Abstract
Main Conclusion
The combined photoinhibitory and PSII-reaction centre quenching against light stress is an important mechanism that allows the green macroalga Ulva rigida to proliferate and form green tides in coastal ecosystems.
Abstract
Eutrophication of coastal ecosystems often stimulates massive and uncontrolled growth of green macroalgae, causing serious ecological problems. These green tides are frequently exposed to light intensities that can reduce their growth via the production of reactive oxygen species (ROS). To understand the physiological and biochemical mechanisms leading to the formation and maintenance of green tides, the interaction between inorganic nitrogen (Ni) and light was studied. In a bi-factorial physiological experiment simulating eutrophication under different light levels, the bloom-forming green macroalga Ulva rigida was exposed to a combination of ecologically relevant nitrate concentrations (3.8–44.7 µM) and light intensities (50–1100 µmol photons m−2 s−1) over three days. Although artificial eutrophication (≥ 21.7 µM) stimulated nitrate reductase activity, which regulated both nitrate uptake and vacuolar storage by a feedback mechanism, nitrogen assimilation remained constant. Growth was solely controlled by the light intensity because U. rigida was Ni-replete under oligotrophic conditions (3.8 µM), which requires an effective photoprotective mechanism. Fast declining Fv/Fm and non-photochemical quenching (NPQ) under excess light indicate that the combined photoinhibitory and PSII-reaction centre quenching avoided ROS production effectively. Thus, these mechanisms seem to be key to maintaining high photosynthetic activities and growth rates without producing ROS. Nevertheless, these photoprotective mechanisms allowed U. rigida to thrive under the contrasting experimental conditions with high daily growth rates (12–20%). This study helps understand the physiological mechanisms facilitating the formation and persistence of ecologically problematic green tides in coastal areas.
Similar content being viewed by others
Introduction
Marine macroalgae or seaweeds are the dominant primary producers and habitat-formers of temperate coastal regions worldwide, supplying ~ 50% of energy to higher trophic levels and providing habitat, food and shelter for invertebrates and fish (Bartsch et al. 2008; Christie et al. 2009; Iken 2012). Since recent estimates showed that macroalgae alone account for 20% of the coastal net primary production worldwide, they have an impact on the global carbon cycling and possibly climate change (Duarte et al. 2022). However, global- and regional-scale climate change factors, such as ocean acidification, ocean warming and eutrophication, are great threats to many seaweed communities, which, therefore, will undergo substantial ecological reorganisations with changes in net primary production (Harley et al. 2012; Duarte et al. 2022). In coastal environments with reduced water exchange (e.g. in lagoons, fjords, estuaries and bays), eutrophication can cause rapid proliferation of opportunistic green macroalgae, such as Ulva, Chaetomorpha and Cladophora, which is an increasing global problem (Bischof et al. 2006; Teichberg et al. 2010; Smetacek and Zingone 2013; Perrot et al. 2014; Nelson et al. 2015; Wallace and Gobler 2015; Hiraoka 2021). Green tides or green macroalgal blooms are densely arranged mats of green macroalgae, which often consist of ten to hundreds of thousands of tonnes of fresh biomass. There are steep gradients of light, oxygen and nutrients within these dense mats, creating different physiological conditions within a few centimetres (Malta et al. 2003; Bischof et al. 2006). Anoxic conditions in the water column due to bacterial decomposing of floating Ulva blooms stimulate the microbial production of H2S, which accumulates in the sediment. The release of the toxic H2S gas from the sediment causes the death of benthic and planktic organisms. This is not only an ecological disaster, but it also leads to economic losses in the affected regions (Smetacek and Zingone 2013).
Bloom-forming green macroalgae have morphological and physiological properties (i.e. high surface area-to-volume ratios and fast nutrient uptake rates) that allow them to exploit the available inorganic nitrogen (Ni) sources efficiently and to ‘outcompete’ other marine macrophytes and even fast-growing phytoplankton (Rosenberg and Ramus 1984; Runcie et al. 2003; Han and Liu 2014). Because Ulva synthesises (almost) exclusively somatic, photosynthetically active tissues, species of this genus can reach high daily growth rates by dividing up to twice a day under optimal abiotic environmental conditions (Hiraoka et al. 2020; Rautenberger 2022).
An efficient nitrogen physiology [i.e. high uptake and assimilation rates at low inorganic nitrogen (Ni) concentrations] is crucial to the formation of green tides (Naldi and Viaroli 2002; Van Alstyne et al. 2015). After nitrate (NO3–) enters the cells by specific transport systems, it is either directly assimilated or stored internally in the vacuoles (Runcie et al. 2003; Lartigue and Sherman 2005; Kennison et al. 2011). Ulva has a comparatively low storage capacity for nitrate, which reaches only up to 500 µmol g−1 DW in Ulva rigida, reducing its capacity of overcoming periods of nitrogen deficiency to only a few days (Borum 1996; Naldi and Viaroli 2002). The first step of nitrate incorporation is catalysed by nitrate reductase (NR) in the cytosol (Berges 1997). To reduce nitrate to nitrite (NO2–), reduction power provides two electrons that derive from the mitochondrial respiratory carbon flow to NR (Huppe and Turpin 1994). In contrast to the processes in higher plants and microalgae in which both NADH and NADPH act as electron donors of NR, NADH is utilised exclusively in Ulva (Huppe and Turpin 1994; Hurd et al. 1995). The subsequent reduction of nitrite to ammonium (NH4+) by nitrite reductase (NiR) in the chloroplast requires six electrons that are provided by the photosynthetic electron transport as reduced ferredoxin (Fdred). Another two photosynthetic electrons are needed for the assimilation of ammonium by glutamate synthase (GS) to produce amino acids (Huppe and Turpin 1994). In higher plants, ~ 8% of the photosynthetically transported electrons are used for nitrogen assimilation (Fridlyand and Scheibe 1999). Since these chloroplast-based steps depend on photosynthesis, a modulation of either of these primary physiological processes may thus affect the other.
Green tides of Ulva floating on the sea surface or laying as mats on shores are frequently exposed to extreme and rapidly changing radiation regimes during the day. Hence, the light intensities reaching Ulva’s surface throughout a day can range from being limited to excessive for photosynthesis, which affects growth (Cruces et al. 2019). At light intensities below or close to their photosynthetic saturation (Ek = 300–600 µmol photons m−2 s−1), the majority of light energy absorbed by the light-harvesting complexes of PSII (i.e. LHCII) is utilised by carbon assimilation in the Calvin–Benson cycle to produce carbohydrates for growth (Beer et al. 2000; Rautenberger et al. 2015). Beyond this critical point, however, most of the absorbed light energy cannot be used for carbon assimilation, resulting in an activation of photoprotective mechanisms to avoid photodamage (Wilhelm and Selmar 2011). This usually occurs on sunny days, particularly during midday in summer, when the ground-level light intensities reach more than 1000 µmol photons m−2 s−1. For example, the mean summer (December–February) light intensities in Dunedin, southeastern New Zealand, ranged between 1346 and 1947 µmol photons m−2 s−1 in 2012 (R. Rautenberger, unpublished data).
Different photoprotective mechanisms become active under excess light. As an early response to light stress, the majority of the excessively absorbed light energy is dissipated by non-photochemical quenching (NPQ) mechanisms (e.g. the xanthophyll cycle), the activity of photosystem II (PSII) is down-regulated to change the photosynthetic electron transport rate (ETR) (Wilhelm and Selmar 2011). In Ulva, the cyclic electron transport around PSII might also become a crucial photoprotective mechanism when the activity of the xanthophyll cycle is limited or non-existent (Franklin and Badger 2001). Under prolonged light stress, the light absorption capacity is reduced by decreasing the sizes of the light-harvesting antennae attached to PSII (Yamazaki et al. 2005; Mou et al. 2013; Zhang et al. 2013). Nevertheless, despite the efficiency of these photoprotective mechanisms, photosynthesis often becomes ‘super-saturated’ under light stress. Photosynthesis generates reactive oxygen species (ROS) in excess by transferring electrons to molecular oxygen (i.e. the Mehler reaction) via Fdred (Asada 1999; Cruces et al. 2019). If ROS is not sufficiently ‘detoxified’ by the network of metabolic antioxidants (e.g. glutathione) and ROS-scavenging enzymes (e.g. superoxide dismutase, SOD) of the Mehler-ascorbate peroxidase pathway, highly reactive peroxides (O22–) and free radicals (e.g. ˙OH) oxidise pigments, proteins, lipids and nucleic acids (Bischof and Rautenberger 2012). Although SOD detoxifies superoxide anions, the produced hydrogen peroxide (H2O2) is removed by ascorbate peroxidase with water as end-product of the reaction (Bischof et al. 2006; Cruces et al. 2019). In addition, Ulva rigida excretes H2O2 produced through photosynthesis under light stress in its surrounding environment (Collén et al. 1995).
