natl Acad Sci USA 101:4356–4360
Lyn H, Pierre P, Bennett AJ, Fears S, Woods R, Hopkins WD (2011) Planum temporale grey matter asymmetries in chimpanzees (Pan troglodytes), vervet (Chlorocebus aethiops sabaeus), rhesus (Macaca mulatta) and bonnet (Macaca radiata) monkeys. Neuropsychologia 49:2004–2012
Madalosso SH, Pérez-Villegas EM, Armengol JA (2005) Naturally occurring neuronal death during the postnatal development of Purkinje cells and their precerebellar afferent projections. Brain Res Rev 49:267–279
Mann DMA, Yates PO, Stamp JE (1978) The relationship between lipofuscin pigment and ageing in the human nervous system. J Neurol Sci 37:83–93
McHolm GB, Aguilar MJ, Norris FH (1984) Lipofuscin in amyotrophic lateral sclerosis. Arch Neurol 41:1187–1188
McManus JFA, Mowry RW (1960) Staining methods: histologic and histochemical. Harper and Row, New York
Miquel J, Oro J, Bensch KG, Johnson JE (1990) Lipofuscin: fine-structural and biochemical studies. In: Pryor WA (ed) Free radicals in biology, vol 3. Academic Press, New York, pp 133–182
Mormino EC, Brandel MG, Madison CM, Marks S, Baker SL, Jagust WJ (2012) A beta deposition in aging is associated with increases in brain activation during successful memory encoding. Cereb Cortex 22:1813–1823
Morrison JH, Hof PR (1997) Life and death of neurons in the aging brain. Science 278:412–419
Mrak RE, Griffin WST, Graham DI (1997) Aging-associated changes in human brain. J Neuropathol Exp Neurol 56:1269–1275
Müller U, Heinsen H (1984) Regional differences in the ultrastructure of Purkinje cells of the rat. Cell Tissue Res 235:91–98
Myeku N, Figueiredo-Pereira ME (2009) Ubiquitin/proteasome and autophagy/lysosome pathways: comparison and role in neurodegeneration. In: Banik N, Ray S (eds) Handbook of neurochemistry and molecular neurobiology: brain and spinal cord trauma. Springer, New York, pp 514–524
Naguro T, Iwashita K (1992) Olfactory epithelium in young-adult and aging rats as seen with high-resolution scanning electron-microscopy. Microsc Res Techn 23:62–75
Nakano M, Mizuno T, Gotoh S (1993) Accumulation of cardiac lipofuscin in crab-eating monkeys (Macaca fasicularis): the same rate of lipofuscin accumulation in several species of primates. Mech Ageing Develop 66:243–248
Nakano M, Oenzil F, Mizuno T, Gotoh S (1995) Age-related changes in the lipofuscin accumulation of brain and heart. Gerontology 41(Suppl. 2):69–79
Nandy K (1981) Morphological changes in the cerebellar cortex of aging Macaca nemestrina. Neurobiol Aging 2:61–64
Nandy K, Mostofsky DI, Idrobo F, Blatt L, Nandy S (1988) Experimental manipulations of lipofuscin formation in aging mammals. In: Zs–Nagy I (ed) Lipofuscin—1987: state of the art. Elsevier, Amsterdam, pp 289–304
Nimchinsky EA, Gilissen E, Allman JM, Perl DP, Erwin JM, Hof PR (1999) A neuronal morphologic type unique to humans and great apes. Proc Natl Acad Sci USA 96:5268–5273
Nishimiya J (1988) CT evaluation of cerebellar atrophy with aging in healthy persons [Article in Japanese]. No To Shinkei 40:585–591
Nixon RA (2006) Autophagy in neurodegenerative disease: friend, foe or turncoat? Trends Neurosci 29:528–535
Nixon RA (2007) Autophagy, amyloidogenesis and Alzheimer disease. J Cell Sci 120:4081–4091
Nixon RA, Cataldo AM (1993) The lysosomal system in neuronal cell death: a review. Ann NY Acad Sci 679:87–109
Nixon RA, Yang D-S (2011) Autophagy failure in Alzheimer’s disease-locating the primary defect. Neurobiol Dis 43:38–45
Nixon RA, Cataldo AM, Paskevich PA, Hamilton DJ, Wheelock TR, Kanaley-Andrews L (1992) The lysosomal system in neurons. Involvement at multiple stages of Alzheimer’s disease pathogenesis. Ann NY Acad Sci 674:65–88
Nixon RA, Cataldo AM, Mathews PM (2000) The endosomal-lysosomal system of neurons in Alzheimer’s disease pathogenesis: a review. Neurochem Res 25:1161–1172
