Abstract
Main conclusion
Autophagy is involved in developmentally programmed cell death and is identified during the early development of phloem, as well as xylem with a dual role, as both an inducer and executioner of cell death.
Abstract
The regulation of primary and secondary development of roots and stems is important for the establishment of root systems and for the overall survival of trees. The molecular and cellular basis of the autophagic processes, which are used at distinct moments during the growth of both organs, is crucial to understand the regulation of their development. To address this, we use Populus trichocarpa seedlings grown in a rhizotron system to examine the autophagy processes involved in root and stem development. To monitor the visual aspects of autophagy, transmission electron microscopy (TEM) and immunolocalization of AuTophaGy-related protein (ATG8) enabled observations of the phenomenon at a structural level. To gain further insight into the autophagy process at the protein and molecular level, we evaluated the expression of ATG gene transcripts and ATG protein levels. Alternations in the expression level of specific ATG genes and localization of ATG8 proteins were observed during the course of root or stem primary and secondary development. Specifically, ATG8 was present in the cells exhibiting autophagy, during the differentiation and early development of xylem and phloem tissues, including both xylary and extraxylary fibers. Ultrastructural observations revealed tonoplast invagination with the formation of autophagic-like bodies. Additionally, the accumulation of autophagosomes was identifiable during the differentiation of xylem in both organs, long before the commencement of cell death. Taken together, these results provide evidence in support of the dual role of autophagy in developmental PCD. A specific role of the controller of cell death, which is a committed step with the release of hydrolytic enzymes from the vacuole and final digestion of protoplast, from which there is no return once initiated, is only attributed to mega-autophagy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autophagy, as an intracellular process responsible for the degradation of macromolecules, whole cytoplasmic compartments, and organelles, is perhaps best known for its participation in the process of eliminating dysfunctional, damaged, or toxic components from cells (Yoshimoto 2012). During several tissue development, autophagy can be engaged in proper vacuole biogenesis (Inoue et al. 2006; Yano et al. 2007). It occurs constitutively during formation of both cortical tissue (Yano et al. 2007) and sieve elements of phloem (Yang et al. 2015), but does not lead to cell death. The dual nature of autophagy causes on one hand the appropriate functioning of every living plant cell, but on the other hand it is also involved in initiating a program of cellular degradation during programmed cell death (PCD).
Programmed cell death occurs during the course of developmental processes (dPCD) and also in response to exogenous factors (Kacprzyk et al. 2011). Autophagy is a part of a highly regulated response to adapt to and survive adverse abiotic stress conditions (Han et al. 2011; Luo et al. 2017; Shangguan et al. 2018; Pillajo et al. 2018), biotic stresses (Liu et al. 2005; Hayward et al. 2009; Lenz et al. 2011a; Zhou et al. 2014), and senescence (Avila-Ospina et al. 2014; Sobieszczuk-Nowicka et al. 2018; Wojciechowska et al. 2018). It can occur in the whole organism, a specific tissue, or in single cells. The most relevant studies of autophagy during development concern xylogenesis, where structural and biochemical evidence of the role of dPCD in tracheary formation have been provided in Zinnia elegans suspension cultures that were forced to transdifferentiate into tracheary elements (Fukuda et al. 1993, 1998; Fukuda 1996, 2000, 2004; Kuriyama and Fukuda 2006) and later confirmed in planta for Arabidopsis (Avci et al. 2008; Kwon et al. 2010) and Populus (Courtois-Moreau et al. 2009; Bagniewska-Zadworna et al. 2012, 2014a). Only few studies on programmed cell semi-death of phloem sieve cells were performed in developing caryopsis of Titicum aestivum, indicating that selective autophagy can be involved in that developmental process which does not end with cell death (Wang et al. 2008; Yang et al. 2015). Other studies of the role of dPCD have been conducted in relation to leaf morphogenesis (Gunawardena et al. 2004; Wertman et al. 2012), male reproductive development in plants (Hanamata et al. 2014), and plant embryogenesis (Bozhkov et al. 2005; Di Berardino et al. 2018). As a result, the ultrastructural, physiological, and molecular changes associated with several developmental processes have been identified and described as being a typical autophagy connected with dPCD. Although it has been widely documented and described, the underlying mechanism of PCD has not been fully understood. Detailed evidence, however, with comparative studies for the role of autophagy in different plant organ development is lacking. Determining exactly when and how various components of autophagy may affect tissue development resulting from dPCD has not been documented.
