Abstract
Classification of head and neck tumors has evolved in recent decades including a widespread application of molecular testing in tumors of the salivary glands, sinonasal tract, oropharynx, nasopharynx, and soft tissue. Availability of new molecular techniques allowed for the definition of multiple novel tumor types unique to head and neck sites. Moreover, the expanding spectrum of immunohistochemical markers facilitates a rapid identification of diagnostic molecular abnormalities. As such, it is currently possible for head and neck pathologists to benefit from a molecularly defined classifications, while making diagnoses that are still based largely on histopathology and immunohistochemistry. This review highlights some principal molecular alterations in head and neck neoplasms presently available to assist pathologists in the practice of diagnosis, prognostication and prediction of response to treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Classification of head and neck tumors has expanded dramatically in recent decades with identification of many new entities and widespread application of molecular testing in tumors of the salivary glands, sinonasal tract, oropharynx, nasopharynx, and soft tissue of the head and neck. Molecular testing has disclosed pathogenetic processes of well-established but previously enigmatic entities, and clarified the relationships between various neoplasms. The current 5th edition of the WHO Classification of Head and Neck Tumours relies heavily on molecular data to support the inclusion of several new tumor entities and their subtypes, and to provide prognostic and pathogenetic information [1]. Furthermore, the description of molecular alterations in various subgroups of tumors and the application of molecular testing in large case cohorts in a research setting has substantially clarified the boundaries of many existing histologic entities and allowed for the identification of specific morphologic and immunohistochemical features of new tumor types. On the other hand, it must be emphasized that this molecular revolution in head and neck pathology has not made pathologists entirely dependent on molecular testing in making their diagnoses. An expanding spectrum of immunohistochemical markers based on new molecular information has improved diagnostic options using immunohistochemistry, and such markers also facilitate a rapid identification of useful diagnostic molecular tests. Thus, head and neck pathologists currently benefit from a molecularly defined classification, while making diagnoses that are still based largely on histopathology and immunohistochemistry.
In recent years, considerable progress in salivary gland and sinonasal tumor taxonomy has taken place with the discovery of tumor type-specific fusion oncogenes generated by chromosomal translocations. This review covers molecular alterations important in head and neck neoplasms from a practical viewpoint in diagnosis, prognostication and prediction of response to treatment.
Salivary gland neoplasms
Salivary gland tumors represent a challenging field in diagnostic head and neck pathology. These rare tumors exhibit a diverse array of morphological features, comprising almost 40 subtypes in the current World Health Organization (WHO) Classification of Head and Neck Tumours [1]. Traditionally, salivary neoplasms are diagnosed on morphological grounds, with the help of ancillary stains and immunohistochemistry. Several salivary gland neoplasms are characterized by recurrent genomic alterations, particularly gene fusions. These include fusions of the ETV6 gene in secretory carcinoma (SC) [2,3,4], MYB/MYBL1::NFIB in adenoid cystic carcinoma (AdCC) [5], CRTC1/CRTC3::MAML2 in mucoepidermoid carcinoma (MEC) [6, 7], and EWSR1::ATF1 in hyalinizing clear cell carcinoma [8] as the most frequent and best characterized gene fusions (Table 1).
Molecular alterations with prognostic and predictive significance
Salivary gland secretory carcinoma (SC), previously called mammary analogue SC, is a mostly low-grade malignancy characterized by well-defined morphology and an immunohistochemical (Fig. 1A-D) and genetic profile identical to SC of the breast [2]. Translocation t(12;15)(p13;q25) resulting in the ETV6::NTRK3 gene fusion is characteristic for SC, along with S100 protein and mammaglobin immunopositivity. The spectrum of genetic alterations in rare cases of SC continues to evolve including cases with ETV6::RET [3], ETV6::MET [9], and VIM::RET [10] fusions. Other rare alterations in low-grade SC include CTNNB1::ALK fusion [11] and dual fusions of ETV6::NTRK3 and ETV6:: MAML3 in the same tumor [12]. All these rearrangements can be used as diagnostically useful molecular markers in establishing the diagnosis of SC. Immunohistochemical positivity for S100, SOX10 and mammaglobin is a practical way to diagnose SC, and molecular testing may not be needed in most cases.
Secretory carcinoma (SC) of grade 1 with classical microcystic morphology with production of eosinophilic material (A). SC of grade 3 morphology forming solid nests with minimal or no secretion (B). The fusions joining of ETV6 gene exon 5 with NTRK3 gene exon 15 (C) and ETV6 gene exon 6 with RET gene exon 12 (D) are illustrated. Protein domains are depicted
Furthermore, in cases with ETV6::NTRK3 and ETV6::RET gene fusions in SC the introduction of targeted therapies using NTRK [13,14,15] and RET inhibitors [16] has implicated these fusions also as relevant predictive markers.
Salivary mucoepidermoid carcinoma (MEC) is a malignant salivary gland neoplasm characterized by mucous, intermediate and epidermoid (squamoid) tumor cells forming cystic and solid growth patterns, and usually associated with MAML2 gene rearrangement. Most MECs harbor a tumor type-specific translocation at t(11;19)(q21;p13) resulting in CRTC1::MAML2 fusion gene [6, 7, 17]. Rare cases display a t(11;15)(q21;q26) translocation with CRTC3::MAML2 fusion [18] (Fig. 2A-C) or a highly unusual t(6;22)(p21;q12) translocation with EWSR1::POU5F1 fusion [19]. The CRTC1::MAML2 fusion is seen in most low- and intermediate-grade and some high-grade tumors. CRTC1/3::MAML2 fusions were originally considered as a biomarker for favorable overall survival in patients with salivary gland MEC [7]. More recent studies, however, have challenged these conclusions and current grading systems have not been fully satisfying in predicting the aggressiveness of MECs. A positive FISH-result for MAML2-rearrangement can be highly useful in confirming the diagnosis of MEC in difficult cases. Further evidence may be gained from NGS and PCR testing. However, negative FISH results are inconclusive, and cannot be used in differential diagnosis. The immunohistochemical profile of MEC includes positivity for p63, p40, and may be useful in differential diagnosis from other salivary gland tumors. In one recent study, KMT2A gene rearrangements in MECs identified aggressive tumor behavior regardless of histologic grade obtained with conventional grading systems [20]. Furthermore, these results may suggest the possibility of treating KMT2A-rearranged MECs using azacytidine and venetoclax, as these treatments have shown promising outcomes in the treatment of KMT2A-rearranged leukemias [21].
Classical mucoepidermoid carcinoma (MEC) consisting of cystic structures with plenty of mucoid cells settled within intermediate cells (A). Clear cell variant of MEC with solid morphology and only few mucoid cells (B). The fusions joining of CRTC1 or CRTC3 genes both exon 1 with MAML2 gene exon 2 (C) are illustrated. Protein domains are depicted
Adenoid cystic carcinoma (AdCC) is an invasive carcinoma composed of epithelial and myoepithelial neoplastic cells arranged in tubular, cribriform, and solid patterns associated with an eosinophilic extracellular matrix and reduplicated basement membrane materials, often associated with gene fusions involving the MYB, MYBL1 and NFIB genes. The genomic hallmarks of AdCC are t(6;9) or t(8;9) translocations, resulting in MYB::NFIB and MYBL1::NFIB fusions, respectively [5, 22] (Fig. 3A-D). The former alteration is found in > 80% of cases and the latter in approximately 5% of cases [5]. MYB/MYBL1 activation due to gene fusion or other mechanisms is a key event in pathogenesis of AdCC [22]. Losses of 1p, 6q, and 15q are associated with high-grade tumours, and loss of 14q is seen exclusively in low-grade tumors [23, 24]. Next-generation sequencing identified mutations with mostly low level of recurrence in genes of the FGF/IGF/PI3K, chromatin remodelling, and NOTCH signaling pathways [25, 26]. Use of FISH techniques for MYB, MYBL1 or NFIB-rearrangments can be very useful for specific identification of AdCC. Immunohistochemical antibodies to altered MYB can also be used.
Adenoid cystic carcinoma with cribriform to tubular morphology containing basophilic matrix and reduplicated basement membrane material (A). Solid pattern characterized by dense cellular tumour nests without pseudocystic spaces, this case showed NOTCH1 gene mutation (B). The fusions joining of MYB gene exon 14 with NFIB gene exon 9 (C) and MYBL1 gene exon 14 with NFIB gene exon 9 (D) are illustrated. Protein domains are depicted
Traditional therapeutic agents for patients with advanced, recurrent, or metastatic AdCC have demonstrated poor efficacy in prolonging survival. The presence of NOTCH alterations in AdCC associate with poor survival. Patients with these aberrations might potentially benefit from anti-NOTCH drugs, such as bronticuzumab [27].