The consumption of photosynthetic electrons by certain physiological processes might become an important strategy to protect algae against high light stress when the photosynthetic light and carbon reactions become unbalanced (Wilhelm and Selmar 2011). Amongst them, the stimulation of Ni assimilation (e.g. resulting from eutrophication) could require an increased amount of photosynthetic electrons with consequences for the ROS production at PSI. The availability of fewer of these electrons is expected to reduce the production of ROS under high light stress, which could sustain high growth rates of green, bloom-forming macroalgae such as Ulva. However, despite mechanistic interactions between Ni assimilation and photosynthesis, their impact on growth is not yet well understood in green macroalgae (Cabello-Pasini and Figueroa 2005; Xu et al. 2014). Increased Ni assimilation can also affect macroalgal photoacclimation by providing N-rich compounds (e.g. amino acids) for the biosynthesis of proteins during repair processes of damaged D1 proteins when the PSII repair cycle is active (Henley et al. 1991; Bouchard et al. 2006). Therefore, increased Ni input by eutrophication may help Ulva to respond better to periods of light stress and thereby sustain its fast growth for the formation and maintenance of green tides.
The aim of this study was to gain understanding of the dynamics of green tides by investigating the interaction between nitrogen assimilation and photosynthesis on growth of the bloom-forming green macroalga U. rigida. Eutrophication in coastal marine ecosystems was simulated in the laboratory by transferring U. rigida from low to higher, ecologically realistic seawater nitrate concentrations under different light intensities. It is hypothesised that the stimulation of nitrate assimilation by eutrophication reduces both photoinhibition of photosynthesis and ROS production under light stress due to higher electron demands. Moreover, higher nitrate assimilation rates may also support photoacclimation by providing N-rich cell compounds for biosynthetic repair processes. Consequently, eutrophication under high light stress has a potential to sustain high growth rates of U. rigida as a pre-requisite for its proliferation. The present study helps explain how two of the most prominent environmental factors, light and Ni, control the formation and maintenance of green tides of Ulva in coastal areas. This can be instrumental in the management of harmful green tides in coastal marine ecosystems.
Materials and methods
Macroalgal material
A male gametophytic individual of the green macroalga Ulva rigida C.Agardh (Chlorophyta; strain SBDN 247, for details see Rautenberger et al. 2015) was collected in October 2011 from the upper subtidal (3 m below sea surface) at Aramoana Spit (Otago Harbour), South Island, New Zealand (45°47ʹS, 170°42ʹE) and transported in a dark box to the laboratory in the Department of Botany, University of Otago. Macroalgal discs of 3 cm2 each (~ 25 mg FW) were cut from the thallus and raised as clonal stock cultures in 5 L of continuously aerated seawater in cylindrical glass jars over eight months. Seawater (salinity 34, pH 8.05), collected at Portobello Marine Laboratory (Otago Harbour), was enriched with a third of the full ion strength of the phosphate, iron-EDTA, trace metals II and vitamin stock solution of the ESNW formula and adjusted to pH 7.97 (Berges et al. 2001). Clonal stock cultures were kept at 5 μM of nitrate and 50 μmol photons m−2 s−1 (Philips TLD 18W/840 Cool White; Philips, Amsterdam, The Netherlands) in a 12h:12h light:dark cycle at 12 ± 1 °C in a climate-controlled cabinet (Contherm Scientific Ltd., Lower Hutt, New Zealand).
Experimental set-up
The entire experiment, which consisted of all treatment combinations (see below), was repeated three times within four weeks, resulting in three independent replicates (n = 3). For each experimental cycle, 144 Ulva discs of similar size (3 cm2, 25 mg FW) were cut from the clonal stock cultures and evenly distributed amongst nine cylindrical, acid-washed (10% HCl) glass jars, containing 5 L of nutrient-enriched seawater each (see above). The seawater was continuously aerated with filtered air (0.45 µm) from the bottom of the jars and changed each morning at the same time (between 9:30 and 10:30). Ulva discs were exposed to 52 ± 1 μmol photons m−2 s−1 (n = 3; 12h:12h light:dark cycle) and incubated at 3.8 ± 0.6 µM nitrate (n = 8) at 12 ± 1 °C in a climate-controlled walk-in cabinet (Contherm Scientific Ltd.). Light was provided from the top of the cabinet by ten quartz metal halide lamps (HPI-T Plus 400 W, Philips).
After three days of pre-experimental incubation to acclimate Ulva to these growth conditions, the cylindrical jars containing 16 Ulva discs each in 5 L of aerated seawater were transferred to one of the nine possible experimental conditions of both factors (light × nitrate) with their three levels (low light, LL: 52 ± 1 µmol photons m−2 s−1; saturation light, SL: 612 ± 9 µmol photons m−2 s−1; high light, HL: 1,111 ± 22 µmol photons m−2 s−1; nitrate: 3.8 ± 0.6 µM, 21.7 ± 1.0 µM, 44.7 ± 4.2 µM; Table 1). Seawater phosphate and ammonium concentrations remained constant throughout the experiment at 7.4 ± 0.9 µM and 0.8 ± 0.2 μM, respectively. The experiment was performed for three days to study the effects of the nitrate assimilation on short-term photoacclimation. The position of the jars was changed randomly in each experimental cycle. The experiment was performed at 12 ± 1 °C. Macroalgal samples for biochemical analyses were taken on the first and third day of the experiment, frozen with liquid nitrogen and stored either at – 20 °C (for pigment analysis) or – 80 °C (for enzyme analyses). Photosynthetic measurements were performed before the experiment started, after eight hours and before the experiment ended on the third day.
Macroalgal growth
Growth was determined by comparing the total FW of Ulva discs in each jar at the beginning and the end of the experiment. After the discs were blotted dry, FW was determined using a 3-point balance (Sartorius, Göttingen, Germany). Relative growth rates (RGRs, in % d−1) were calculated after Lüning (1990).
Nitrate uptake rates
Nitrate uptake rates were measured on days 1 and 3 of the experiment. After seawater had been changed in each 5 L glass culture jar in the mornings to avoid nutrient limitation, initial water samples (10 mL at t0) were taken before Ulva discs were added again. Further water samples (10 mL) were taken 30, 60, 120, 240, 360 and 480 min after the seawater had been renewed. Samples were stored in 10% HCl-washed plastic tubes at – 20 °C until analysis. Nitrate, ammonium and phosphate concentrations in seawater were determined colorimetrically using a ‘QuikChem 8500 series 2’ flow-injection analysis (FIA) system (Lachat Instruments, Loveland, CO, USA) after Parsons et al. (1984). The average nitrate uptake rate (in μmol g−1 FW h−1) of each replicate over the 480 min sampling period was calculated from plots of nitrate concentration vs time using a linear function. The slopes representing the disappearance of nitrate from the seawater by uptake of Ulva were divided by the total macroalgal FW in a jar during the sampling period and multiplied with the volume of seawater in a jar at the beginning of sampling (i.e. 5 L).
Nitrate reductase activity
Frozen algae (– 80 °C) were ground to a fine powder (12–62 mg; Mini-Beadbeater-96, BioSpec Products, Bartlesville, OK, USA) in liquid nitrogen. NR was extracted from crude extracts (1.0 mL 10 mg−1 FW) in 200 mM Na2HPO4/NaH2PO4 buffer (pH 7.9), containing 3% (w/v) BSA, 2 mM DTT, 0.3% (w/v) polyvinylpyrrolidone, 5 mM Na2–EDTA and 1% (v/v) Triton X-100. Extracts were not centrifuged (Hurd et al. 1995).
For the NR assay, 100 µL of the crude extract was added to 290 µL of 200 mM Na2HPO4/NaH2PO4 buffer (pH 7.9), 50 µL of 2 mM NADH (freshly prepared), and 10 µL of 1 mM FAD (freshly prepared). The assay was started by adding 50 µL of 100 mM KNO3. After a 15 min incubation at 12 ± 1 °C, the reaction was stopped by precipitation with 250 µL of 1M Zn-acetate at room temperature (RT). For control (intracellular nitrate), 250 µL of 1M Zn-acetate was added to the reaction mixture before the starter was added and identically treated as samples afterwards (Young et al. 2005). After the precipitate was centrifuged (600 g, 15 min, RT), 500 µL of 58 mM sulphanilamide (dissolved in 10% HCl) was added to 500 µL of the clear supernatant. Afterwards, 500 µL of 3.9 mM N-(1-naphthyl)ethylenediamine (NED; dissolved in H2O) was added to produce a pink azo dye that was measured photometrically at 543 nm. Nitrite concentration (in U g−1 FW) was calculated using a standard curve of known nitrite concentrations treated identically with sulphanilamide and NED.