Nixon RA, Yang D-S, Lee J-H (2008) Neurodegenerative lysosomal disorders. A continuum from development to late age. Autophagy 4:590–599
Obersteiner H (1903) Über das hellgelbe Pigment in den Nervenzellen und das Vorkommen weiterer fettähnlicher Körper im Centralnervensystem. Arbeiten aus dem neurologischen Institut Wien 10:245–274
Oenzil F, Kishikawa M, Mizuno T, Nakano M (1994) Age-related accumulation of lipofuscin in 3 different regions of rat brain. Mech Age Dev 76:157–163
Ogomori K, Kitamoto T, Tateishi J, Sato Y, Suetsugu M, Abe M (1989) Beta-protein amyloid is widely distributed in the central nervous system of patients with Alzheimer’s disease. Am J Pathol 134:243–251
Oldham MC, Horvath S, Geschwind DH (2006) Conservation and evolution of gene coexpression networks in human and chimpanzee brains. Proc Natl Acad Sci 103:17973–17978
Palay SL, Chan-Palay V (1974) Cerebellar Cortex. Cytology and organization. Springer, New York
Pannese E (2011) Morphological changes in nerve cells during normal aging. Brain Struct Funct 216:85–89
Patterson N, Richter DJ, Gnerre S, Lander ES, Reich D (2006) Genetic evidence for complex speciation of humans and chimpanzees. Nature 441:1103–1108
Patterson N, Richter DJ, Gnerre S, Lander ES, Reich D (2008) Patterson et al. reply. Nature 452:E4
Pearse AGE (1972) Histochemistry. Theoretical and applied. J. & A. Churchill, London
Perez SE, Raghanti MA, Hof PR, Kramer L, Ikonomovic MD, Lacor PN, Erwin JM, Sherwood CC, Mufson EJ (2013) Alzheimer’s disease pathology in the neocortex and hippocampus of the western lowland gorilla (Gorilla gorilla gorilla). J Comp Neurol 521:4318–4338. doi:10.1002/cne.23428
Porta EA (1987) Tissue lipoperoxidation and lipofuscin accumulation as influenced by age, type of dietary fat and levels of vitamin E in rats. In: Totaro EA, Glees P, Pisanti FA (eds) Advances in age pigments research. Pergamon Press, Oxford, pp 37–73
Porta EA, Hartroft WS (1969) Lipid pigments in relation to aging and dietary factors (lipofuscins). In: Wolman M (ed) Pigments in pathology. Academic Press, New York, pp 191–235
Powell SR, Wang P, Divald A, Teichberg S, Haridas V, McCloskey TW, Davies KJA, Katzeff H (2005) Aggregates of oxidized proteins (lipofuscin) induce apoptosis through proteasome inhibition and dysregulation of proapoptotic proteins. Free Rad Biol Med 38:1093–1101
Rideout HJ, Lang-Rollin I, Stefanis L (2004) Involvement of macroautophagy in the dissolution of neuronal inclusions. Int J Biochem Cell Biol 36:2551–2562
Rogers J, Silver MA, Shoemaker WJ, Bloom FE (1980) Senescent changes in a neurobiological model system: cerebellar Purkinje cell electrophysiology and correlative anatomy. Neurobiol Aging 1:3–11
Rosen RF, Farberg AS, Gearing M, Dooyema J, Long PM, Anderson DC, Davis-Turak J, Coppola G, Geschwind DH, Paré JF, Duong TQ, Hopkins WD, Preuss TM, Walker LC (2008) Tauopathy with paired helical filaments in an aged chimpanzee. J Comp Neurol 509:259–270
Rossi F, Tempia F (2006) Unravelling the Purkinje neuron. Cerebellum 5:75–76
Rossi F, Gianola S, Corvetti L (2006) The strange case of Purkinje axon regeneration and plasticity. Cerebellum 5:174–182
Rugarli EI, Langer T (2012) Mitochondrial quality control: a matter of life and death for neurons. EMBO J 31:1336–1349
Samorajski T, Ordy JM, Rady-Reimer P (1968) Lipofuscin pigment accumulation in the nervous system of aging mice. Anat Rec 160A:555–574
Sanchez M, Sillitoe RV, Attwell PJE, Ivarsson M, Rahman S, Yeo CH, Hawkes R (2002) Compartmentation of the rabbit cerebellar cortex. J Comp Neurol 444:159–173
Schmucker DL, Sachs H (2002) Quantifying dense bodies and lipofuscin during aging: a morphologist’s perspective. Arch Gerontol Geriatr 34:249–261
Sherwood CC, Duka T (2012) Now that we’ve got the map, where are we going? Moving from gene candidate lists to function in studies of brain evolution. Brain Behav Evol 80:167–169