Salivary duct carcinoma (SDC) is an aggressive malignancy resembling mammary ductal carcinoma, most typically with an apocrine phenotype. The most frequent genetic alterations are mutations in TP53 (55%), HRAS (23%), PIK3CA (23%), and an amplification of HER2/neu (35%) [28]. Due to low recurrency of these mutations, molecular testing for them is not indicated for diagnostic purposes. In diagnostic practice, immunohistochemical androgen receptor positivity is still a useful marker for SDC. PLAG1 or HMGA2 rearrangements due to a pre-existing pleomorphic adenoma (PA) can be identified in about half of SDCs [29, 30]. A small subset of SDCs harbors a rearrangement of ALK [31]. Given the therapeutic relevance of ALK fusions, inclusion of ALK immunohistochemistry in any atypical-looking or androgen receptor poor SDC and high-grade adenocarcinoma, not otherwise specified is recommended. Because systemic therapies targeting androgen receptor, HER2/neu amplification, phosphatidylinositol 3 kinase (PI3K) pathway, including mutations of PIK3CA and BRAF p.V600E, and loss of phosphatase and tensin homolog (PTEN) are presently under study, molecular testing for them may become warranted [32,33,34].
Molecular alterations with differential diagnostic significance
Hyalinizing clear cell carcinoma (HCCC) is a carcinoma composed of clear and eosinophilic cells in a variably hyalinized stroma, usually associated with EWSR1 rearrangement. Most HCCCs harbor EWSR1::ATF1 gene fusion [8], while EWSR1::CREM fusion is less common [35] (Fig. 4A-D). Although the finding of infiltrative nests of low-grade clear tumor cells within a hyalinized or cellular fibrous stroma is quite characteristic of HCCC, some cases may have also abundant mucocytes and/or clear cell differentiation, or the hyalinization is minimal or absent. Differential diagnosis in such cases includes a wide spectrum of salivary gland tumors, namely clear cell mucoepidermoid carcinoma, epithelial-myoepithelial carcinoma, myoepithelial carcinoma, oncocytoma, myoepithelioma, and even metastatic kidney clear cell carcinoma, and the distinction of these benefits from FISH studies [36] on EWSR1-rearrangment. Conventional immunohistochemistry may also be helpful, but not always conclusive.
Hyalinizing clear cell carcinoma is characterized by clear cell morphology (A) or eosinophilic cells (B) creating solid nestes embedded within two types of stroma hylinized basement membrane-like (A) and desmoplastic or fibrocellular (B). The fusions joining of EWSR1 gene exon 8 with ATF1 gene exon 4 (C) and EWSR1 gene exon 14 with CREM gene exon 6 (D) are illustrated. Protein domains are depicted
Epithelial-myoepithelial carcinoma (EMC) is a salivary gland malignancy characterized by biphasic tubular structures and usually a multinodular growth pattern. The histological hallmark of EMC is a biphasic arrangement of inner (luminal) eosinophilic ductal epithelial cells and outer (abluminal) cells which are often clear myoepithelial cells. There are, however, several histologic variants with cribriform, basaloid, sebaceous, apocrine/oncocytic, or double-clear appearances, or with squamous differentiation resulting in differential diagnostic challenges. The salivary gland tumor entities to be differentiated include those with biphasic or/and clear cell morphology, such as adenoid cystic carcinoma, basal cell adenocarcinoma, pleomorphic adenoma, myoepithelial carcinoma, and clear cell carcinoma [37]. Because clear myoepithelial cells can be seen in many benign and malignant salivary gland tumors, the differential diagnosis of epithelial-myoepithelial carcinoma is challenging. Testing for HRAS mutations is useful when discriminating EMC from its mimics [38]. Immunohistochemistry is often not conclusive.
Polymorphous adenocarcinoma (PAC) (previously known as polymorphous low-grade adenocarcinoma) is a malignant epithelial tumor characterized by cytological uniformity, morphological diversity, and an infiltrative growth pattern. It is predominantly seen in minor salivary glands. Cribriform adenocarcinoma (CAC) was initially reported at the base of the tongue [39] and later in other minor salivary gland locations [40]. CAC is characterized by a multinodular growth pattern separated by fibrous septa, a relatively uniform solid, cribriform and microcystic architecture, and optically clear nuclei. Glomeruloid and papillary structures, peripheral palisading and clefting may be observed. Although CAC and classic PAC have molecular alterations affecting the same PRKD gene family, there are notable differences. PACs harbor recurrent PRKD1 E710D hotspot mutations in > 70% of cases, whereas 80% of CACs display PRKD1/2/3 fusions with partner genes including ARID1A, DDX3X or STRN3 [41, 42]. Histologically, fusion-positive tumors show papillary growth in a high percentage of cases, while arrangement in single cell filing is seen in a low percentage. CACs display a propensity for base of tongue location and frequent (50%) lymph node metastasis compared with the hot spot mutation-related tumors which have a low risk for nodal metastasis. Compared with classic PAC, CAC has a high propensity for location in the base of the tongue and a higher risk for lymph node metastasis [43]. Genetic analysis of PRKD genes appears to be useful in characterizing this spectrum of tumors, and identifying those tumors with a high risk for nodal metastasis. Immunohistochemistry may not be conclusive with this regard.
Pleomorphic adenoma (PA) is a benign tumor characterized by cytomorphological and architectural diversity with an admixture of ductal and myoepithelial cells usually embedded in a chondromyxoid or fibrous stroma. PAs harbour recurrent translocations or intrachromosomal rearrangements resulting in gene fusions involving PLAG1 on 8q12 (> 50%) or HMGA2 on 12q14.3 (10–15%) [44, 45]. Antibodies to PLAG1 [46] and HMGA2 [47], respectively, are emerging as practical, sensitive and specific immunohistochemical markers for PA.
Diagnosis of PA is straightforward in most cases but sometimes PA is difficult to differentiate from low-grade epithelial-myoepithelial carcinoma. Molecular testing for PLAG1/HMGA2 rearrangement versus HRAS mutation can be very useful.
Microsecretory adenocarcinoma (MSA) is a newly identifed low-grade salivary adenocarcinoma (Fig. 5A-C) characterized by distinctive morphology and a specific MEF2C::SS18 fusion [48].
Microsecretory carcinoma is composed of small tubules and microcysts lined by flat intercalated duct-like cells and containing abundant basophilic luminal secretions (A). The nuclei are uniform, oval, and lack prominent nucleoli (B). The fusion joining of MEF2C gene with SS18 gene is illustrated (C)
Sinonasal neoplasms
Sinonasal tract comprising the nasal cavity, paranasal sinuses and the skull base is a region characterized by a broad spectrum of tumors that exhibit a significant diversity of molecularly defined entities (Table 2). The recent WHO Classification of Head and Neck Tumors includes new entities in which molecular genetics has an important diagnostic role, including HPV-related multiphenotypic sinonasal carcinoma and SWI/SNF-deficient sinonasal carcinoma and adenocarcinoma [1], and a subset of emerging and developing entities including IDH-mutated malignancies in the category of sinonasal undifferentiated carcinoma. Molecular genetics also plays a diagnostic role in the context of hereditary syndromes that can be manifested in the sinonasal tract.
Non-keratinizing squamous cell carcinoma (NKSCC) with DEK::AFF2 gene fusion. Sinonasal NKSCC is in 36–58% driven by transcriptionally active HPV, most commonly type 16 [1]. DEK::AFF2 carcinoma is currently classified as an emerging entity under NKSCC category, localized especially in the sinonasal tract but also few cases were reported in the middle ear [49, 50]. Despite bland looking morphology this tumor behaves in and aggressive fashion with high-risk of local recurrence, nodal metastatic dissemination and distant spread. FISH testing for DEK- or AFF2 rearrangements is essential for diagnosis. DEK::AFF2 carcinoma has an excellent response to immune checkpoint inhibitors (ICI) – anti-PD-L1 [49].
NUT Carcinoma is a highly aggressive, mostly lethal malignancy with a monotonous poorly differentiated morphology.