Photosynthesis
Photosynthetic performance was measured using a PAM-2000 chlorophyll fluorometer (Walz GmbH, Effeltrich, Germany) at 12 ± 1°C (Rautenberger et al. 2009). Minimum (F0) and maximum fluorescence (Fm) were measured after 5 min of dark adaptation and maximum PSII-quantum yield, i.e. Fv/Fm, was calculated (Schreiber et al. 1995). Before dark adaption, a 5-s far red light was applied (30 µmol m−2 s−1). Electron (e–) transport rate vs irradiance (i.e. ETR-E) curves were subsequently recorded by incrementally increasing actinic light intensities after applying a saturation pulse (> 9000 µmol photons m−2 s−1, 0.8 s) every 30 s. ETRs were calculated by multiplying incident actinic light intensities (E, in µmol photons m−2 s−1), the proportion of E that was absorbed by one Ulva disc, the fraction of absorbed actinic light that was most probably received by PSII (i.e. 0.5) and the PSII-operating efficiency (Grzymski et al. 1997; Baker 2008). Maximum ETR (ETRmax, in µmol e– m−2 s−1), the light saturation point of photosynthesis (Ek, in µmol photons m−2 s−1), and the initial slope of the ETR-E curves (α, in µmol e– µmol−1 photons) were estimated from ETR-E curves fitted to the model of Jassby and Platt (1976) using R version 2.15 (R Core Team, http://www.R-project.org) according. NPQ was calculated from maximum fluorescence yields of dark- (Fm) and light-adapted (Fm′) Ulva discs measured at 497 µmol photons m−2 s−1.
Pigment analysis
Chlorophylls of frozen samples (– 20 °C) were extracted in 100% N-N-dimethylformamide (DMF; BDH Laboratory Supplies, Farham, Nottingham, UK) in the dark at 4 °C for 24 h. Chl a and b were analysed spectrophotometrically (Ultrospec 3000, Pharmacia Biotech, Cambridge, UK) at 663.8 and 646.8 nm at RT with 100% DMF as reference. Readings at 750.0 nm were used as a correction factor for scattered light. Both Chl a and b contents (in μg mg−1 FW) were calculated according to Porra et al. (1989).
Antioxidant enzyme activities
Frozen samples (– 80 °C, 35–62 mg FW) were ground to a fine powder in liquid nitrogen using mortar and pestle. Antioxidant enzymes were extracted with 1 mL of 100 mM KH2PO4 (pH 7.5/KOH/4 °C) containing 1 mM K2-EDTA, 0.1% (v/v) Triton X-100 and Complete Protease Inhibitor Cocktail tablets (EDTA-free, 1 tab 50 mL−1; Roche Diagnostics, Mannheim, Germany). After centrifugation (13,000 g, 20 min, 4 °C), aliquots of the supernatants were frozen with liquid nitrogen and stored at – 80 °C.
Total SOD activities were determined according to the xanthine oxidase-cytochrome c reduction method of McCord and Fridovich (1969), as described for Ulva by Rautenberger and Bischof (2006). Fifty microliters of the crude extract was added to 920 μL of the assay mixture (50 mM KH2PO4, pH 7.8/KOH/25 °C, 0.1 mM K2-EDTA, 0.01 mM cytochrome c and 0.05 mM xanthine). The assay was started by adding 30 μL of xanthine oxidase (Sigma-Aldrich, Munich, Germany) to the assay mixture and the increase in absorbance was recorded photometrically at 550 nm (Ultrospec 3000, Pharmacia Biotech). Xanthine oxidase was adjusted to give an increase in absorbance of 0.025 ± 0.005 min−1 at 550 nm and 25 °C in the blank. Total SOD activities (in U mg−1 protein) were calculated using an inhibition curve with purified Fe-SOD (Sigma-Aldrich). One unit of SOD activity is defined as the amount of enzyme required to inhibit the rate of cytochrome c reduction by 50% in 3 mL (McCord and Fridovich 1969).
Total glutathione reductase (GR) activities were analysed after Gutterer et al. (1999): 20 μL of 10 mM NADPH (freshly prepared in 100 mM KH2PO4, pH 7.0/KOH/20 °C) and 100 μL of 10 mM oxidised glutathione (GSSG; dissolved in H2O) were added to 840 μL of 100 mM KH2PO4 (pH 7.0/KOH/20 °C). The reaction was started after adding 40 μL of the crude extract to the assay mixture. The decrease in absorbance was followed photometrically at 340 nm and 20 °C. GR activity (in U mg−1 protein) was calculated using the extinction coefficient for NADPH of 6220 M−1 cm−1.
The total soluble protein content in crude extracts was determined photometrically at 595 nm (Bradford 1976) and calculated by comparing it with a standard curve of BSA (ICPbio Ltd, Auckland, New Zealand).
Statistical analysis
Statistically significant differences within and between growth light intensities and seawater nitrate concentrations were tested with a model I (fixed effects) two-way ANOVA, one- and two-way repeated measures ANOVA (ANOVA-RM), and a multivariate analysis of variance (MANOVA) using the statistical software JMP Pro versions 10.0 and JMP 11.0 (SAS Institute Inc., Cary, NC, USA). The Shapiro–Wilk W test and the Levene test were used to test on normal distribution and homoscedasticity, respectively. Log transformations were performed when data were not normally distributed. Tukey–Kramer Honestly Significantly Different (HSD) test was used as a post hoc test. A 5% significance level (P = 0.05) was applied in all tests.
Results
Macroalgal growth
Growth of Ulva rigida, measured by the increase in FW over three days, was strongly controlled by the growth light intensity (P = 0.0002, 2-way ANOVA) rather than by nitrate concentration (P = 0.8222, 2-way ANOVA) or an interaction between both factors (P = 0.8346, 2-way ANOVA; Fig. 1). Relative growth rates (RGRs) of the low light (9.1 ± 0.9% d−1) and high light-grown algae (12.3 ± 0.9% d−1) were similar but lower than those of U. rigida exposed to the saturating light intensity (20.0 ± 0.7% d−1).
Relative growth rates (RGR) of Ulva rigida after three days of exposure to different growth light intensities and nitrate concentrations in seawater. Treatments: LL: 52 µmol photons m−2 s−1; SL: 612 µmol photons m−2 s−1; HL: 1,111 µmol photons m−2 s−1; nitrate concentration: 3.8 µM nitrate (white columns), 21.7 µM nitrate (light grey columns) and 44.7 µM nitrate (dark grey columns). Error bars represent SDs of three replicates per treatment (n = 3). Different lower case letters above the columns indicate statistically significant differences of means between treatments (P < 0.001, 2-way ANOVA, Tukey HSD)
Nitrate uptake rates
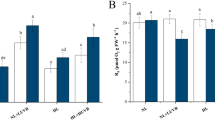
In all nitrate concentrations and light intensities tested, uptake rates were constant over the 480 min incubation period indicated by the continuous linear decrease in seawater nitrate concentrations (data not shown). Nitrate uptake rates of U. rigida changed only in response to the initial nitrate concentration in seawater (i.e. 3.8, 21.7 and 44.7 µM) over the course of the experiment (P < 0.0001, ANOVA-RM), but not due to the exposed light intensities (P = 0.9034, ANOVA-RM). On the first day, nitrate uptake of U. rigida was uniform across all light intensities at the initial concentration of 3.8 µM (3.1–3.6 µmol nitrate g−1 FW h−1) and increased by 20 to 50% to similar rates ranging between 3.9 and 5.1 µmol nitrate g−1 FW h−1 two days later (Fig. 2A, B). At initial seawater nitrate concentrations of 21.7 and 44.7 µM, nitrate uptake rates were significantly higher than at 3.8 µM under all light conditions (13.3–23.0 µmol nitrate g−1 FW h−1) on day 1. They decreased equally to 8.0–13.5 µmol nitrate g−1 FW h−1 two days later, at the end of the experiment (P < 0.0001, ANOVA-RM), without the applied light regime showing any effects. However, uptake rates remained higher than those measured at 3.8 µM.
Average nitrate uptake rates of Ulva rigida over 480 min at different seawater nitrate concentrations and growth light intensities (A) on the first and (B) the third day of the experiment. Error bars denote SDs of three replicates per treatment (n = 3). Different lower case letters above the columns indicate statistically significant differences between treatments (P < 0.05, 2-way ANOVA with repeated measures). For details of experimental treatments see Fig. 1 and Table 1
Nitrate reductase activity
On the third day of the experiment, NR activity was regulated by nitrate concentrations in seawater alone (P = 0.0018, 2-way ANOVA) and not by growth light intensities (P = 0.1959, 2-way ANOVA) or an interaction (P = 0.3602, 2-way ANOVA). Activities of NR were the lowest at 3.8 µM (24.4 ± 7.5 mU mg−1 FW) and increased when nitrate concentration was 21.7 µM (35.5 ± 6.6 mU mg−1 FW) and 44.7 µM (40.5 ± 4.6 mU mg−1 FW) but without showing significant differences between these two nitrate treatments (Fig. 3).