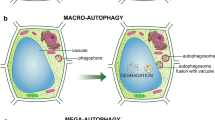
The conceptual framework of dPCD is complicated by the fact that three types of autophagy are distinguished in plants, and their frequently overlooked plurality may affect our understanding of organ development because such variation can be caused through several independent mechanisms. Microautophagy is characterized by the occurrence of small portions of cytoplasm that are sequestered in a vacuole through tonoplast invagination, forming autophagic bodies within the vacuole. During macroautophagy, larger compartments are engulfed within a double-membrane structure called an autophagosome, which later transports its contents and merges with a vacuole for completion of the degradative process. Lastly, mega-autophagy is characterized by the permeabilization or rupture of the tonoplast, resulting in the release of substantial amounts of hydrolytic enzymes from the vacuole and subsequent degradation of the general cytoplasm (van Doorn and Woltering 2005; van Doorn and Papini 2013). Typically, more than one type of autophagy can occur within the same cell, with mega-autophagy occurring after the two other types (Bagniewska-Zadworna et al. 2012, 2014c).
The plurality of autophagic processes is often overlooked. As a result, macroautophagy is the most frequently studied, especially in the context of selective autophagy of particular components and the maintenance of homeostasis in plant cells (Li and Vierstra 2012; Masclaux-Daubresse 2016; Masclaux-Daubresse et al. 2017; Yoshimoto and Ohsumi 2018). In plants, a principal characteristic of autophagy in PCD processes is its dual role as an initiator and executioner in PCD (Minina et al. 2014; Wojciechowska et al. 2018). Thus, its pro-survival and pro-death functions are recognized (Avila-Ospina et al. 2014; van Doorn and Woltering 2010). Previous studies have revealed the complexity of the molecular events underlying autophagosome formation (Han et al. 2011). Among genes encoding specific AuTophaGy (ATG)-related proteins, one subset, including ATG1 to ATG10, ATG12 to ATG14, and ATG16 to ATG18, is required, and the corresponding gene products are referred to as the core machinery for autophagosomes formation (Xie and Klionsky 2007; Han et al. 2011; Masclaux-Daubresse et al. 2017). The identification of many autophagy-related genes in plants has significantly enhanced our molecular understanding of the process of autophagy.
To date, formation and the occurrence of autophagic structures in plants were observed using transmission electron microscopy (TEM) (Bagniewska-Zadworna et al. 2012; van Doorn and Papini 2013) and were detected by the immunolocalization of the ATG8 protein, which functions in autophagosome assembly by binding autophagic membranes and enters the vacuole via active autophagy flux (Ryabovol and Minibayeva 2016; Li et al. 2018). ATG8 could also contribute to the diversification of selective autophagy pathways in plants (Kellner et al. 2017).
Advances in molecular technologies are providing opportunities to revisit autophagy functioning in plants. Studies combining both molecular and structural approaches in the study of autophagy in plants in a comparative manner, however, are relatively limited; especially with regard to the functioning of autophagy in developmental processes in different plant organs. Therefore, in the current study, the occurrence of autophagy during pioneer root and stem development in Populus trichocarpa was identified and analyzed, including several events related to primary and secondary root and stem development. To gain further insight into autophagy during root and shoot development in P. trichocarpa, TEM and immunolocalization of ATG8 were used at a structural level, in addition to protein and transcript characterization at a molecular level. Given that developmental studies of roots do not mirror analogous studies in stems, a comparison of their primary and secondary development was important to take into account. For the present study, the overall objective was to increase our knowledge of the role and function of autophagy in the development of different plant organs and tissues, not only connected with cell death.
Materials and methods
Plant material and experimental design
All experiments utilized P. trichocarpa (Torr. & A. Gray ex Hook.), grown from seeds at the Institute of Dendrology, Polish Academy of Sciences in Kórnik (52°14′40″N 17°06′27″E). Seeds were obtained from the FLORPAK Młynki Seeds Store, Poland. Seedlings were initially grown in a plant growth chamber (Conviron GR96) at 18 °C day/14 °C night and a 16 h day/8 h night photoperiod. After 3 months, plants were transferred into rhizotrons. The rhizotrons (30 × 50 cm) were constructed of two transparent polycarbonate plates held 2–3 cm apart by thick-walled plastic tubing to allow a wide growing space. Waterlogging was avoided by placement of a drainage hole in the bottom of the rhizotron, which permitted both soil aeration and drainage of excess water. The rhizotrons were placed in an underground chamber. Roots were grown in the clear-walled chambers that were filled with natural soil, while shoots were grown above the rhizotron in the air. Rhizotrons were installed in a semi-open, foil greenhouse to prevent flooding and heat stress. Pioneer roots in all of the experiments were divided into segments corresponding to their developmental stage as follows: 0–2 cm—root tips with apical meristem; 4–6 cm—primary development; and, 13–16 cm—secondary development. A similar classification of segments was used for stems: 0–2 cm—apical meristem with primary development; 15–20 cm—secondary development; and, 30–35 cm—isolated secondary xylem. Root tips were treated as a negative control for the process of xylogenesis, while isolated secondary xylem served as a positive control.