NUT carcinoma is genetically characterized by rearrangement of NUTM1 gene on 15q14 [51]. The NUT gene is physiologically expressed in mature spermatogonia. The most common fusion partners of NUT are genes involved in transcription and chromosome regulation belonging to the BET family (BRD2, BRD3, BRD4 and BRDT)[52]. In 75% of cases the fusion partner of NUTM1 is the BRD4 gene (in 19p13.1), and in 15% the BRD3 gene (in 9q34.2) [53, 54]. FISH techniques or other molecular testing may be used to demonstrate NUTM1- and the various BRD-rearrangements. However, the monoclonal NUT antibody is highly specific for NUT carcinoma [55], and offers a simple and reliable way to diagnose this malignancy.
In a subset of cases, NUT is fused with non-BRD genes. In 6% of cases fusion involves the NSD3 gene (in 8p11.23) [56], while in 2% of cases, genes for zinc finger containing proteins, (ZNF532 on 18q21.32, or ZNF592 on 15q25.3) are involved in NUT fusion [57].
There is no known effective treatment for NUT carcinoma which has a median survival of 6.7 months [58]. The first targeted drugs for NUT carcinoma were histone deacetylase inhibitors (HDACi) (vorinostat) and BET inhibitors (BETi) [59].
SWI/SNF complex deficient sinonasal carcinoma and other malignancies
The chromatin remodeling Switch/Sucrose non-fermentable complex (SWI/SNF) is a pleomorphic complex of over 20 tumor suppressors [60]. There are five different subtypes of SWI/SNF complex deficient sinonasal/base of skull malignancies, including SMARCB1-deficient sinonasal carcinoma; SMARCB1-deficient sinonasal adenocarcinoma; SMARCA4-deficient sinonasal carcinoma, a subset of SMARCA4-deficient teratocarcinosarcomas and poorly differentiated chordomas.
These tumors are usually poorly differentiated or undifferentiated malignancies with a highly aggressive clinical course and poor outcome.
The mortality of SMARCA4-deficient sinonasal carcinomas is higher than in other tumors of this family [61, 62]. SWI/SNF mutations are involved in many cancer-related pathways mostly related to epigenetic alterations (Fig. 6A-C). SWI/SNF complex deficient malignancies can be preferentially identified by immunohistochemical antibodies to SMARCB1 (INI1) and SMARCA4 (BRG1) which are sensitive diagnostic tools [63].The therapeutic option rests in local control of the disease with polychemotherapy and radiotherapy [61, 62].
Recent findings indicate a promising role for immunomodulators and immune checkpoint inhibitors as potential drugs in patients with SWI/SNF related malignancies [60, 64]. Tumor cells with loss of SMARCB1 demonstrate a constitutive EZH2 activation, and EZH2 inhibitors may modulate tumor immunogenicity and anti-tumor immune response [60, 64, 65].
Sinonasal undifferentiated carcinoma (SNUC) is a high-grade epithelial neoplasm without signs of cell differentiation, and the diagnosis is made only by exclusion of other sinonasal and non-sinonasal malignancies. Many undifferentiated epithelial neoplasms in previous WHO classifications were included under this term until advances in molecular pathology allowed for their identification as proper entities. Recently, isocitrate dehydrogenase 1 or 2 (IDH1/2) mutations were identified in a subset of SNUC [66, 67] including three main hotspot mutations of IDH1 R132, IDH2 R140 and IDH2 R172 [68]. Monoclonal or multi-specific antibodies for immunohistochemical detection of IDH1/2 mutations represent a cheaper alternative to molecular genetic testing for these mutations. However, immunohistochemistry lacks the ability to detect the full spectrum of IDH1/2 mutations [69].
SNUCs are aggressive diseases with poor outcome. The standard therapeutical approach is a combination of surgery, chemotherapy and radiation [70]. Tumors with IDH2-mutations show better outcome than other SNUCs [70]. IDH mutations provide alternative therapeutic options including selective small molecule inhibitors (e.g. Enasidenib for IDH2 mutations and Ivosidenib for IDH1 mutations).
HPV-associated multiphenotypic sinonasal carcinoma (HMSC) is an epithelial tumor almost exclusively localized in sinonasal tract and harboring high-risk HPV [71]. The most common serovariety is type 33. P16 immunohistochemistry can be used to identify the HPV-association. HMSC is histologically very pleomorphic and may mimic various salivary and non-salivary tumor types. The histological appearance of HMSC is usually high-grade and associated with destructive growth and propensity for local recurrence. Despite the aggressive appearance, HMSC has low metastatic potential and little tendency to lethal behavior [72].
Soft tissue neoplasms with head and neck predilection
Molecular alterations with differential diagnostic significance
The head and neck can be host to a wide range of soft tissue neoplasms with molecular findings, but a discussion of all these entities is beyond the scope of this review. We have included a selection of soft tissue tumors, often of recent molecular definition, that harbor diagnostically obviously useful molecular alterations. All discussed entities are listed in Table 3.
Biphenotypic sinonasal sarcoma (BSS) is a low-grade mesenchymal neoplasm with neurogenic and myogenic differentiation localized exclusively in the sinonasal region. BSS is molecularly defined by rearrangement of PAX3 gene [73]. The most common fusion is PAX3::MAML3 in more than half of the cases, with alternative fusion partners including NCOA1, NCOA2, FOXO1, FOXO6 and WWTR1 [74,75,76].
BSS are usually low-grade tumors with frequent local recurrence, sometimes many years after diagnosis [77]. Three cases of BSS with high-grade transformation have been published [78,79,80], one of which developed into rhabdomyosarcoma (RMS). Molecular testing of BSS and RMS showed similar gene fusions including PAX3::FOXO1 and PAX3::NCOA1 [74].
Nasal chondromesenchymal hamartoma (NCMH) is a pediatric benign mesenchymal sinonasal tumor which in a subset of cases is associated with DICER1 syndrome [81]. NCMH is usually an indolent tumor but when syndromic, patients have an increased risk for pleuropulmonary blastoma, ovarian sex cord-stromal tumor, renal tumors, genitourinary embryonal rhabdomyosarcoma, brain tumors and ciliary body medulloepithelioma. When diagnosing NCMH, genetic testing for germline DICER1 is recommended.
Inflammatory myofibroblastic tumor (IMT) is a low-grade neoplasm which in approximately 15% of cases arises in the head and neck with favorable prognosis and very infrequent recurrences [82, 83]. IMT is driven by constitutive activity of ALK or other receptor tyrosine kinase. Immunostaining for ALK-1 serves as a valuable screening tool, and ALK rearrangement can be confirmed with FISH techniques. Rare cases of IMT with fusions of other tyrosine kinase genes suggest that in cases of ALK-1 immunonegativity targeted RNA-sequencing would be a more suitable test.
Ectomesenchymal chondromyxoid tumor (ECT) (aka RREB1::MRTFB-rearranged neoplasm) is a tumor of uncertain malignant potential located predominantly in the tongue and rarely arising in extraglossal locations [84, 85]. The immunoprofile is non-specific, and therefore molecular testing is the preferred method when diagnosing ECT. In most cases it is characterized by a RREB1::MRTFB fusion, while EWSR1 gene rearrangement is seen in a small subset of cases [86, 87]. These tumors are genetically and histologically linked to soft tissue myoepithelial tumors [86] and may be mistaken for other mesenchymal tumors with dominant spindle cell morphology [88]. ECT usually follows benign course with no metastasis. Surgery is curative in most cases, even though local recurrences may occur [84, 85].
GLI1-altered soft tissue neoplasms are tumors of uncertain histogenesis, epithelioid morphology and non-specific immunoprofile, presenting in the head and neck in 40% of cases. In two thirds of cases these tumors harbor GLI1 fusions including ACTB::GLI1, PTCH1::GLI1, MALAT1::GLI1, and DERA::GLI1, while the rest harbor GLI1 amplification [1, 89, 90]. The various fusion partners to GLI1 are best determined by targeted RNA-sequencing but GLI1 amplification can also be detected by FISH. Awareness of a potential co-amplification of neighboring genes (CDK4, MDM2, DDIT3, etc.) on chromosome 12 detectable by FISH has to be taken into account [91]. GLI1 immunostaining shows high specificity and good sensitivity for GLI1-rearranged mesenchymal tumors. However, the sensitivity is unsatisfactory in plexiform fibromyxoma [92]. Biologic behavior varies from indolent tumors to metastasizing malignancies. Local recurrence or distant spread may appear in approximately 20% of cases [90, 93]. Potential targeted therapeutic options in GLI1-altered neoplasms are sonic hedgehod (Shh) signaling pathway inhibitors [94].