Sherwood CC, Bauernfeind AL, Bianchi S, Raghanti MA, Hof PR (2012) Human brain evolution writ large and small. In: Hofman MA, Falk D (eds) Progress in brain research, vol 195. Elsevier, Amsterdam, pp 237–254
Shima A, Tomonaga M (1988) Microfluorimetric characterization of in situ autofluorescence of lipofuscin granules in the aged human brains. In: Zs–Nagy I (ed) Lipofuscin—1987: state of the art. Elsevier, Amsterdam, pp 147–157
Sillitoe RV, Malz CR, Rockland K, Hawkes R (2004) Antigenic compartmentation of the primate and tree shrew cerebellum: a common topography of zebrin II in Macaca mulatta and Tupaia belangeri. J Anat 204:257–269
Sitte N, Huber M, Grune T, Ladhoff A, Doecke WD, Von Zglinicki T, Davies KJ (2000) Proteasome inhibition by lipofuscin/ceroid during postmitotic aging of fibroblasts. FASEB J 14:1490–1498
Sohal RS, Wolfe LS (1986) Lipofuscin: characteristics and significance. In: Swaab DF, Fliers E, Mirmiran M, Van Gool WA, Van Haaren F (eds) Progress in brain research, vol 70. Elsevier, Amsterdam, pp 171–183
Stojanovic A, Roher AE, Ball MJ (1994) Quantitative analysis of lipofuscin and neurofibrillary tangles in the hippocampal neurons of Alzheimer’ disease brains. Dementia 5:229–233
Stoppini M, Andreola A, Foresti G, Bellotti V (2004) Neurodegenerative diseases caused by protein aggregation: a phenomenon at the borderline between molecular evolution and ageing. Pharmacol Res 50:419–431
Stubel H (1911) Die Fluoreszenz tierischer Gewebe in ultraviolettem Licht. Pflueg Arch Ges Physiol Mensch Tiere 142:1–14
Suraweera A, Münch C, Hanssum A, Bertolotti A (2012) Failure of amino acid homeostasis causes cell death following proteasome inhibition. Mol Cell 48:1–12
Szweda PA, Camouse M, Lundberg KC, Oberley TD, Szweda LI (2003) Aging, lipofuscin formation, and free radical-mediated inhibition of cellular proteolytic systems. Aging Res Rev 2:383–405
Tai H-C, Schuman EM (2008) Ubiquitin, the proteasome and protein degradation in neuronal function and dysfunction. Nat Rev Neurosci 9:826–838
Tandler CJ, Ríos H, Pellegrino de Iraldi A (1997) Differential staining of two subpopulations of Purkinje neurons in rat cerebellum with acid dyes. Biotech Histochem 72:231–239
Terman A (2001) Garbage catastrophe theory of aging: imperfect removal of oxidative damage? Redox Rep 6:15–26
Terman A, Brunk UT (1998a) Lipofuscin: mechanisms of formation and increase with age. APMIS 106:265–276
Terman A, Brunk UT (1998b) On the degradability and exocytosis of ceroid/lipofuscin in cultured rat cardiac myocytes. Mech Ageing Dev 100:145–156
Terman A, Brunk UT (2004a) Lipofuscin. Int J Biochem Cell Biol 36:1400–1404
Terman A, Brunk UT (2004b) Aging as a catabolic malfunction. Int J Biochem Cell Biol 36:2365–2375
Terman A, Gustafsson B, Brunk UT (2007) Autophagy, organelles and ageing. J Pathol 211:134–143
Terman A, Kurz T, Navratil M, Ariaga EA, Brunk UT (2010) Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial-lysosomal axis theory of aging. Antiox Redox Sign 12:503–535
Tewari HB, Bourne GH (1963) Histochemical studies on the «dark» and «light» cells of the cerebellum of rat. Acta Neuropathol 3:1–15
Thal DR, Rüb U, Orantes M, Braak H (2002) Phases of Abeta-deposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800
Treff WM (1974) Das Involutionsmuster des Nucleus dentatus cerebelli. In: Platt D (ed) Altern. Schattauer, Stuttgart, pp 37–54
Trzesniewska K, Brzyska M, Elbaum D (2004) Neurodegenerative aspects of protein aggregation. Acta Neurobiol Exp 64:41–52
Uddin M, Wildman DE, Liu G, Xu W, Johnson RM, Hof PR, Kapatos G, Grossman LI, Goodman M (2004) Sister grouping of chimpanzees and humans as revealed by genome-wide phylogenetic analysis of brain gene expression profiles. Proc Natl Acad Sci USA 101:2957–2962