Nitrate reductase (NR) activities of Ulva rigida after three days of an exposure to different nitrate concentrations in seawater and growth light intensities. Error bars denote SDs of three replicates per treatment (n = 3). Different lower case letters above the columns indicate statistically significant differences of means between treatments (P < 0.05, 2-way ANOVA, Tukey HSD). For details of experimental treatments, see Fig. 1 and Table 1
Photosynthetic performance
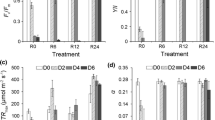
After the ‘pre-experimental’ acclimation and before U. rigida was exposed to different nitrate and light regimes, U. rigida showed Fv/Fm values of 0.787 ± 0.012 (P = 0.4709, 2-way ANOVA) that remained constant under LL throughout the experiment (P = 0.4802, 1-way ANOVA-RM; Fig. 4A). After 8 h under SL on the first day, Fv/Fm decreased by 25% to 0.587 ± 0.033 (P < 0.0001, 2-way ANOVA-RM) but increased again by 13% to 0.665 ± 0.035 over the following two days (Fig. 4B). A strong decline in Fv/Fm by 83% to 0.137 ± 0.057 (P < 0.0001, 2-way ANOVA-RM) was measured after 8 h in the HL treatment (day 1). However, Fv/Fm markedly increased by 200% to 0.411 ± 0.067 (P < 0.0001, 2-way ANOVA-RM) following two days in the HL treatment (Fig. 4C).
Changes in Fv/Fm of Ulva rigida exposed to A limiting (LL: 52 µmol photon m−2 s−1), B saturating (SL: 612 µmol photon m−2 s−1) and C high light intensities (HL: 1,111 µmol photon m−2 s−1) at three seawater nitrate concentrations (3.8, 21.7 and 44.7 µM) over three days. Error bars represent SDs of three replicates per treatment (n = 3). Different lower case letters above the columns indicate statistically significant differences of means between treatments (P < 0.0001, 2-way ANOVA, Tukey HSD). For details of experimental treatments, see Fig. 1 and Table 1
Photosynthesis by U. rigida was solely influenced by the growth light intensities—neither nitrate concentration (all P > 0.05, 2-way ANOVA) nor an interaction between the two factors (all P > 0.05, 2-way ANOVA) had an effect on photosynthetic parameters. Although ETRmax of LL- (16.3 ± 1.5 µmol e– m−2 s−1) and SL-grown (18.5 ± 3.7 µmol e– m−2 s−1) U. rigida were similar after three days, ETRmax decreased significantly in the HL treatment (9.4 ± 1.5 µmol e– m−2 s−1; P = 0.0086, 2-way ANOVA; Fig. 5A). The light intensity at which ETRs of U. rigida were saturated (Ek) reflects a typical light-dependent pattern: Ek under LL (78 ± 7 µmol photons m−2 s−1) was the lowest and increased under both SL (177 ± 28 µmol photons m−2 s−1) and HL (159 ± 18 µmol photons m−2 s−1; P = 0.029, 2-way ANOVA; Fig. 5B). The electron transport efficiency, which is represented by the initial slope of ETR-E curves (α), gradually decreased with an increasing growth light intensity (P < 0.0001, 2-way ANOVA; Fig. 5C). LL-grown algae showed highest α values (0.209 ± 0.002 μmol e– μmol−1 photons) that were reduced by 50% and 71% under SL (0.104 ± 0.008 μmol e– μmol−1 photons) and HL (0.061 ± 0.008 μmol e– μmol−1 photons), respectively.
Photosynthetic parameters and chlorophyll a/b ratio of Ulva rigida after three days of exposure to three different light conditions and three nitrate concentrations in seawater. A Maximum electron (e–) transport rates (ETRmax). B Light saturation points (Ek) of electron transport rates. C Initial slopes of electron transport rate vs. irradiance (ETR-E) curves. D Chlorophyll a/b ratios. Error bars denote SDs of three replicates per treatment (n = 3). Different lower case letters above the columns indicate statistically significant differences of means between treatments (P < 0.05, 2-way ANOVA, Tukey HSD)
Chlorophylls
After three days, both Chl a and b contents of LL-grown algae were higher than those of algae exposed to both SL and HL, which were almost identical amongst each other (both P < 0.001, 2-way ANOVA). Neither the content of Chl a nor of Chl b was affected by seawater nitrate concentrations (both P > 0.05, 2-way ANOVA) or an interaction between growth light intensity and seawater nitrate (both P > 0.05, 2-way ANOVA). In LL-grown algae, 1.21 ± 0.09 µg Chl a mg−1 FW and 0.72 ± 0.01 µg Chl b mg−1 FW were determined. When U. rigida was exposed to higher light intensities (i.e. SL and HL), contents of Chl a and b decreased by 57% (0.69 ± 0.08 µg Chl a mg−1 FW) and by 46% (0.33 ± 0.03 µg Chl b mg−1 FW), respectively (data not shown).
The Chl a/b ratio showed a clear light effect (P = 0.0124, 2-way ANOVA) with no influence of the different nitrate concentrations in seawater (P > 0.9278, 2-way ANOVA) nor an interaction between both factors (P = 0.8154, 2-way ANOVA; Fig. 5D). Chl a/b ratios in LL-grown algae (1.67 ± 0.11) were lower than in algae grown under both SL (2.09 ± 0.09) and HL (2.11 ± 0.04; Fig. 5D).
Non-photochemical quenching (NPQ)
NPQ in U. rigida was strongly regulated by the growth light intensity alone (P < 0.0001, MANOVA) and increased significantly as a consequence of the prolonged light stress on day 3 (P = 0.0199, MANOVA). Whilst NPQ remained unchanged compared to LL (0.319 ± 0.047) after eight hours of exposure to SL (0.381 ± 0.100) on day 1, NPQ in the HL treatment declined significantly to 0.021 ± 0.015 (Fig. 6A). Two days later (day 3), NPQ increased significantly to 0.750 ± 0.136 in response to prolonged SL exposure. Although HL also caused a significant increase in NPQ compared to day 1 (0.283 ± 0.117), it did not rise above the level measured of LL-grown algae (0.293 ± 0.025). Neither seawater nitrate concentration (P < 0.2559, 2-way ANOVA) nor an interaction between both factors (P < 0.4001, 2-way ANOVA) had an effect on NPQ in U. rigida.
Non-photochemical quenching (NPQ) of Ulva rigida exposed to three different light conditions and seawater nitrate concentrations on the first (A) and the third day (B) of the experiment. Error bars denote SDs of three replicates per treatment (n = 3). Different lower case letters above the columns indicate statistically significant differences of means between treatments (P < 0.05, 2-way ANOVA, Tukey HSD). For details of experimental treatments, see Fig. 1 and Table 1
Activities of antioxidant enzymes
On the third day of the experiment, both total SOD (35 ± 10 U mg−1 protein) and GR (0.17 ± 0.04 U mg−1 protein) activities remained unchanged in all light and nitrate treatments compared to LL (SOD: P = 0.2632; GR: P = 0.6061, both 2-way ANOVA) (data not shown).
Discussion
The present study reveals that the stimulation of Ni assimilation caused by enhanced seawater nitrate concentrations had no effects on photoinhibition and ROS production in Ulva rigida to maintain its high growth rates. Thus, photosynthesis and nitrogen assimilation did not affect each other in this important bloom-forming green macroalga, even though both physiological processes are interconnected. Although eutrophication of coastal seawater can have a strong impact on the physiology of marine macroalgae by stimulating photosynthesis and growth (Gordillo 2012), this was not the case for the strain of U. rigida used in this study. Its photosynthesis and growth were clearly affected by the incident light intensities, whereas seawater nitrate concentrations solely increased nitrate uptake and reduction, without showing interactions between these two major environmental factors. Therefore, it seems to be unlikely for this U. rigida strain that mechanistic interactions between the nitrogen metabolism and photosynthesis based on a common electron share might have an influence on the formation and maintenance of green tides.
Regulation of the nitrogen metabolism by nitrate reductase activity
Nitrate uptake, internal storage in the vacuoles, its reduction and assimilation are closely coupled processes in green macroalgae. The acclimation of the nitrogen physiology to changing nitrate concentrations in seawater by adjusting the activity of the nitrate-reducing enzyme, NR, strongly controls both uptake and storage by a feedback mechanism (Kennison et al. 2011). At an initial seawater nitrate concentration of 3.8 µM, constant low uptake rates and NR activities (~ 25 mU mg−1 FW) indicate that the nitrate taken up from seawater was entirely assimilated rather than internally stored. This implies that U. rigida constantly assimilates the available nitrate in the oligotrophic coastal waters of the collection site where nitrate concentrations occasionally exceed 5 µM in winter (Phillips and Hurd 2003; Dean and Hurd 2007; Kregting et al. 2008).