Rhabdomyosarcomas (RMS) are a clinically, prognostically and biologically heterogeneous group of tumors categorized morphologically into four main subtypes [95]. However, molecular-genetic findings delineate six distinct subtypes; embryonal RMS with unknown driver mutations/fusions, alveolar RMS with FOXO1 fusions, MYOD1-mutated RMS with MYOD1 activating mutations, VGLL2/VGLL3/NCOA2-rearranged RMS, pleomorphic RMS with a complex genetic background, and finally TFCP2-rearranged RMS with EWSR1 or FUS fusion partners [96]. Immunohistochemically, spindle cell/sclerosing RMS are usually positive (at least focally) for cytokeratins and myogenic markers, mainly MyoD1, myogenin, desmin and PAX7.
VGLL2/VGLL3/NCOA2-rearranged RMS belong to spindle cell/sclerosing RMS, primarily affecting newborns or infants with head and neck predilection. The characteristic molecular genetic events involve VGLL2::CITED3, VGLL2::NCOA2, TEAD1::NCOA2, or SRF::NCOA2 gene fusions [97, 98]. Recently, novel VGLL3 rearrangements with TCF12, EP300 and PPARGC1A as fusion partners have been described [99]. Complete surgical resection is usually curative, while RMS-type chemotherapy is recommended for unresectable cases.
TFCP2-rearranged RMS (TFCP2-RMS) are spindle cell/sclerosing and very aggressive mesenchymal tumors with rhabdomyoblastic differentiation and EWSR1/FUS::TFCP2 rearrangements [100]. In a subset of cases, hemizygous deletion or amplification of the ALK gene are described [101]. TFCP2-RMS tumors are predominantly localized in the jaws and the skull of young adults. The tumors have a rapid clinical course with a poor prognosis of 3-year overall survival at 28% [102]. Treatment options include surgery. chemotherapy and radiotherapy. The combination of radiation and ALK inhibitors has shown partial response in anecdotal cases [103, 104].
Adamantinoma-like Ewing sarcoma (ALES) is a controversial variant of Ewing sarcoma (ES) defined by the presence of t(11;22) and EWSR1::FLI1 fusion [105]. It is speculated whether they represent an epithelial or mesenchymal neoplasm, as they commonly express epithelial markers (pancytokeratins and p63/p40) as well as ES-related markers (CD99 and NKX2.2) [106]. Many tumors are treated with surgery and adjuvant polychemotherapy according to ES-specific protocols.
NTRK- and other kinase gene-rearranged spindle cell neoplasms encompass a wide morphological spectrum ranging from benign-appearing to high-grade tumors, consisting of spindled cells often immunopositive for S100 protein and CD34, and resembling infantile or adult-type fibrosarcoma, malignant peripheral nerve sheath tumor, or lipofibromatosis-like neural tumor. Superficial and deep soft tissues including salivary glands, as well as the skeletal component of the head and neck, are some of the more common locations of these tumors [107, 108].
While ETV6::NTRK3 is a well-established genetic driver of infantile fibrosarcoma, other fusions of NTRK1/3 and various other kinase genes including MET, RET, RAF1, or BRAF have been implicated in the pathogenesis of these tumors. Most cases are only locally aggressive and distant metastasis is an uncommon event in high-grade cases [108]. PanTrk immunohistochemistry seems to be a useful screening tool in NTRK1/3 rearranged cases with an overall sensitivity of 88% [109]. FISH detection of ETV6::NTRK3 is sufficient to confirm the diagnosis of infantile fibrosarcoma. However, given the number of possible kinase genes and their fusion partners when other tumor entities come into question, targeted RNA-sequencing is the method of choice [108]. Importantly, small inhibitory molecules targeting NTRK and RET have been used in the treatment of locally advanced inoperable tumors or cases with metastatic spread.
EWSR1/FUS::POU2AF3(COLCA2) sarcomas are newly recognized aggressive neoplasms with tendency to both local recurrence and metastatic spread despite multimodal treatment. They affect adult patients and commonly arise in the head and neck, particularly within the sinonasal tract. Their morphological spectrum of these tumors is wide from spindle cells, through biphasic tumors with spindled and round cells with features of neuroendocrine differentiation, to purely round cell tumors with high nuclear grade [110,111,112]. Approximately half of the cases display pan-cytokeratin positivity [110,111,112], possibly leading to diagnostic confusion with synovial sarcoma. As EWSR1 fusions with various partners are frequent in soft tissue tumors, FISH analysis of an EWSR1 break is not a sufficient diagnostic test. Instead, targeted RNA-sequencing is advised to render the correct diagnosis.
HPV- And EBV-Associated tumors of the nasopharynx and the oropharynx
The expression of late viral genes plays a role in the pathogenesis of epithelial and lymphoproliferative neoplasms of the oropharyngeal and nasopharyngeal areas. Only a considerably small subset of patients who acquired the infection during their lifetime develop any related tumor, highlighting the major influence of other genetic and environmental factors in their pathogenesis.
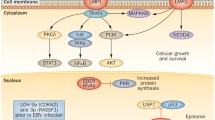
Nasopharyngeal carcinoma (NPC) is a rare neoplasm with high incidence in high-risk (endemic) populations including ethnic Chinese (southern China and Singapore) and Inuits of Alaska, Canada and Greenland. Low-risk (non-endemic) populations with rare occurrence of NPC include Europeans and Americans of Caucasian ethnicity [1]. In most cases of non-keratinizing NPC in high-risk populations, EBV infection is an early pathogenetic event [113]. Other important pathogenetic influences include genetic and environmental factors [114]. In low-risk populations, fewer cases are EBV positive, while high-risk HPV positive and virus-negative cases, particularly in keratinizing NPC are seen [115, 116]. In-situ hybridization with EBV-encoded small RNAs (EBER) or RNA-ISH for high-risk HPV can be used to detect the viral load in tumor cells. Immunohistochemistry for LMP-1 has low sensitivity and is not recommended. Serum EBV DNA and antibody levels have prognostic value, and patients with high viral load fare worse than those with lower load or absence of viral DNA [114]. In EBV- and HPV-positive cases patients have shown better outcome than in virus-negative cases [115].
HPV-associated squamous cell carcinoma of the oropharynx has an increasing incidence especially in developed countries. In North America and Northern Europe it is more prevalent now than the HPV-independent oropharyngeal squamous cell carcinoma [117]. High-risk HPV16 is responsible for most cases of HPV-associated carcinoma [118]. The patients are generally younger, have a higher socioeconomic status and have a better prognosis (5-year overall survival of 80%) than patients with HPV-independent carcinoma [1, 119, 120]. However, similar to HPV-independent cases, patients with HPV-associated squamous cell carcinoma are often active or past smokers [119]. The HPV-associated carcinomas most commonly originate from the squamous epithelium of tonsillar crypts and lack keratinization [121]. Histologic grading is not recommended as it does not carry significant prognostic value. In addition, the HPV-associated neuroendocrine carcinoma is a distinct aggressive tumor type that must be differentiated from squamous cell carcinoma. This can be facilitated by positive immunohistochemical staining for neuroendocrine markers such as synaptophysin, chromogranin and INSM1. Immunohistochemistry for the surrogate marker p16 is sufficient to detect HPV infection in most cases [122]. Strong diffuse positivity in tumor cell cytoplasm and nuclei must be present in more than 70% of cells to diagnose the lesion as positive. In problematic instances (equivocal p16 positivity, discrepancy between morphology and immunoprofile), PCR genotyping or in-situ hybridization methods can be employed to confirm the presence of high-risk HPV in the tumor.
Extranodal NK/T-cell lymphoma, nasal type is an aggressive tumor that infiltrates and destroys the nasal cavity, the nasopharynx, the oropharynx and structures of the oral cavity. It belongs to EBV-positive lymphoproliferations, and in-situ hybridization for EBER is positive in most viable tumor cells [1]. While a 5-year overall survival can be achieved using chemoradiotherapy in a majority of patients, the detection of persistent post-treatment serum EBV DNA suggests a worse prognosis [123].
EBV-associated smooth muscle tumor and EBV-positive mucocutaneous ulcer are two indolent EBV-positive lesions that can be found in the oral or nasal cavities, pharynx and larynx of immunosuppressed patients. Both display positivity for EBER in-situ hybridization and can be treated with conservative surgical removal. In addition, some cases might regress spontaneously with decreased immunosuppression [1, 124, 125].
In conclusion, a tremendous development has taken place in the classification of head and neck tumors with widespread application of molecular testing. In many cases, an expanding spectrum of immunohistochemical surrogates helps with histopathological diagnosis, and also with rapid identification of diagnostic molecular abnormalities. Therefore, pathologists currently benefit from the molecular underpinnings of tumors while still making diagnoses largely based on histology and immunohistochemistry.