qRT-PCR analysis of gene expression
RNA isolation was performed with a Ribospin Plant kit (GeneAll Biotechnology Co., Ltd, Seoul, South Korea) according to the manufacturer’s recommendations. Synthesis of cDNA was performed using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Thermo Fisher Scientific Inc., Waltham, MA, USA) following the protocol supplied by the manufacturer. qRT-PCR was carried out using an SYBR Green Master Mix kit (Applied Biosystems) in a CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) using the following amplification program: denaturation by a hot start at 95 °C for 10 min, 40 cycles of a two-step program (denaturation at 95 °C for 15 and annealing/extension at 60 °C for 1 min). ACTIN, UBQ (ubiquitin) and 18S rRNA were selected as housekeeping genes and for normalization of expression values, because they exhibited the lowest sample to sample variation and high stable expression in all samples types and time points. The sequences of the gene-specific primer pairs utilized in the qRT-PCR analyses are listed in Table 1. ATG genes which change in expression in the organs studied were selected for further analyses. Data analyses were performed according to the previously described method (Bagniewska-Zadworna and Stelmasik 2015). The average cycle threshold (Ct) values of the reference genes were subtracted from the corresponding Ct value of each gene to obtain a Ct value, and the relative expression levels were calculated using the Ct method. All gene expression analyses were performed using three technical replicates of each of the three biological replicates of each experimental variant. Statistical analyses were performed using Statistica 10 software (StatSoft Poland Inc., Tulsa, OH, USA).
Protein extraction, gel electrophoresis, and immunoblot analysis
A phenol-based method was used for the extraction of total protein as described by Wojciechowska et al. (2018). Protein concentration was measured with a 2-D Quant Kit (GE Healthcare, Piscataway, NJ, USA) and proteins were separated by SDS-PAGE on 4–20% Mini-PROTEAN TGX precast gels (Bio-Rad), with an equal amount of protein (20 µg) in each lane. Transfer to PVDF membrane was performed using Trans-Blot® Turbo™ (Bio-Rad). Primary rabbit antibody—anti-ATG8 (AS14 2769, Agrisera, Vännäs, Sweden) was diluted 1:10,000 in 2% skimmed milk powder. Incubation with secondary antibodies was performed using antibody conjugated with horseradish peroxidase (HRP), goat, anti-rabbit (Agrisera) diluted 1:10,000 in 2% skimmed milk powder. The PVDF membrane was incubated with Clarity western ECL substrate chemiluminescent detection reagent (Bio-Rad) for 5 min prior to image registration in G-BOX CHEMI XR5 (Syngene, Cambridge, UK).
Immunofluorescence
Freshly collected pioneer root and stem segments were sectioned (30 µm) using a vibratome (Leica VT 1200S, Leica Biosystems, Nussloch, Germany) and immediately fixed for 1 h in 0.5% glutaraldehyde with 4% formaldehyde (Polysciences, Warrington, FL, USA) in 0.1 M PBS buffer. The material was rinsed in PBS and sections were blocked with 2% bovine serum albumin (BSA, Sigma) in PBS for 20 min. Subsequently, the material was treated with a primary ATG8 rabbit antibody that was diluted 1:1000 with 0.2% BSA/PBS, overnight at 4 °C. The samples were rinsed five times in PBS, and subsequently incubated at 36 °C for 1 h with poly-HRP-conjugated secondary antibody (Thermo Fisher Scientific, Carlsbad, CA, USA). A tyramine signal amplification (TSA) technique, as described by Wojciechowska et al. (2018), was also used to assess the localization of ATG8 protein due to its high level of sensitivity. After rinsing in PBS, the material was mounted in Prolong Gold (Life Technologies, Thermo Fisher Scientific). Lignin autofluorescence was monitored at the same time that immunolocalization was assessed. At least five root and stem segments were harvested from each developmental category for the analysis of the immunofluorescence of each antibody. The results were registered with a Leica TCS SP5 confocal microscope (Leica Biosystems) using lasers: 405 diode emitting light at wavelengths of 405 and argon emitting light at wavelengths 488 to observe fluorescence of lignins and ATG8, respectively. Two negative controls were performed, omitting primary antibody anti-ATG8 and without antibodies conjugated with HRP.