When U. rigida was transferred from pre-experimental (3.8 µM) to higher nitrate concentrations (≥ 21.7 µM), the instant increase in nitrate uptake rates (4–6 ×) can be primarily ascribed to the filling of the intracellular nitrate pools (i.e. vacuoles). At this point, the low NR activity resulting from the pre-incubation was not able to reduce nitrate to nitrite as fast as it entered the cells, which results in the storage of nitrate in the vacuoles (McGlathery et al. 1996; Lartigue and Sherman 2005; Kennison et al. 2011). Higher nitrate concentrations increased the NR activities through enhanced transcription of the corresponding gene as demonstrated for Ulva prolifera (Guo et al. 2017). Constant uptake rates during the first eight hours after the transfer suggest that nitrate pools were slowly and continuously filled without reaching capacity within this period of time. This is in contrast to other Ulva species in which nitrate uptake rates decreased within 3–6 h after having been exposed to higher nitrate concentrations since nitrate pools reached capacity along with maximum NR activities (Lartigue and Sherman 2005; Kennison et al. 2011). Hence, NR activity in U. rigida seems to adjust more slowly to increasing nitrate concentrations than other Ulva species due to the filling of larger nitrate pools (Naldi and Viaroli 2002; Lartigue and Sherman 2005). Two days later, nitrate pools were filled to capacity as indicated by significantly lower uptake rates and, meanwhile, NR activity increased to the maximum rate of 35–41 mU mg−1 FW. This shows that nitrate uptake decreases when NR reached its maximum activity; then uptake rates seem to be equivalent to the maximum NR activity, which, in turn, does not require nitrate to be stored temporarily in the intracellular pools. This indicates that the activity of NR is the rate-limiting step of the entire nitrate-assimilating pathway in U. rigida. A ‘negative feedback regulation process’ of uptake, storage and reduction/assimilation was demonstrated to be present in green macroalgae, including U. rigida (McGlathery et al. 1996; Naldi and Viaroli 2002; Lartigue and Sherman 2005; Kennison et al. 2011). It seems that the acclimation of U. rigida to nitrate concentrations ≥ 21.7 µM is strongly associated with the adjustment of NR activities to control uptake rates.
NR activity is often regulated by the synthesis of the enzyme due to gene expression, which is triggered by different environmental factors (nitrate, light, CO2) and the intracellular availability of carbon skeletons (Huppe and Turpin 1994). In U. rigida, NR activity was mainly regulated by nitrate, stimulating its synthesis at concentrations ≥ 21.7 µM. Because growth and nitrogen uptake/assimilation were not coupled, U. rigida seems to be saturated with Ni under oligotrophic conditions. In nitrate-replete Ulva lactuca, excess Ni, which is not used for growth, was allocated to protein synthesis, increasing enzyme contents and other nitrogen-containing metabolites (Teichberg et al. 2007). Because growth rates of U. rigida did not seem to be associated with internal Ni pools as in other Ulva species, U. rigida can proliferate and potentially form green tides even under oligotrophic conditions due to constant Ni assimilation rather than by Ni pool sizes (Teichberg et al. 2010). Therefore, eutrophication does not seem to be the primary factor for its fast growth when other factors (e.g. light) are limiting.
Photoprotection and photoacclimation are regulated by light to sustain macroalgal growth
NPQ is the most important photoprotective mechanism that dissipates excessively absorbed light energy as heat to protect the photosynthetic apparatus, mainly PSII, from photodamage (Wilhelm and Selmar 2011). In Ulva rigida, PSII-photodamage under sudden light stress as measured by the fast decline in Fv/Fm can be ascribed to the inactive NPQ. In both Ulva fasciata and U. lactuca, a similar sharp decline in Fv/Fm and NPQ was accompanied with a rapid and immense degradation of the PSII-intrinsic D1 protein after these macroalgae was transferred from low to high light (80–1500 µmol photons m−2 s−1). After 2 h of high light stress, 50–70% of the initially active D1 protein were damaged and degraded before the rapidly declining Fv/Fm levelled off at values ~ 0.1 (Carr and Björk 2007). Since the decrease in Fv/Fm did not entirely reflect the damages of the D1 protein by showing lower values than the actual D1 protein contents, an additional mechanism was apparently active: some of the remaining, non-degraded PSII-RCs were still active and fully functional in photochemistry, whilst others were inactive but not damaged. Photochemically active PSII are thought to be responsible for the low Fv/Fm values measured in U. lactuca (0.07) and U. fasciata (0.09–0.13), which are similar to 0.137 under HL in U. rigida (this study). Inactive, non-damaged PSII-RCs can dissipate excess excitation energy from the ‘still’-coupled LHCII as heat and, in turn, protect neighbouring PSII from photoinactivation to avoid further structural damages (Öquist et al. 1992; Franklin 1994; Franklin and Larkum 1997; Chow et al. 2002). Despite the lack of NPQ, photoprotection by both photoinhibition and PSII-RC quenching seems to be very effective not only in U. fasciata and U. lactuca but also in U. rigida.
The production of superoxide anions (O2*–) remained low in U. rigida due to effective photoprotection. Neither SOD nor GR activities increased after three days of light stress, which indicates that ROS production was low and could be sufficiently detoxified by the Mehler-ascorbate peroxidase pathway. This finding further supports the currently discussed hypothesis that the photodamage of PSII is caused by a light-induced inactivation of the PSII-associated oxygen-evolving complex rather than by ROS produced due to an over-reduction of the primary electron acceptor (i.e. QA) or a charge recombination in PSII (Ohnishi et al. 2005; Murata et al. 2012). The present study suggests that photoinhibition in combination with the PSII-RC quenching seem to be crucial to U. rigida’s ability to respond to high light stress when the NPQ processes such as the xanthophyll cycle are ‘inactive’.
After two days of exposure to light stress, the marked photoacclimation of PSII activity for U. rigida measured by the recovery of Fv/Fm can be attributed to the fast restoration of the PSII-RCs by the PSII repair cycle that exceeds the rate of photodamage. Since higher rates of photodamage and degradation of the D1 protein are followed by a faster restoration of the PSII-RCs under HL compared to SL, the rates of the PSII repair via de novo synthesis of the D1 protein apparently depend on the amount of damaged and degraded PSII-RCs that lack the D1 protein (Tyystjärvi 2013). The restoration of PSII activity by the PSII repair cycle apparently influences the regain of NPQ: the increase in NPQ under both SL and HL could result from the build-up of the proton gradient (ΔpH) across the thylakoid membrane due to the increasing PSII activity. After the lumen pH dropped below ~ 6, violaxanthin de-epoxidase is activated and converts violaxanthin to eventually zeaxanthin in the xanthophyll cycle. Concurrently, the PSII-associated PsbS and LHCSR proteins in Ulva become protonated, which allows the zeaxanthin-PsbS/LCHSR aggregates to dissipate excess excitation energy in LHCII as heat rather than to transfer excitons to other chlorophylls and form singlet oxygen (Mou et al. 2013; Zhang et al. 2013). However, how far this scenario of NPQ reactivation is involved in the photoacclimation under prolonged high light stress in Ulva requires further investigation. As a long-term photoacclimation to light stress, U. rigida reduced the absorption cross section of PSII (i.e. size of LHCII) after three days of expose to both SL and HL significantly. Smaller PSII antennae, which are indicated by high Chl a/b ratios of 2.09–2.11 under SL and HL compared to 1.67 under LL, decrease the absorption of light and limit energy transfer to PSII to protect PSII-RCs from light-induced oxidative stress.
All these short-term photoprotective and long-term photoacclimation mechanisms together allow U. rigida to sustain its photosynthetic activity, which has direct consequences for its growth. Whilst photoprotection and -acclimation maintained a high photosynthetic activity under SL to enable growth rates of 20% d−1, the HL-induced photoinhibition was clearly responsible for the growth reduction of U. rigida’s by 60% (RGR = 12% d−1). Because high growth rates of ≥ 20% d−1 favour the proliferation, light intensities ranging between 300 and 600 µmol photons m−2 s−1 seem to be optimal for the formation of green tides by U. rigida (Beer et al. 2000; Rautenberger et al. 2015). Although such light intensities were shown to favour the proliferation of U. prolifera by relatively high growth rates (20–40% d−1), its combination with other local environmental conditions, such as optimal temperature and salinity regimes, allow the green macroalga’s to bloom (Xiao et al. 2016; Wu et al. 2018, 2022). Nevertheless, growth rates of 9–12% d−1 may allow U. rigida to outcompete most macroalgae, whose growth rate are usually lower (Teichberg et al. 2010).
It is striking that the different, ecologically relevant nitrate concentrations in seawater had no supporting effect on both photoprotection and photoacclimation under SL and HL in U. rigida, which suggests that these mechanisms were saturated at seawater nitrate concentrations of 3.8 µM. This is supported by the Ni-dependent pattern of photoacclimation in Ulva rotundata: whilst PSII activity was able to acclimate to high light stress over seven days under sufficient Ni supply (15 µM NH4+), photoinhibition persisted when ammonium concentration (1.5 µM NH4+) remained low (Henley et al. 1991). As the pattern of high light acclimation in Ni-replete U. rotundata was very similar to that of U. rigida in the present study, it can be concluded once again that U. rigida was sufficiently Ni-supplied at nitrate concentrations ≥ 3.8 of nitrate to support the PSII repair cycle with sufficient amino acids for the biosynthesis of the D1 protein (Litchman et al. 2002; Davison et al. 2007).
Does increased nitrogen assimilation function as a photoprotective mechanism in Ulva rigida?