Data availability
Data supporting the findings of this study are available within the article. The complete datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
WHO Classification of Tumours Editorial Board (2023) Head and neck tumours. Lyon (France): International Agency for Research on Cancer; forthcoming. (WHO classification of tumours series, 5th edn, vol. 9). https://publications.iarc.fr
Skalova A, Vanecek T, Sima R et al (2010) Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol 34:599–608. https://doi.org/10.1097/PAS.0b013e3181d9efcc
Skalova A, Vanecek T, Martinek P et al (2018) Molecular Profiling of Mammary Analog Secretory Carcinoma Revealed a Subset of Tumors Harboring a Novel ETV6-RET Translocation: Report of 10 Cases. Am J Surg Pathol 42:234–246. https://doi.org/10.1097/PAS.0000000000000972
Skalova A, Vanecek T, Majewska H et al (2014) Mammary analogue secretory carcinoma of salivary glands with high-grade transformation: report of 3 cases with the ETV6-NTRK3 gene fusion and analysis of TP53, beta-catenin, EGFR, and CCND1 genes. Am J Surg Pathol 38:23–33. https://doi.org/10.1097/PAS.0000000000000088
Fujii K, Murase T, Beppu S et al (2017) MYB, MYBL1, MYBL2 and NFIB gene alterations and MYC overexpression in salivary gland adenoid cystic carcinoma. Histopathology 71:823–834. https://doi.org/10.1111/his.13281
Jee KJ, Persson M, Heikinheimo K et al (2013) Genomic profiles and CRTC1-MAML2 fusion distinguish different subtypes of mucoepidermoid carcinoma. Mod Pathol 26:213–222. https://doi.org/10.1038/modpathol.2012.154
Okumura Y, Miyabe S, Nakayama T et al (2011) Impact of CRTC1/3-MAML2 fusions on histological classification and prognosis of mucoepidermoid carcinoma. Histopathology 59:90–97. https://doi.org/10.1111/j.1365-2559.2011.03890.x
Antonescu CR, Katabi N, Zhang L et al (2011) EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosomes Cancer 50:559–570. https://doi.org/10.1002/gcc.20881
Rooper LM, Karantanos T, Ning Y et al (2018) Salivary Secretory Carcinoma With a Novel ETV6-MET Fusion: Expanding the Molecular Spectrum of a Recently Described Entity. Am J Surg Pathol 42:1121–1126. https://doi.org/10.1097/PAS.0000000000001065
Skalova A, Baneckova M, Thompson LDR et al (2020) Expanding the Molecular Spectrum of Secretory Carcinoma of Salivary Glands With a Novel VIM-RET Fusion. Am J Surg Pathol 44:1295–1307. https://doi.org/10.1097/PAS.0000000000001535
Sasaki E, Masago K, Fujita S et al (2020) Salivary Secretory Carcinoma Harboring a Novel ALK Fusion: Expanding the Molecular Characterization of Carcinomas Beyond the ETV6. Gene Am J Surg Pathol 44:962–969. https://doi.org/10.1097/PAS.0000000000001471
Guilmette J, Dias-Santagata D, Nose V et al (2019) Novel gene fusions in secretory carcinoma of the salivary glands: enlarging the ETV6 family. Hum Pathol 83:50–58. https://doi.org/10.1016/j.humpath.2018.08.011
Cocco E, Scaltriti M, Drilon A (2018) NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol 15:731–747. https://doi.org/10.1038/s41571-018-0113-0
Lassen U (2019) How I treat NTRK gene fusion-positive cancers. ESMO Open 4:e000612. https://doi.org/10.1136/esmoopen-2019-000612
Drilon A (2019) TRK inhibitors in TRK fusion-positive cancers. Ann Oncol 30:viii23–viii30. https://doi.org/10.1093/annonc/mdz282
Drilon A, Hu ZI, Lai GGY et al (2018) Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol 15:151–167. https://doi.org/10.1038/nrclinonc.2017.175
Seethala RR, Dacic S, Cieply K et al (2010) A reappraisal of the MECT1/MAML2 translocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol 34:1106–1121. https://doi.org/10.1097/PAS.0b013e3181de3021
Nakayama T, Miyabe S, Okabe M et al (2009) Clinicopathological significance of the CRTC3-MAML2 fusion transcript in mucoepidermoid carcinoma. Mod Pathol 22:1575–1581. https://doi.org/10.1038/modpathol.2009.126
Moller E, Stenman G, Mandahl N et al (2008) POU5F1, encoding a key regulator of stem cell pluripotency, is fused to EWSR1 in hidradenoma of the skin and mucoepidermoid carcinoma of the salivary glands. J Pathol 215:78–86. https://doi.org/10.1002/path.2327
Othman BK, Steiner P, Leivo I et al (2023) Rearrangement of KMT2A Characterizes a Subset of Pediatric Parotid Mucoepidermoid Carcinomas Arising Metachronous to Acute Lymphoblastic Leukemia. Fetal Pediatr Pathol 42:796–807. https://doi.org/10.1080/15513815.2023.2241903
Cheung LC, Aya-Bonilla C, Cruickshank MN et al (2023) Preclinical efficacy of azacitidine and venetoclax for infant KMT2A-rearranged acute lymphoblastic leukemia reveals a new therapeutic strategy. Leukemia 37:61–71. https://doi.org/10.1038/s41375-022-01746-3
Persson M, Andren Y, Mark J et al (2009) Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci U S A 106:18740–18744. https://doi.org/10.1073/pnas.0909114106
Persson M, Andren Y, Moskaluk CA et al (2012) Clinically significant copy number alterations and complex rearrangements of MYB and NFIB in head and neck adenoid cystic carcinoma. Genes Chromosomes Cancer 51:805–817. https://doi.org/10.1002/gcc.21965
Steiner P, Andreasen S, Grossmann P et al (2018) Prognostic significance of 1p36 locus deletion in adenoid cystic carcinoma of the salivary glands. Virchows Arch 473:471–480. https://doi.org/10.1007/s00428-018-2349-6
Ho AS, Kannan K, Roy DM et al (2013) The mutational landscape of adenoid cystic carcinoma. Nat Genet 45:791–798. https://doi.org/10.1038/ng.2643
Ho AS, Ochoa A, Jayakumaran G et al (2019) Genetic hallmarks of recurrent/metastatic adenoid cystic carcinoma. J Clin Invest 129:4276–4289. https://doi.org/10.1172/JCI128227
Miller LE, Au V, Mokhtari TE, et al. (2022) A Contemporary Review of Molecular Therapeutic Targets for Adenoid Cystic Carcinoma. Cancers (Basel) 14. https://doi.org/10.3390/cancers14040992
Dalin MG, Desrichard A, Katabi N et al (2016) Comprehensive Molecular Characterization of Salivary Duct Carcinoma Reveals Actionable Targets and Similarity to Apocrine Breast Cancer. Clin Cancer Res 22:4623–4633. https://doi.org/10.1158/1078-0432.CCR-16-0637
Bahrami A, Perez-Ordonez B, Dalton JD et al (2013) An analysis of PLAG1 and HMGA2 rearrangements in salivary duct carcinoma and examination of the role of precursor lesions. Histopathology 63:250–262. https://doi.org/10.1111/his.12152
Chiosea SI, Thompson LD, Weinreb I et al (2016) Subsets of salivary duct carcinoma defined by morphologic evidence of pleomorphic adenoma, PLAG1 or HMGA2 rearrangements, and common genetic alterations. Cancer 122:3136–3144. https://doi.org/10.1002/cncr.30179
Agaimy A, Baneckova M, Ihrler S et al (2021) ALK Rearrangements Characterize 2 Distinct Types of Salivary Gland Carcinomas: Clinicopathologic and Molecular Analysis of 4 Cases and Literature Review. Am J Surg Pathol 45:1166–1178. https://doi.org/10.1097/PAS.0000000000001698
Santana T, Pavel A, Martinek P et al (2019) Biomarker immunoprofile and molecular characteristics in salivary duct carcinoma: clinicopathological and prognostic implications. Hum Pathol 93:37–47. https://doi.org/10.1016/j.humpath.2019.08.009