Transmission electron microscopy (TEM)
The root and stem samples were fixed in a mixture of 2% glutaraldehyde and 2% formaldehyde (pH 6.8) for 2 h at room temperature and overnight at 4 °C. The samples were subsequently rinsed three times with cacodylate buffer (0.05 M; pH 6.8, Polysciences) and postfixed with 1% osmium tetroxide for 2 h at room temperature. The fixed material was counterstained and embedded in low-viscosity Spurr’s resin as described by Bagniewska-Zadworna et al. (2012). Ultrathin sections (0.1 µm) were stained with uranyl acetate and lead citrate, and examined with an HT7700 transmission electron microscope (Hitachi, Tokyo, Japan), operating at an accelerating voltage of 100 kV. At least five root and stem segments from each developmental category were subjected to cytological analysis. An average of three copper grids per sample was examined using TEM.
Results
Expression of ATG genes at different stages of root and stem development
During the initial screening tests, ATG genes which change in expression in the organs studied were selected for further analyses. The expression analysis of ATG genes revealed significant differences between the expression profile in specific root and stem segments (Figs. 1 vs. 2). The expressions of ATG7, ATG8C, ATG8D, ATG8G, ATG8H, ATG11, and ATG18D were analyzed (Figs. 1, 2). Several characteristic profiles were observed related to the autophagy during root and stem development. Statistically significant differences in the expression of three ATG genes (ATG8C, ATG8D, ATG18D) were observed in pioneer roots along their development gradient (Fig. 1). Expression of these genes increased, relative to their expression in root tips, with primary and secondary tissue development (Fig. 1). Other genes exhibited a shift in their expression only in primary root (ATG7) or secondary root tissues (ATG11), while two genes did not show their expression increase (ATG8H, ATG8G) (Fig. 1). A slightly different pattern of expression was observed in stem tissues. In contrast to roots, the expression of all of the examined ATG genes (except ATG18D) exhibited up-regulation during secondary growth of the stem (Fig. 2). Only two genes (ATG8H, ATG18D) exhibited a shift in expression in isolated secondary xylem; while one was uniformly expressed (ATG11) in xylem tissues. The level of expression of other genes was significantly lower in xylem tissues (Fig. 2).
The relative expression of different ATG genes in P. trichocarpa root tips and pioneer roots during primary and secondary tissue development. The name of each gene is indicated at the bottom of the histogram. Means labeled with the same letter do not differ significantly according to a Tukey’s post hoc test (p < 0.05, ± SE)
The relative expression of different ATG genes in P. trichocarpa stems during primary and secondary tissue development, as well as in isolated secondary xylem tissues. The name of each gene is indicated at the bottom of the histogram. Means labeled with the same letter do not differ significantly according to a Tukey’s post hoc test (p < 0.05, ± SE)
Distribution and localization of ATG8 protein
Based on the significantly increased expression of ATG genes in both roots and stems along a developmental gradient, the amount and localization of ATG8 protein, which is necessary for appropriate autophagosome formation, were examined by immunoblot (Western blot) and immunolocalization analyses. The ATG8 protein can be detected either as a protein conjugated to phosphatidylethanolamine (PE) on an autophagosomal membrane or as a free protein without PE. The level of ATG8 protein in both pioneer roots and stems changed along the developmental gradient represented by the sampled segments (Fig. 3). Results indicated that the amount of ATG8 protein was highest in the root tips and the secondary tissues of roots and stems, as well as in isolated secondary xylem elements. The dominant form in root tips was free ATG8, whereas ATG8 protein conjugated to phosphatidylethanolamine (ATG8-PE) was predominant in secondary tissues of roots and stems (Fig. 3).