In microalgae, the non-linear relationship between the photosynthetic ETR and oxygen production or carbon incorporation rates at supersaturating light intensities was ascribed to ‘alternative’ sinks of photosynthetic electrons, protecting them from photoinhibition (Wilhelm et al. 2006; Napoléon and Claquin 2012). Whilst photorespiration is largely suppressed in green algae (e.g. Ulva) and diatoms that employ an efficient carbon-concentrating mechanism, large amounts of photosynthetic electrons can be transferred to oxygen at the acceptor side of PSII under high light (Claquin et al. 2004; Waring et al. 2010). In nitrate-replete diatoms, excess photosynthetic energy can be channelled to nitrate reduction, resulting in the release of ‘non-nutritionally’ reduced nitrate into the surrounding environment (Lomas and Gilbert 1999).
Whether or not the stimulation of the nitrogen metabolism can function as a photoprotective mechanism by redirecting a higher proportion of photosynthetic electrons to nitrate assimilation is still unclear for macroalgae despite the hint that high seawater nitrate affects the relationship between ETR and oxygen production in U. rigida (Cabello-Pasini and Figueroa 2005). Since photosynthesis provides eight electrons by Fdred to NiR and glutamate synthase (GS) to assimilate one mole of nitrate in photosynthetically active tissue, an increase in nitrogen assimilation rates (e.g. due to eutrophication) may require a higher amount of photosynthetic electrons. However, in this study the conspicuous lack of any interaction between the nitrate and light treatments suggests that there is no substantial increase in electron consumption by NiR and GS under higher nitrate concentrations. This is because NR activity, as the rate-limiting step of the entire nitrogen-assimilating pathway, controls the rates of the subsequent reactions steps, including those of NiR and GS. Since NR activity in U. rigida increases only slowly and moderately (~ + 50%) to higher nitrate concentrations, NiR and GS activities cannot rise significantly to create a higher electron sink than at 3.8 µM. Moreover, an excretion of nitrate into the surrounding seawater was not detected under any experimental treatment, which suggests that only a basic and constant amount of photosynthetic electrons is delivered to assimilate nitrogen, regardless the incident light intensity. Furthermore, a decreased PSII activity under photoinhibition (at SL and HL) and the remaining low photosynthetic activity under chronic photoinhibition (at HL) photosynthetic electrons are only provided in sufficient quantities to be utilised by the Calvin-Benson cycle. Therefore, nitrate assimilation does not seem to act as a photoprotective mechanism in U. rigida.
Conclusion
Ulva rigida is a green macroalgal species that is able to form large green tides in coastal marine areas. This can cause huge ecological and economic problems when green tides persist and spread out (Smetacek and Zingone 2013). Because growth of U. rigida is strongly controlled by light intensity rather than the seawater nitrate concentrations resulting from Ni-saturation at oligotrophic conditions (3.8 µM), effective photoprotection and photoacclimation are required to maintain its photosynthetic activity and thus high growth rates. Apparently, a purposeful damage and degradation of the D1 protein (i.e. photoinhibition) in combination with the PSII-RC quenching reduces the ‘excitation pressure’ in PSII efficiently when heat dissipation of excess light energy by NPQ is inactive. By these short-term photoprotective mechanisms, U. rigida was able to respond rapidly to light stress before long-term photoacclimation could become effective. The re-invigoration of heat dissipation by regaining NPQ activity along with the reduction of the PSII absorption cross section allowed U. rigida to keep its daily growth rates high. These physiological mechanisms combined can be regarded to as a pre-requisite for the formation of green tides. Being arranged in dense mats, U. rigida is exposed to a steep light gradient. Whilst the light-exposed top and ‘sub-canopy’ layers could maintain high growth rates due to effective photoprotection and -acclimation, efficient light capture strategies (e.g. increased PSII antenna sizes) may allow U. rigida to reach sufficient growth rates at bottom layer despite light-limitation. The present study demonstrates that the high phenotypic plasticity of U. rigida with respect to the regulation of the photosynthetic activity is crucial to maintain its massive growth. Although proliferation can cause enormous and economic losses in coastal marine ecosystems, it could be also beneficially applied to remove or neutralise pollutants under bioremediation.
Data availability
The data sets generated and/or analysed during this study are available from the corresponding author on reasonable request.
Abbreviations
- ETR:
-
Electron transport rate
- HL:
-
High light
- LL:
-
Low light
- NiR:
-
Nitrite reductase
- NR:
-
Nitrate reductase
- SL:
-
Saturation light
- SOD:
-
Superoxide dismutase
References
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639. https://doi.org/10.1146/annurev.arplant.50.1.601
Baker N (2008) Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113. https://doi.org/10.1146/annurev.arplant.59.032607.092759
Bartsch I, Wiencke C, Bischof K, Buchholz CM, Buck BH, Eggert A, Feuerpfeil P, Hanelt D, Jacobsen S, Karez R, Karsten U, Molis M, Roleda MY, Schubert H, Schumann R, Valentin K, Weinberger F, Wiese J (2008) The genus Laminaria sensu lato: recent insights and developments. Eur J Phycol 43:1–86. https://doi.org/10.1080/09670260701711376
Beer S, Larsson C, Poryan O, Axelsson L (2000) Photosynthetic rates of Ulva (Chlorophyta) measured by pulse amplitude modulated (PAM) fluorometry. Eur J Phycol 35:69–74. https://doi.org/10.1080/09670260010001735641
Berges J (1997) Miniview: algal nitrate reductases. Eur J Phycol 32:3–8. https://doi.org/10.1080/09541449710001719315
Berges JA, Franklin DJ, Harrison PJ (2001) Evolution of an artificial seawater medium: improvements in enriched seawater, artificial water over the last two decades. J Phycol 37:1138–1145. https://doi.org/10.1046/j.1529-8817.2001.01052.x
Bischof K, Rautenberger R (2012) Seaweed responses to environmental stress: reactive oxygen and antioxidative strategies. In: Wiencke C, Bischof K (eds) Seaweed biology Novel insights into ecophysiology, ecology and utilization. Springer-Verlag, Berlin, pp 109–132. https://doi.org/10.1007/978-3-642-28451-9_6
Bischof K, Rautenberger R, Brey L, Pérez-Lloréns JL (2006) Physiological acclimation to gradients of solar irradiance within mats of the filamentous green macroalga Chaetomorpha linum from southern Spain. Mar Ecol Progr Ser 306:165–175. https://doi.org/10.3354/meps306165
Borum J (1996) Shallow waters and land/sea boundaries. In: Jørgensen BB, Richardson K (eds) Eutrophication in coastal marine ecosystems. American Geographical Union, Washington, pp 179–203. https://doi.org/10.1029/CE052p0179
Bouchard JN, Roy S, Campbell DA (2006) UVB Effects on the photosystem II-D1 protein of phytoplankton and natural phytoplankton communities. Photochem Photobiol 82:936–951. https://doi.org/10.1562/2005-08-31-IR-666
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Cabello-Pasini A, Figueroa FL (2005) Effect of nitrate concentration on the relationship between photosynthetic oxygen evolution and electron transport rate in Ulva rigida (Chlorophyta). J Phycol 41:1169–1177. https://doi.org/10.1111/j.1529-8817.2005.00144.x
Carr H, Björk M (2007) Parallel changes in non-photochemical quenching properties, photosynthesis and D1 levels at sudden, prolonged irradiance exposures in Ulva fasciata Delile. J Photochem Photobiol 87:18–26. https://doi.org/10.1016/j.jphotobiol.2006.12.005
Chow WS, Lee H-Y, Park Y-I, Park Y-M, Hong Y-N, Anderson JM (2002) The role of inactive photosystem-II-mediated quenching in a last-ditch community defence against high light stress in vivo. Philos Trans R Soc Lond (b Biol Sci) 357:1441–1450. https://doi.org/10.1098/rstb.2002.1145