Uijen MJM, Lassche G, van Engen-van Grunsven ACH et al (2020) Systemic therapy in the management of recurrent or metastatic salivary duct carcinoma: A systematic review. Cancer Treat Rev 89:102069. https://doi.org/10.1016/j.ctrv.2020.102069
Rahman M, Griffith CC (2021) Salivary Duct Carcinoma: An Aggressive Salivary Gland Carcinoma with Morphologic Variants Newly Identified Molecular Characteristics, and Emerging Treatment Modalities. Surg Pathol Clin 14:111–126. https://doi.org/10.1016/j.path.2020.09.010
Chapman E, Skalova A, Ptakova N et al (2018) Molecular Profiling of Hyalinizing Clear Cell Carcinomas Revealed a Subset of Tumors Harboring a Novel EWSR1-CREM Fusion: Report of 3 Cases. Am J Surg Pathol 42:1182–1189. https://doi.org/10.1097/PAS.0000000000001114
Skalova A, Stenman G, Simpson RHW et al (2018) The Role of Molecular Testing in the Differential Diagnosis of Salivary Gland Carcinomas. Am J Surg Pathol 42:e11–e27. https://doi.org/10.1097/PAS.0000000000000980
Nakaguro M, Nagao T (2021) Epithelial-Myoepithelial Carcinoma. Surg Pathol Clin 14:97–109. https://doi.org/10.1016/j.path.2020.10.002
Urano M, Nakaguro M, Yamamoto Y et al (2019) Diagnostic Significance of HRAS Mutations in Epithelial-Myoepithelial Carcinomas Exhibiting a Broad Histopathologic Spectrum. Am J Surg Pathol 43:984–994. https://doi.org/10.1097/PAS.0000000000001258
Michal M, Skalova A, Simpson RH et al (1999) Cribriform adenocarcinoma of the tongue: a hitherto unrecognized type of adenocarcinoma characteristically occurring in the tongue. Histopathology 35:495–501. https://doi.org/10.1046/j.1365-2559.1999.00792.x
Skalova A, Sima R, Kaspirkova-Nemcova J et al (2011) Cribriform adenocarcinoma of minor salivary gland origin principally affecting the tongue: characterization of new entity. Am J Surg Pathol 35:1168–1176. https://doi.org/10.1097/PAS.0b013e31821e1f54
Sebastiao APM, Xu B, Lozada JR et al (2020) Histologic spectrum of polymorphous adenocarcinoma of the salivary gland harbor genetic alterations affecting PRKD genes. Mod Pathol 33:65–73. https://doi.org/10.1038/s41379-019-0351-4
Owosho AA, Baker E, Wood CB et al (2023) A novel STRN3::PRKD1 fusion in a cribriform adenocarcinoma of salivary gland with high-grade transformation. Genes Chromosomes Cancer 62:624–628. https://doi.org/10.1002/gcc.23181
Hahn E, Xu B, Katabi N et al (2023) Comprehensive Molecular Characterization of Polymorphous Adenocarcinoma, Cribriform Subtype: Identifying Novel Fusions and Fusion Partners. Mod Pathol 36:100305. https://doi.org/10.1016/j.modpat.2023.100305
Stenman G, Fehr A, Skalova A, et al. (2022) Chromosome Translocations, Gene Fusions, and Their Molecular Consequences in Pleomorphic Salivary Gland Adenomas. Biomedicines 10. doi: https://doi.org/10.3390/biomedicines10081970
Stenman G (2013) Fusion oncogenes in salivary gland tumors: molecular and clinical consequences. Head Neck Pathol 7(Suppl 1):S12-19. https://doi.org/10.1007/s12105-013-0462-z
Katabi N, Xu B, Jungbluth AA et al (2018) PLAG1 immunohistochemistry is a sensitive marker for pleomorphic adenoma: a comparative study with PLAG1 genetic abnormalities. Histopathology 72:285–293. https://doi.org/10.1111/his.13341
Mito JK, Jo VY, Chiosea SI et al (2017) HMGA2 is a specific immunohistochemical marker for pleomorphic adenoma and carcinoma ex-pleomorphic adenoma. Histopathology 71:511–521. https://doi.org/10.1111/his.13246
Bishop JA, Weinreb I, Swanson D et al (2019) Microsecretory Adenocarcinoma: A Novel Salivary Gland Tumor Characterized by a Recurrent MEF2C-SS18 Fusion. Am J Surg Pathol 43:1023–1032. https://doi.org/10.1097/PAS.0000000000001273
Yang W, Lee KW, Srivastava RM et al (2019) Immunogenic neoantigens derived from gene fusions stimulate T cell responses. Nat Med 25:767–775. https://doi.org/10.1038/s41591-019-0434-2
Todorovic E, Truong T, Eskander A et al (2020) Middle Ear and Temporal Bone Nonkeratinizing Squamous Cell Carcinomas With DEK-AFF2 Fusion: An Emerging Entity. Am J Surg Pathol 44:1244–1250. https://doi.org/10.1097/PAS.0000000000001498
French CA, Miyoshi I, Kubonishi I et al (2003) BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res 63:304–307
Pivot-Pajot C, Caron C, Govin J et al (2003) Acetylation-dependent chromatin reorganization by BRDT, a testis-specific bromodomain-containing protein. Mol Cell Biol 23:5354–5365. https://doi.org/10.1128/MCB.23.15.5354-5365.2003
French CA, Ramirez CL, Kolmakova J et al (2008) BRD-NUT oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene 27:2237–2242. https://doi.org/10.1038/sj.onc.1210852
Huang QW, He LJ, Zheng S et al (2019) An Overview of Molecular Mechanism Clinicopathological Factors, and Treatment in NUT Carcinoma. Biomed Res Int 2019:1018439. https://doi.org/10.1155/2019/1018439
Haack H, Johnson LA, Fry CJ et al (2009) Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol 33:984–991. https://doi.org/10.1097/PAS.0b013e318198d666
French CA, Rahman S, Walsh EM et al (2014) NSD3-NUT fusion oncoprotein in NUT midline carcinoma: implications for a novel oncogenic mechanism. Cancer Discov 4:928–941. https://doi.org/10.1158/2159-8290.CD-14-0014
Alekseyenko AA, Walsh EM, Zee BM et al (2017) Ectopic protein interactions within BRD4-chromatin complexes drive oncogenic megadomain formation in NUT midline carcinoma. Proc Natl Acad Sci U S A 114:E4184–E4192. https://doi.org/10.1073/pnas.1702086114
Bauer DE, Mitchell CM, Strait KM et al (2012) Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res 18:5773–5779. https://doi.org/10.1158/1078-0432.CCR-12-1153
Filippakopoulos P, Qi J, Picaud S et al (2010) Selective inhibition of BET bromodomains. Nature 468:1067–1073. https://doi.org/10.1038/nature09504
Wang X, Haswell JR, Roberts CW (2014) Molecular pathways: SWI/SNF (BAF) complexes are frequently mutated in cancer–mechanisms and potential therapeutic insights. Clin Cancer Res 20:21–27. https://doi.org/10.1158/1078-0432.CCR-13-0280
Agaimy A, Hartmann A, Antonescu CR et al (2017) SMARCB1 (INI-1)-deficient Sinonasal Carcinoma: A Series of 39 Cases Expanding the Morphologic and Clinicopathologic Spectrum of a Recently Described Entity. Am J Surg Pathol 41:458–471. https://doi.org/10.1097/PAS.0000000000000797
Agaimy A, Jain D, Uddin N et al (2020) SMARCA4-deficient Sinonasal Carcinoma: A Series of 10 Cases Expanding the Genetic Spectrum of SWI/SNF-driven Sinonasal Malignancies. Am J Surg Pathol 44:703–710. https://doi.org/10.1097/PAS.0000000000001428
Agaimy A (2023) SWI/SNF-deficient Sinonasal Carcinomas. Adv Anat Pathol 30:95–103. https://doi.org/10.1097/PAP.0000000000000372
Ngo C, Postel-Vinay S (2022) Immunotherapy for SMARCB1-Deficient sarcomas: current evidence and future developments. Biomedicines 10. doi: https://doi.org/10.3390/biomedicines10030650
Aspeslagh S, Morel D, Soria JC et al (2018) Epigenetic modifiers as new immunomodulatory therapies in solid tumours. Ann Oncol 29:812–824. https://doi.org/10.1093/annonc/mdy050
Jo VY, Chau NG, Hornick JL et al (2017) Recurrent IDH2 R172X mutations in sinonasal undifferentiated carcinoma. Mod Pathol 30:650–659. https://doi.org/10.1038/modpathol.2016.239