UN Department of International Economic and Social Affairs (1985) The world aging situation: strategies and policies. E85/14/5. UN, New York
von Economo C (1926) Eine neue Art Spezialzellen des Lobus cinguli und Lobus insulae. Zschr ges Neurol Psychiat 100:706–712
Wakeley J (2008) Complex speciation of humans and chimpanzees. Nature 452:E3
Wang HY, D’Andrea MR, Nagele RG (2002) Cerebellar diffuse amyloid plaques are derived from dendritic Aβ42 accumulations in Purkinje cells. Neurobiol Aging 23:213–223
Watt AJ, Cuntz H, Mori M, Nusser Z, Sjöström PJ, Häusser M (2009) Traveling waves in developing cerebellar cortex mediated by asymmetrical Purkinje cell connectivity. Nat Neurosci 12:463–473
Wildman DE, Uddin M, Liu G, Grossman LI, Goodman M (2003) Implications of natural selection in shaping 99.4% nonsynonymous DNA identity between humans and chimpanzees: enlarging genus Homo. Proc Natl Acad Sci USA 100:7181–7188
Wolfe DM, Lee J-H, Kumar A, Lee S, Orenstein SJ, Nixon RA (2013) Autophagy failure in Alzheimer’s disease and the role of defective lysosomal acidification. Eur J Neurosci 37:1949–1961
Yin D (1995) Studies on age pigments evolving into a new theory of biological aging. Gerontology 41(Suppl. 2):159–172
Yin D (1996) Biochemical basis of lipofuscin, ceroid, and age pigment-like fluorophores. Free Rad Biol Med 21:871–888
Yu WH, Kumar A, Peterhoff CM, Kulnane LS, Uchiyama Y, Lamb BT, Cuervo AM, Nixon RA (2004) Autophagic vacuoles are enriched in amyloid precursor protein-secretase activities: implications for beta-amyloid peptide over-production and localization in Alzheimer’s disease. Int J Biochem Cell Biol 36:2531–2540
Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee J-H, Mohan PS, Mercken M, Farmery MR, Tjernberg LO, Jiang Y, Duff K, Uchiyama Y, Näslund J, Mathews PM, Cataldo AM, Nixon RA (2005) Macroautophagy-a novel beta-amyloid peptide-generating pathway activated in Alzheimer’s disease. J Cell Biol 171:87–98
Zetzsche T, Baimbridge KG, Möhler H, Chan-Palay V (1990) Subsets of GABA neurons in the human cerebellum in normal controls and senile dementia of the Alzheimer type patients detected by glutamate decarboxylase, GABAA/benzodiazepine receptor protein, calbindin D-28k and parvalbumin immunocytochemistry. Dementia 1:237–252
Zhang C, Zhu Q, Hua T (2010) Aging of cerebellar Purkinje cells. Cell Tissue Res 341:341–347
Acknowledgments
The authors greatly thank Virginie Stygelbout, Valérie Suain and Helmut Heinsen for expert advices and for bibliographical references. The semithin section (Fig. 1) was realized by Ludmilla Staneva-Dobrovski (University of Düsseldorf). Alison Mortimer, University of the Witwatersrand (South Africa) provided technical assistance. Funding was provided by the Iris and Ellen Hodges Trust (South Africa) and the Medical Research Council (South Africa) to EG and the Diane program (Walloon region) (816856), the Fonds de la Recherche Scientifique Médicale (3.4504.10) to J-PB and performed in the frame of the IAP program (P7/16) of the Belgian Federal Science Policy Office (J-PB). Primate brain materials used in this study were loaned by the Great Ape Aging Project (NIH/NIA grant AG014308, J. Erwin, PI) from zoological gardens that are accredited by the Association of Zoos and Aquariums (AZA) and that participate in the Ape Taxon Advisory Group (Ape-TAG). We especially appreciate the contribution of zoo veterinarians and staff in collecting and providing specimens. The manuscript does not contain clinical studies or patient data.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gilissen, E.P., Leroy, K., Yilmaz, Z. et al. A neuronal aging pattern unique to humans and common chimpanzees. Brain Struct Funct 221, 647–664 (2016). https://doi.org/10.1007/s00429-014-0931-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-014-0931-5