Christie H, Norderhaug KM, Fredriksen S (2009) Macrophytes as habitat for fauna. Mar Ecol Progr Ser 396:221–233. https://doi.org/10.3354/meps08351
Claquin P, Kromkamp JC, Martin-Jezequel V (2004) Relationship between photosynthetic metabolism and cell cycle in a synchronized culture of the marine alga Cylindrotheca fusiformis (Bacillariophyceae). Eur J Phycol 39:33–41. https://doi.org/10.1080/0967026032000157165
Collén J, Jiménez del Río M, García-Reina G, Pedersén M (1995) Photosynthetic production of hydrogen peroxide by Ulva rigida C. Ag. (Chlorophyta). Planta 196:225–230. https://doi.org/10.1007/BF00201378
Cruces E, Rautenberger R, Cubillos VM, Ramírez-Kushel E, Rojas-Lillo Y, Lara C, Montory JA, Gómez I (2019) Interaction of photoprotective and acclimation mechanisms in Ulva rigida (Chlorophyta) in response to diurnal changes in solar radiation in southern Chile. J Phycol 55:1011–1027. https://doi.org/10.1111/jpy.12894
Davison IR, Jordan TL, Fegley JC, Grobe CW (2007) Response of Laminaria saccharina (Phaeophyta) growth and photosynthesis to simultaneous ultraviolet radiation and nitrogen limitation. J Phycol 43:636–646. https://doi.org/10.1111/j.1529-8817.2007.00360.x
Dean PR, Hurd CL (2007) Seasonal growth, erosion rates, and nitrogen and photosynthetic ecophysiology of Undaria pinnatifida (Heterokontophyta) in southern New Zealand. J Phycol 43:1138–1148. https://doi.org/10.1111/j.1529-8817.2005.00144.x
Duarte CM, Gattuso J-P, Hancke K, Gundersen H, Filbee-Dexter K, Pedersen MF, Middelburg JJ, Burrows MT, Krumhansl KA, Wernberg T, Moore P, Pessarrodona A, Ørberg SB, Pinto IS, Assis J, Queirós AM, Smale DA, Bekkby T, Serrão EA, Krause-Jensen D (2022) Global estimates of the extent and production of macroalgal forests. Glob Ecol Biogeogr 31:1422–1439. https://doi.org/10.1111/geb.13515
Franklin LA (1994) The effect of temperature acclimation on the photoinhibitory responses of Ulva rotundata Blid. Planta 192:324–331. https://doi.org/10.1007/BF00198567
Franklin LA, Badger M (2001) A comparison of photosynthetic electron transport rates in macroalgae by pulse amplitude modulated chlorophyll fluorescence and mass spectrometry. J Phycol 37:756–767. https://doi.org/10.1046/j.1529-8817.2001.00156.x
Franklin LA, Larkum AWD (1997) Multiple strategies for a high light existence in a tropical marine macroalga. Photosynth Res 53:149–159. https://doi.org/10.1023/A:1005820203585
Fridlyand LE, Scheibe R (1999) Controlled distribution of electrons between acceptors in chloroplasts: a theoretical consideration. Biochim Biophys Acta 1413:31–42. https://doi.org/10.1016/S0005-2728(99)00079-1
Gordillo FJL (2012) Environment and algal nutrition. In: Wiencke C, Bischof K (eds) Seaweed biology. Novel insights into ecophysiology, ecology and utilization. Springer-Verlag, Berlin, pp 67–86. https://doi.org/10.1007/978-3-642-28451-9_4
Grzymski J, Johnsen G, Sakshaug E (1997) The significance of intracellular self-shading on the biooptical properties of brown, red, and green macroalgae. J Phycol 33:408–414. https://doi.org/10.1111/j.0022-3646.1997.00408.x
Guo Y, Wang HZ, Wu CH, Fu HH, Jiang P (2017) Cloning and characterization of nitrate reductase gene in Ulva prolifera (Ulvophyceae, Chlorophyta). J Phycol 53:1035–1043. https://doi.org/10.1111/jpy.12556
Gutterer J, Dringen R, Hirrlinger J, Hamprecht B (1999) Purification of glutathione reductase from bovine brain, generation of an antiserum, and immunocytochemical localization of the enzyme in neural cells. J Neurochem 73:1422–1430. https://doi.org/10.1046/j.1471-4159.1999.0731422.x
Han Q, Liu D (2014) Macroalgae blooms and their effects on seagrass ecosystems. J Ocean Univ China 13:791–798. https://doi.org/10.1007/s11802-014-2471-2
Harley CDG, Anderson KM, Demes KW, Jorve JP, Kordas RL, Coyle TA, Graham MH (2012) Effects of climate change on global seaweed communities. J Phycol 48:1064–1078. https://doi.org/10.1111/j.1529-8817.2012.01224.x
Henley WJ, Levavasseur G, Franklin LA, Osmond CB, Ramus J (1991) Photoacclimation and photoinhibition in Ulva rotundata as influenced by nitrogen availability. Planta 184:235–243. https://doi.org/10.1007/BF00197952
Hiraoka M (2021) Massive Ulva green tides caused by inhibition of biomass allocation to sporulation. Plants 10:2482. https://doi.org/10.3390/plants10112482
Hiraoka M, Kinoshita Y, Higa M, Tsubaki S, Monotilla AP, Onda A, Dan A (2020) Fourfold daily growth rate in multicellular marine alga Ulva meridionalis. Sci Rep 10:12606. https://doi.org/10.1038/s41598-020-69536-4
Huppe HC, Turpin DH (1994) Integration of carbon and nitrogen metabolism in plant and algal cells. Annu Rev Plant Physiol Plant Mol Biol 45:577–607. https://doi.org/10.1146/annurev.pp.45.060194.003045
Hurd CL, Berges JA, Osborne J, Harrison PJ (1995) An in vitro nitrate reductase assay for marine microalgae: optimization and characterization of the enzyme for Fucus gardneri (Phaeophyta). J Phycol 31:835–843. https://doi.org/10.1111/j.0022-3646.1995.00835.x
Iken K (2012) Grazers on benthic seaweeds. In: Wiencke C, Bischof K (eds) Seaweed biology. Novel insights into ecophysiology, ecology and utilization. Springer-Verlag, Berlin, pp 157–175. https://doi.org/10.1007/978-3-642-28451-9_8
Jassby A, Platt R (1976) Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol Oceanogr 21:540–541. https://doi.org/10.4319/lo.1976.21.4.0540
Kennison RL, Kamer K, Fong P (2011) Rapid nitrate uptake rates and large short-term storage capacities may explain why opportunistic green macroalgae dominate shallow eutrophic estuaries. J Phycol 47:483–494. https://doi.org/10.1111/j.1529-8817.2011.00994.x
Kregting LT, Hepburn CD, Hurd CL, Pilditch CA (2008) Seasonal patterns of growth and nutrient status of the macroalga Adamsiella chauvinii (Rhodophyta) in soft sediment environments. J Exp Mar Biol Ecol 360:94–102. https://doi.org/10.1016/j.jembe.2008.04.001
Lartigue J, Sherman TD (2005) Response of Enteromorpha sp. (Chlorophyceae) to a nitrate pulse: nitrate uptake, inorganic nitrogen storage and nitrate reductase activity. Mar Ecol Progr Ser 292:147–157. https://doi.org/10.3354/meps292147
Litchman E, Neale PJ, Banaszak AT (2002) Increased sensitivity to ultraviolet radiation in nitrogen-limited dinoflagellates: photoprotection and repair. Limnol Oceanogr 47:86–94. https://doi.org/10.4319/lo.2002.47.1.0086
Lomas MW, Gilbert PM (1999) Temperature regulation of nitrate uptake: A novel hypothesis about nitrate uptake and reduction in cool-water diatoms. Limnol Oceanogr 44:556–572. https://doi.org/10.4319/lo.1999.44.3.0556
Lüning K (1990) Seaweeds. Their environment, biogeography, and ecophysiology. Wiley, New York
Malta E, Rijstenbil JW, Brouwer PEM, Kromkamp JC (2003) Vertical heterogeneity in physiological characteristics of Ulva spp. mats. Mar Biol 143:1029–1038. https://doi.org/10.1007/s00227-003-1134-4
McCord J, Fridovich I (1969) Superoxide dismutase – an enzymatic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
McGlathery KJ, Pedersen MF, Borum J (1996) Changes in intracellular nitrogen pools and feedback controls on the nitrogen uptake in Chaetomorpha linum (Chlorophyta). J Phycol 32:393–401. https://doi.org/10.1111/j.0022-3646.1996.00393.x
Mou S, Zhang X, Dong M, Fan X, Xu J, Cao S, Xu D, Wang W, Ye N (2013) Photoprotection in the green tidal alga Ulva prolifera: Role of LHCSR and PsbS proteins in response to high light stress. Plant Biol 15:1033–1039. https://doi.org/10.1111/j.1438-8677.2012.00712.x
Murata N, Allakhverdiev SI, Nishiyama Y (2012) The mechanism of photoinhibition in vivo: re-evaluation of the roles of catalase, α-tocopherol, non-photochemical quenching, and electron transport. Biochim Biophys Acta 1817:1127–1133. https://doi.org/10.1016/j.bbabio.2012.02.020
Naldi M, Viaroli P (2002) Nitrate uptake and storage in the seaweed Ulva rigida C.Agardh in relation to nitrate availability and thallus nitrate content in a eutrophic coastal lagoon (Sacca di Goro, Po River Delta, Italy). J Exp Mar Biol Ecol 269:65–83. https://doi.org/10.1016/S0022-0981(01)00387-2
Napoléon C, Claquin P (2012) Multi-Parametric relationships between PAM measurements and carbon incorporation, an in situ approach. PLoS ONE 7:e40284. https://doi.org/10.1371/journal.pone.0040284