Dogan S, Chute DJ, Xu B et al (2017) Frequent IDH2 R172 mutations in undifferentiated and poorly-differentiated sinonasal carcinomas. J Pathol 242:400–408. https://doi.org/10.1002/path.4915
Riobello C, Lopez-Hernandez A, Cabal VN et al (2020) IDH2 Mutation Analysis in Undifferentiated and Poorly Differentiated Sinonasal Carcinomas for Diagnosis and Clinical Management. Am J Surg Pathol 44:396–405. https://doi.org/10.1097/PAS.0000000000001420
Mito JK, Bishop JA, Sadow PM et al (2018) Immunohistochemical Detection and Molecular Characterization of IDH-mutant Sinonasal Undifferentiated Carcinomas. Am J Surg Pathol 42:1067–1075. https://doi.org/10.1097/PAS.0000000000001064
Chambers KJ, Lehmann AE, Remenschneider A et al (2015) Incidence and survival patterns of sinonasal undifferentiated carcinoma in the United States J Neurol Surg B. Skull Base 76:94–100. https://doi.org/10.1055/s-0034-1390016
Bishop JA, Ogawa T, Stelow EB et al (2013) Human papillomavirus-related carcinoma with adenoid cystic-like features: a peculiar variant of head and neck cancer restricted to the sinonasal tract. Am J Surg Pathol 37:836–844. https://doi.org/10.1097/PAS.0b013e31827b1cd6
Rodarte AI, Parikh AS, Gadkaree SK et al (2019) Human Papillomavirus Related Multiphenotypic Sinonasal Carcinoma: Report of a Case with Early and Progressive Metastatic Disease. J Neurol Surg Rep 80:e41–e43. https://doi.org/10.1055/s-0039-3399571
Wang X, Bledsoe KL, Graham RP et al (2014) Recurrent PAX3-MAML3 fusion in biphenotypic sinonasal sarcoma. Nat Genet 46:666–668. https://doi.org/10.1038/ng.2989
Fritchie KJ, Jin L, Wang X et al (2016) Fusion gene profile of biphenotypic sinonasal sarcoma: an analysis of 44 cases. Histopathology 69:930–936. https://doi.org/10.1111/his.13045
Le Loarer F, Laffont S, Lesluyes T et al (2019) Clinicopathologic and Molecular Features of a Series of 41 Biphenotypic Sinonasal Sarcomas Expanding Their Molecular Spectrum. Am J Surg Pathol 43:747–754. https://doi.org/10.1097/PAS.0000000000001238
Nichols MM, Alruwaii F, Chaaban M et al (2023) Biphenotypic Sinonasal Sarcoma with a Novel PAX3::FOXO6 Fusion: A Case Report and Review of the Literature. Head Neck Pathol 17:259–264. https://doi.org/10.1007/s12105-022-01479-w
Kominsky E, Boyke AE, Madani D et al (2023) Biphenotypic Sinonasal Sarcoma: A Case Report and Review of Literature. Ear Nose Throat J 102:385–390. https://doi.org/10.1177/0145561321999196
Bell D, Phan J, DeMonte F et al (2022) High-grade transformation of low-grade biphenotypic sinonasal sarcoma: Radiological, morphophenotypic variation and confirmatory molecular analysis. Ann Diagn Pathol 57:151889. https://doi.org/10.1016/j.anndiagpath.2021.151889
Hasnie S, Glenn C, Peterson JEG et al (2022) High-Grade Biphenotypic Sinonasal Sarcoma: A Case Report. J Neurol Surg Rep 83:e105–e109. https://doi.org/10.1055/s-0042-1755599
Meyer A, Klubickova N, Mosaieby E et al (2023) Biphenotypic sinonasal sarcoma with PAX3::MAML3 fusion transforming into high-grade rhabdomyosarcoma: report of an emerging rare phenomenon. Virchows Arch 482:777–782. https://doi.org/10.1007/s00428-023-03501-0
Schultz KAP, Williams GM, Kamihara J et al (2018) DICER1 and Associated Conditions: Identification of At-risk Individuals and Recommended Surveillance Strategies. Clin Cancer Res 24:2251–2261. https://doi.org/10.1158/1078-0432.CCR-17-3089
Klubickova N, Michal M, Agaimy A et al (2022) TIMP3::ALK fusions characterize a distinctive myxoid fibroblastic tumor of the vocal cords: a report of 7 cases. Virchows Arch 481:721–729. https://doi.org/10.1007/s00428-022-03389-2
Kerr DA, Thompson LDR, Tafe LJ et al (2021) Clinicopathologic and Genomic Characterization of Inflammatory Myofibroblastic Tumors of the Head and Neck: Highlighting a Novel Fusion and Potential Diagnostic Pitfall. Am J Surg Pathol 45:1707–1719. https://doi.org/10.1097/PAS.0000000000001735
Smith BC, Ellis GL, Meis-Kindblom JM et al (1995) Ectomesenchymal chondromyxoid tumor of the anterior tongue Nineteen cases of a new clinicopathologic entity. Am J Surg Pathol 19:519–530. https://doi.org/10.1097/00000478-199505000-00003
Bubola J, Hagen K, Blanas N et al (2021) Expanding awareness of the distribution and biologic potential of ectomesenchymal chondromyxoid tumor. Head Neck Pathol 15:319–322. https://doi.org/10.1007/s12105-020-01169-5
Argyris PP, Bilodeau EA, Yancoskie AE et al (2016) A subset of ectomesenchymal chondromyxoid tumours of the tongue show EWSR1 rearrangements and are genetically linked to soft tissue myoepithelial neoplasms: a study of 11 cases. Histopathology 69:607–613. https://doi.org/10.1111/his.12973
Dickson BC, Antonescu CR, Argyris PP et al (2018) Ectomesenchymal chondromyxoid tumor: a neoplasm characterized by recurrent RREB1-MKL2 fusions. Am J Surg Pathol 42:1297–1305. https://doi.org/10.1097/PAS.0000000000001096
Agaimy A, Din NU, Dermawan JK et al (2023) RREB1::MRTFB fusion-positive extra-glossal mesenchymal neoplasms: A series of five cases expanding their anatomic distribution and highlighting significant morphological and phenotypic diversity. Genes Chromosomes Cancer 62:5–16. https://doi.org/10.1002/gcc.23082
Dahlen A, Fletcher CD, Mertens F et al (2004) Activation of the GLI oncogene through fusion with the beta-actin gene (ACTB) in a group of distinctive pericytic neoplasms: pericytoma with t(7;12). Am J Pathol 164:1645–1653. https://doi.org/10.1016/s0002-9440(10)63723-6
Papke DJ Jr, Dickson BC, Oliveira AM et al (2023) Distinctive nested glomoid neoplasm: clinicopathologic analysis of 20 cases of a mesenchymal neoplasm with frequent GLI1 alterations and indolent behavior. Am J Surg Pathol 47:12–24. https://doi.org/10.1097/PAS.0000000000001979
Agaram NP, Zhang L, Sung YS et al (2019) GLI1-amplifications expand the spectrum of soft tissue neoplasms defined by GLI1 gene fusions. Mod Pathol 32:1617–1626. https://doi.org/10.1038/s41379-019-0293-x
Parrack PH, Marino-Enriquez A, Fletcher CDM et al (2023) GLI1 Immunohistochemistry Distinguishes Mesenchymal Neoplasms With GLI1 Alterations From Morphologic Mimics. Am J Surg Pathol 47:453–460. https://doi.org/10.1097/PAS.0000000000002018
Xu B, Chang K, Folpe AL et al (2020) Head and Neck Mesenchymal Neoplasms With GLI1 Gene Alterations: A Pathologic Entity With Distinct Histologic Features and Potential for Distant Metastasis. Am J Surg Pathol 44:729–737. https://doi.org/10.1097/PAS.0000000000001439
Antonescu CR, Agaram NP, Sung YS et al (2018) A Distinct Malignant Epithelioid Neoplasm With GLI1 Gene Rearrangements, Frequent S100 Protein Expression, and Metastatic Potential: Expanding the Spectrum of Pathologic Entities With ACTB/MALAT1/PTCH1-GLI1 Fusions. Am J Surg Pathol 42:553–560. https://doi.org/10.1097/PAS.0000000000001010
WHO Classification of Tumours Editorial Board. Soft tissue and bone tumours. Lyon (France): International Agency for Research on Cancer; 2020. (WHO classification of tumours series, 5th ed.; vol. 3). https://publications.iarc.fr/588.