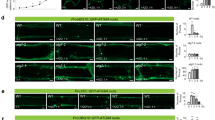
A strong fluorescent signal was observed in the primary tissues of pioneer roots arising from differentiating vascular tissues (phloem and xylem) in the stele (Fig. 4a–c). First, ATG8 was observed in developing primary xylem (Fig. 4a), later also a strong signal came from differentiating primary phloem (Fig. 4b, c). ATG8 in secondary roots was detected in the cambial zone and secondary phloem cells, as well as from fibers and secondary xylem elements differentiating from cambial cells. A stronger signal was observed in xylem cells that was closest to the cambium (Fig. 4d–f).
ATG8 in developing primary stems was located mainly close to and within developing vascular tissues (Fig. 5a–c). The strongest signal was observed in differentiating but not yet lignified extraxylary fibers, while the lowest signal was observed in parenchyma cells located in the center of the stem sections (Fig. 5a–c). A very strong ATG8 signal was detected in secondary stem tissues in the cambium, developing secondary phloem cells, including fibers, and developing xylem elements (Fig. 5d–f). A weaker, disappearing signal was detected along cell walls of the mature phloem. ATG8 protein was also localized in cells of the remaining primary xylem (Fig. 5d).
Intracellular localization of ATG8 revealed the strong signal in the protoplast of developing xylem vessels and fibers along cell wall with dots that revealed the presence of autophagosomes. Representative images are shown in Fig. 6.
Negative control reactions revealed an undetectable signal compared with the standard reactions (Fig. 7).
Ultrastructural observation
Given that ATG proteins were located close to or within xylem tissues, evidence of autophagy was studied in cells undergoing primary and secondary xylem differentiation. At the ultrastructural level, the cytology of autophagy appeared to be the same in all cells, regardless of the organ or process analyzed (Figs. 8, 9). Cytological analysis of the early stages of xylogenesis in roots and stems showed that all of the different types of autophagy were present. Microautophagy, identifiable by tonoplast invagination and autophagic bodies inside vacuoles, was frequently observed (Figs. 8a, b, 9a–d). Some of those invaginations, and subsequently autophagic bodies, contained cytoplasmic material, inclusions that were electron lucent, or multiple vesicles and membrane-built structures differing in size and shape. Many phagophores were evident (Fig. 8c), as well as the presence of autophagosomes in the cytoplasm (Figs. 8d, f, 9e), all of which are characteristic of macroautophagy. Vacuolization in differentiating xylem was also observed and was represented by a large central vacuole in most cases. At this stage of xylogenesis, evidence of micro- and macroautophagy was still observed, and the beginning stages of secondary cell wall development in root tracheary elements (Fig. 8f) and fibers (Fig. 9e) were also visible. In the last stage of xylogenesis, rupture of the tonoplast was observed (Fig. 9f), and due to the release of hydrolytic enzymes, other components of the protoplast appeared in various stages of digestion (mega-autophagy). At the completion of xylogenesis, functional xylem elements were observed (Fig. 9g).
Xylogenesis events in developing pioneer roots of Populus trichocarpa. a, b Microautophagy. Invagination of protoplast contents into vacuoles (arrows) and the formation of autophagic bodies (arrowheads). c, d Macroautophagy. Formation of a membrane structure (also called phagophore) and autophagosome. e–h Increasing vacuolization and secondary cell wall formation in developing xylem elements. AB autophagic-like bodies, AP autophagosomes, CW cell wall, Ph phagophore, V vacuole. Scale bars = 1 µm
Xylogenesis events in developing stems of Populus trichocarpa. a–d Microautophagy. Invagination of protoplast contents into vacuoles (arrows) and the formation of autophagic bodies at different stages of the maturation of tracheary elements and fibers. e Macroautophagy. Formation of autophagosomes present. f Mega-autophagic tonoplast rupture. g Mature xylem element without a protoplast. AB autophagic-like bodies, AP autophagosomes, CW cell wall, TE tracheary element, V vacuole. Scale bars = 1 µm
Discussion
In plants, autophagy is associated during developmental events with both formation and degradation (van Doorn and Woltering 2005), during the process of tissue and organ morphogenesis in ontogeny, and in response to biotic and abiotic stress (Kwon and Park 2008; Wang et al. 2016; Bozhkov 2018). Linking autophagy with plant development processes is not straightforward, and studies on the role of autophagy in plant organogenesis and histogenesis are limited. Research directed at understanding the regulation of cell death is valuable; however, no detailed comparisons of the cytological and molecular events associated with autophagy during development in below- and aboveground organs (roots vs. stems) have been conducted. Therefore, the present research was directed at elucidating the molecular and cellular events of autophagy involved in the regulation of the primary and secondary development of pioneer roots and stems in P. trichocarpa, growing in a rhizotron system.