Nelson W, Neill K, D’Archino R (2015) When seaweeds go bad: an overview of outbreaks of nuisance quantities of marine macroalgae in New Zealand. NZ J Mar Freshw Res 49:472–491. https://doi.org/10.1080/00288330.2015.1064975
Ohnishi N, Allakhverdiev SI, Takahashi S, Higashi S, Watanabe M, Nishiyama Y, Murata N (2005) Two-step mechanism of photodamage to photosystem II: Step 1 occurs at the oxygen-evolving complex and step 2 occurs at the photochemical reaction center. Biochemistry 44:8494–8499. https://doi.org/10.1021/bi047518q
Öquist G, Chow WS, Anderson JM (1992) Photoinhibition of photosynthesis represents a mechanism for the long-term regulation of photosystem II. Planta 186:450–460. https://doi.org/10.1007/BF00195327
Parsons T, Maita Y, Lalli C (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford
Perrot T, Rossi N, Ménesguen A, Dumas F (2014) Modelling green macroalgal blooms on the coasts of Brittany, France to enhance water quality management. J Mar Syst 132:38–53. https://doi.org/10.1016/j.jmarsys.2013.12.010
Phillips JC, Hurd CL (2003) Nitrogen ecophysiology of intertidal seaweeds from New Zealand: N uptake, storage and utilisation in relation to shore position and season. Mar Ecol Progr Ser 264:31–48. https://doi.org/10.3354/meps264031
Porra R, Thompson W, Kriedemann P (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394. https://doi.org/10.1016/S0005-2728(89)80347-0
Rautenberger R (2022) Development of a hatchery for the aquaculture of the green macroalga Ulva sp. in Norway. Final report to ULVA-HATCH project. Norwegian Institute of Bioeconomy Research (NIBIO), Bodø
Rautenberger R, Bischof K (2006) Impact of temperature on UV-susceptibility of two Ulva (Chlorophyta) species from Antarctic and Subantarctic regions. Polar Biol 29:988–996. https://doi.org/10.1007/s00300-006-0141-6
Rautenberger R, Mansilla A, Gómez I, Wiencke C, Bischof K (2009) Photosynthetic responses to UV-radiation of intertidal macroalgae from the Strait of Magellan (Chile). Rev Chil Hist Nat 82:43–61. https://doi.org/10.4067/S0716-078X2009000100003
Rautenberger R, Fernández PA, Strittmatter M, Heesch S, Cornwall CE, Hurd CL, Roleda MY (2015) Saturating light and not increased carbon dioxide under ocean acidification drives photosynthesis and growth in Ulva rigida (Chlorophyta). Ecol Evol 5:874–888. https://doi.org/10.1002/ece3.1382
Rosenberg G, Ramus J (1984) Uptake of inorganic nitrogen and seaweed surface area: volume ratios. Aquat Bot 19:65–72. https://doi.org/10.1016/0304-3770(84)90008-1
Runcie JW, Ritchie RJ, Larkum AWD (2003) Uptake kinetics and assimilation of inorganic nitrogen by Catenella nipae and Ulva lactuca. Aquat Bot 76:155–174. https://doi.org/10.1016/S0304-3770(03)00037-8
Schreiber U, Bilger W, Neubauer C (1995) Chlorophyll fluorescence as a non-intrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze E-D, Caldwell MM (eds) Ecophysiology of photosynthesis. Springer-Verlag, Berlin, pp 49–70. https://doi.org/10.1007/978-3-642-79354-7_3
Smetacek V, Zingone A (2013) Green and golden seaweed tides on the rise. Nature 504:84–88. https://doi.org/10.1038/nature12860
Teichberg M, Heffner L, Fox S, Valiela I (2007) Nitrate reductase and glutamine synthetase activity, internal N pools, and growth of Ulva lactuca: responses to long and short-term N supply. Mar Biol 151:1249–1259. https://doi.org/10.1007/s00227-006-0561-4
Teichberg M, Fox SE, Olsen YS, Valiela I, Martinetto P, Iribarne O, Muto EY, Petti MAV, Corbisier TN, Soto-Jiménez M, Páez-Osuna F, Castro P, Freitas H, Zitelli A, Cardinaletti M, Tagliapietra D (2010) Eutrophication and macroalgal blooms in temperate and tropical coastal waters: nutrient enrichment experiments with Ulva spp. Glob Change Biol 16:2624–2637. https://doi.org/10.1111/j.1365-2486.2009.02108.x
Tyystjärvi E (2013) Photoinhibition of photosystem II. Int Rev Cell Mol Biol 300:243–303. https://doi.org/10.1016/B978-0-12-405210-9.00007-2
Van Alstyne KL, Nelson TA, Ridgway RL (2015) Environmental chemistry and chemical ecology of “Green Tide” seaweed blooms. Integr Comp Biol 55:518–532. https://doi.org/10.1093/icb/icv035
Wallace R, Gobler C (2015) Factors controlling blooms of microalgae and macroalgae (Ulva rigida) in a eutrophic, urban estuary: Jamaica Bay, NY, USA. Estuar Coasts 38:519–533. https://doi.org/10.1007/s12237-014-9818-1
Waring J, Klenell M, Bechtold U, Underwood GJC, Baker NR (2010) Light-induced responses of oxygen photoreduction, reactive oxygen species production and scavenging in two diatom species. J Phycol 46:1206–1217. https://doi.org/10.1111/j.1529-8817.2010.00919.x
Wilhelm C, Selmar D (2011) Energy dissipation is an essential mechanism to sustain the viability of plants: The physiological limits of improved photosynthesis. J Plant Physiol 168:79–87. https://doi.org/10.1016/j.jplph.2010.07.012
Wilhelm C, Büchel C, Fisahn J, Goss R, Jakob T, LaRoche J, Lavaud J, Lohr M, Riebesell U, Stehfest K, Valentin K, Kroth PG (2006) The regulation of carbon and nutrient assimilation in diatoms is significantly different from green algae. Protist 157:91–124. https://doi.org/10.1016/j.protis.2006.02.003
Wu H, Gao G, Zhong Z, Li X, Xu J (2018) Physiological acclimation of the green tidal alga Ulva prolifera to a fast-changing environment. Mar Environ Res 137:1–7. https://doi.org/10.1016/j.marenvres.2018.02.018
Wu H, Liu Y, Beardall J, Zhong Z, Gao G, Xu J (2022) Physiological acclimation of Ulva prolifera to seasonal environmental factors drives green tides in the Yellow Sea. Mar Environ Res 179:105695. https://doi.org/10.1016/j.marenvres.2022.105695
Xiao J, Zhang X, Gao C, Jiang M, Li R, Wang Z, Li Y, Fan S, Zhang X (2016) Effect of temperature, salinity and irradiance on growth and photosynthesis of Ulva prolifera. Acta Oceanol Sin 35:114–121. https://doi.org/10.1007/s13131-016-0891-0
Xu Z, Wu H, Zhan D, Sun F, Sun J, Wang G (2014) Combined effects of light intensity and NH4+-enrichment on growth, pigmentation, and photosynthetic performance of Ulva prolifera (Chlorophyta). Chin J Oceanol Limnol 32:1016–1023. https://doi.org/10.1007/s00343-014-3332-y
Yamazaki J, Suzuki T, Maruta E, Kamimura Y (2005) The stoichiometry and antenna size of the two photosystems in marine green algae, Bryopsis maxima and Ulva pertusa, in relation to the light environment of their natural habitat. J Exp Bot 56:1517–1523. https://doi.org/10.1093/jxb/eri147
Young EB, Lavery PS, van Elven B, Dring MJ, Berges JA (2005) Nitrate reductase activity in macroalgae and its vertical distribution in macroalgal epiphytes of seagrasses. Mar Ecol Progr Ser 288:103–114. https://doi.org/10.3354/meps288103
Zhang X, Ye N, Mou S, Xu D, Fan X (2013) Occurrence of the PsbS and LhcSR products in the green alga Ulva linza and their correlation with excitation pressure. Plant Physiol Biochem 70:336–341. https://doi.org/10.1016/j.plaphy.2013.05.024
Acknowledgements
This work was funded by the Deutsche Forschungsgemeinschaft (DFG) to RR (Research Fellowship grant number RA 2030/1-1,2), and to CLH—a University of Otago Research Grant (UORG), Performance-Based Research (PBRF) funding from the Department of Botany, University of Otago and a Foundation for Research, Science and Technology (FRST) subcontract from the National Institute of Water and Atmospheric Research Ltd., Biodiversity and Biosecurity OBI (C01X0502). We thank Dr David Burritt for his help in providing facilities, and Dr Katja Schweikert for collecting the macroalgal material used in this study.
Funding
Open access funding provided by Norwegian Institute of Bioeconomy Research.
Author information
Authors and Affiliations
Contributions
RR designed the experiments and CLH supervised the research and provided the required equipment and facilities. RR performed the experiments and analysed the data. RR and CLH wrote the manuscript together. All authors read and approved the submitted manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rautenberger, R., Hurd, C.L. Photoprotection by photoinhibitory and PSII-reaction centre quenching controls growth of Ulva rigida (Chlorophyta) and is a pre-requisite for green tide formation. Planta 259, 111 (2024). https://doi.org/10.1007/s00425-024-04389-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-024-04389-z