Leiner J, Le Loarer F (2020) The current landscape of rhabdomyosarcomas: an update. Virchows Arch 476:97–108. https://doi.org/10.1007/s00428-019-02676-9
Mosquera JM, Sboner A, Zhang L et al (2013) Recurrent NCOA2 gene rearrangements in congenital/infantile spindle cell rhabdomyosarcoma. Genes Chromosomes Cancer 52:538–550. https://doi.org/10.1002/gcc.22050
Alaggio R, Zhang L, Sung YS et al (2016) A Molecular Study of Pediatric Spindle and Sclerosing Rhabdomyosarcoma: Identification of Novel and Recurrent VGLL2-related Fusions in Infantile Cases. Am J Surg Pathol 40:224–235. https://doi.org/10.1097/PAS.0000000000000538
Agaimy A, Dermawan JK, Leong I et al (2022) Recurrent VGLL3 fusions define a distinctive subset of spindle cell rhabdomyosarcoma with an indolent clinical course and striking predilection for the head and neck. Genes Chromosomes Cancer 61:701–709. https://doi.org/10.1002/gcc.23083
Watson S, Perrin V, Guillemot D et al (2018) Transcriptomic definition of molecular subgroups of small round cell sarcomas. J Pathol 245:29–40. https://doi.org/10.1002/path.5053
Le Loarer F, Cleven AHG, Bouvier C et al (2020) A subset of epithelioid and spindle cell rhabdomyosarcomas is associated with TFCP2 fusions and common ALK upregulation. Mod Pathol 33:404–419. https://doi.org/10.1038/s41379-019-0323-8
Dehner CA, Broski SM, Meis JM et al (2023) Fusion-driven Spindle Cell Rhabdomyosarcomas of Bone and Soft Tissue: A Clinicopathologic and Molecular Genetic Study of 25 Cases. Mod Pathol 36:100271. https://doi.org/10.1016/j.modpat.2023.100271
Brunac AC, Laprie A, Castex MP et al (2020) The combination of radiotherapy and ALK inhibitors is effective in the treatment of intraosseous rhabdomyosarcoma with FUS-TFCP2 fusion transcript. Pediatr Blood Cancer 67:e28185. https://doi.org/10.1002/pbc.28185
Valerio E, Furtado Costa JL, Perez Fraile NM et al (2023) Intraosseous Spindle Cell/Epithelioid Rhabdomyosarcoma with TFCP2 Rearrangement: A Recent Recognized Subtype with Partial Response to Alectinib. Int J Surg Pathol 31:861–865. https://doi.org/10.1177/10668969221140397
Bridge JA, Fidler ME, Neff JR et al (1999) Adamantinoma-like Ewing’s sarcoma: genomic confirmation, phenotypic drift. Am J Surg Pathol 23:159–165. https://doi.org/10.1097/00000478-199902000-00004
Rooper LM, Bishop JA (2020) Soft Tissue Special Issue: Adamantinoma-Like Ewing Sarcoma of the Head and Neck: A Practical Review of a Challenging Emerging Entity. Head Neck Pathol 14:59–69. https://doi.org/10.1007/s12105-019-01098-y
Xu B, Suurmeijer AJH, Agaram NP et al (2023) Head and Neck Mesenchymal Tumors with Kinase Fusions: A Report of 15 Cases With Emphasis on Wide Anatomic Distribution and Diverse Histologic Appearance. Am J Surg Pathol 47:248–258. https://doi.org/10.1097/PAS.0000000000001982
Davis JL, Al-Ibraheemi A, Rudzinski ER et al (2022) Mesenchymal neoplasms with NTRK and other kinase gene alterations. Histopathology 80:4–18. https://doi.org/10.1111/his.14443
Solomon JP, Linkov I, Rosado A et al (2020) NTRK fusion detection across multiple assays and 33,997 cases: diagnostic implications and pitfalls. Mod Pathol 33:38–46. https://doi.org/10.1038/s41379-019-0324-7
Koshyk O, Dehner CA, van den Hout M et al (2023) EWSR1::POU2AF3(COLCA2) Sarcoma: An aggressive, polyphenotypic sarcoma with a head and neck predilection. Mod Pathol 36:100337. https://doi.org/10.1016/j.modpat.2023.100337
Hiemenz MC, Kaur J, Kuang Z et al (2023) POU2AF3-rearranged sarcomas: A novel tumor defined by fusions of EWSR1 or FUS to a gene formerly designated COLCA2 Genes Chromosomes. Cancer 62:460–470. https://doi.org/10.1002/gcc.23136
Agaimy A, Baneckova M, De Almeida J et al (2023) Recurrent EWSR1::COLCA2 Fusions Define a Novel Sarcoma With Spindle/Round Cell Morphology and Strong Predilection for the sinonasal tract. Am J Surg Pathol 47:361–369. https://doi.org/10.1097/PAS.0000000000002000
Muller E, Beleites E (2000) The basaloid squamous cell carcinoma of the nasopharynx. Rhinology 38:208–211
Zhang J, Shu C, Song Y et al (2016) Epstein-Barr virus DNA level as a novel prognostic factor in nasopharyngeal carcinoma: A meta-analysis. Medicine (Baltimore) 95:e5130. https://doi.org/10.1097/MD.0000000000005130
Ruuskanen M, Irjala H, Minn H et al (2019) Epstein-Barr virus and human papillomaviruses as favorable prognostic factors in nasopharyngeal carcinoma: A nationwide study in Finland. Head Neck 41:349–357. https://doi.org/10.1002/hed.25450
Lin Z, Khong B, Kwok S et al (2014) Human papillomavirus 16 detected in nasopharyngeal carcinomas in white Americans but not in endemic Southern Chinese patients. Head Neck 36:709–714. https://doi.org/10.1002/hed.23362
Carlander AF, Jakobsen KK, Bendtsen SK et al (2021) A Contemporary Systematic Review on Repartition of HPV-Positivity in Oropharyngeal Cancer Worldwide. Viruses 13:1326. https://doi.org/10.3390/v13071326
Mashiana SS, Navale P, Khandakar B et al (2021) Human papillomavirus genotype distribution in head and neck cancer: Informing developing strategies for cancer prevention, diagnosis, treatment and surveillance. Oral Oncol 113:105109. https://doi.org/10.1016/j.oraloncology.2020.105109
Ang KK, Harris J, Wheeler R et al (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363:24–35. https://doi.org/10.1056/NEJMoa0912217
O’Sullivan B, Huang SH, Su J et al (2016) Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol 17:440–451. https://doi.org/10.1016/S1470-2045(15)00560-4
Westra WH (2012) The morphologic profile of HPV-related head and neck squamous carcinoma: implications for diagnosis, prognosis, and clinical management. Head Neck Pathol 6 Suppl 1:S48-54. https://doi.org/10.1007/s12105-012-0371-6
Lewis JS Jr, Beadle B, Bishop JA et al (2018) Human Papillomavirus Testing in Head and Neck Carcinomas: Guideline From the College of American Pathologists. Arch Pathol Lab Med 142:559–597. https://doi.org/10.5858/arpa.2017-0286-CP
Kim SJ, Choi JY, Hyun SH et al (2015) Risk stratification on the basis of Deauville score on PET-CT and the presence of Epstein-Barr virus DNA after completion of primary treatment for extranodal natural killer/T-cell lymphoma, nasal type: a multicentre, retrospective analysis. Lancet Haematol 2:e66-74. https://doi.org/10.1016/S2352-3026(15)00002-2
Hussein K, Rath B, Ludewig B et al (2014) Clinico-pathological characteristics of different types of immunodeficiency-associated smooth muscle tumours. Eur J Cancer 50:2417–2424. https://doi.org/10.1016/j.ejca.2014.06.006
Dojcinov SD, Venkataraman G, Raffeld M et al (2010) EBV positive mucocutaneous ulcer–a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol 34:405–417. https://doi.org/10.1097/PAS.0b013e3181cf8622
Funding
Open access publishing supported by the National Technical Library in Prague. This study was supported by study grant SVV 260652 from the Ministry of Education, Czech Republic (Natálie Klubíčková) and the Cooperatio Program, research area SURG (Natálie Klubíčková), and the project National Institute for Cancer Research – NICR (Programme EXCELES, ID Project No. LX22NPO5102)—Funded by the European Union—Next Generation EU. (Alena Skálová, Martina Bradová), and Turku University Hospital Fund, and Maritza and Reino Salonen Foundation, Finland (Ilmo Leivo).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Informed consent
No patient consent was required for this review article.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Skálová, A., Bradová, M., Michal, M. et al. Molecular pathology in diagnosis and prognostication of head and neck tumors. Virchows Arch 484, 215–231 (2024). https://doi.org/10.1007/s00428-023-03731-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-023-03731-2