Several cellular and molecular markers of autophagy were used to study the process of autophagy during pioneer root and stem development. An analysis of the expression of ATG genes during root and stem development revealed some common patterns. Two ATG8 genes (ATG8C, ATG8D) and ATG18D, among all of the examined ATG genes, exhibited a statistically significant change in their expression in both primary and secondary root segments, while ATG11 was highly expressed only in secondary roots. All of the ATG8 genes analyzed, as well as ATG11, were up-regulated during secondary development of stem tissues. However, only the expressions of ATG8H, as well as ATG11 and ATG18D, were also elevated in isolated secondary xylem, suggesting that these genes may play a role in dPCD during xylogenesis. Functional roles of some of these genes/proteins have been previously reported. ATG11 plays a critical role in phagophore assembly site (PAS) architecture and also appears to play a role in scaffolding the ATG1/13 complex to the PAS (Yorimitsu and Klionsky 2005). In general, ATG11 required for autophagy in Arabidopsis promotes autophagic vesicle delivery to the vacuole. It is also involved in selective autophagy during senescence, indicating that this accessory protein is required during developmentally induced nutrient recycling (Li et al. 2014). Given that in contrast to yeast, Arabidopsis ATG11 protein has an ATG17-like domain, it is plausible that it can function in both general and selective autophagy (Li et al. 2014; Yoshimoto and Ohsumi 2018). ATG18 is absolutely required for autophagosome formation in yeast (Suzuki et al. 2007). Similarly, in plants, Arabidopsis AtATG18a is likely required for proper autophagosome formation during nutrient starvation and senescence (Xiong et al. 2005). AtATG18 is also involved in the autophagy that occurs in response to oxidative stress in Arabidopsis root cells (Xiong et al. 2007).
The largest number of studies has been conducted on ATG8 proteins and the genes encoding them. In contrast to yeasts with only a single ATG8 gene, plants have multigene ATG8 family (Ryabovol and Minibayeva 2016). ATG8 is a key protein in the formation of autophagosomes and ATG8 antibodies can be used to identify autophagosomes, especially when it is fused to a fluorescent protein (Contento et al. 2005) or detected immunocytochemically (Wojciechowska et al. 2018). ATG8 protein can be present in two forms, free and conjugated to phosphatidylethanolamine (PE), participate in autophagosome biogenesis, and regulate the conjugation of ATG8 to PE and its localization to the PAS (Nair et al. 2012). In the present study, the ATG8-PE form, attached to the autophagosome membrane, was the dominant form of ATG8 during stem secondary development as well as in isolated xylem. Considering that PE positively regulates autophagy (Rockenfeller et al. 2015), the participation of autophagy in xylogenesis seems to be indisputable. The analysis revealed similar results for both primary and secondary root and stem tissues. Specifically, ATG8 was detected during the early development of xylem tracheary elements and xylary fibers, as well as in differentiating extraxylary fibers. These cells are obviously less lignified than other nearby cells that are already dead, as well as other functional target cells (Pesquet et al. 2001, 2013; Bagniewska-Zadworna et al. 2014a). A strong ATG8 signal was also observed in cambial tissue, indicating the role that autophagy plays in protoplast complete or partial degradation in developing xylem and phloem sieve cells, respectively. Cells in other tissues did not exhibit such a strong ATG8 signal, indicating that ATG8 protein during the primary and secondary development of roots and stems occurs mainly in cells that have a short life span. ATG7 was also up-regulated during the development of primary tissues in pioneer roots and secondary tissues in stems. ATG7-dependent autophagy has been demonstrated to constitute an “anti-death” (“pro-survival”) mechanism in plants that is used to control and contain the cell death that occurs during an immune response to fungal infection (Lenz et al. 2011b). Considering that all ATG8 (ATG8C, ATG8D, ATG8G, ATG8H) genes analyzed, as well as ATG7 and ATG11, were up-regulated during the development of secondary tissues in stems, and that ATG8H, ATG11, and ATG18D were especially up-regulated in isolated secondary xylem elements, they appear to represent ideal molecular markers for autophagy (dPCD) that are associated with developmental processes in plants. ATG8C, ATG8D, ATG11, and ATG18D were also up-regulated during the development of secondary tissues in pioneer roots. These results provide new insights for developing a better understanding of how autophagy is used to keep cells alive that are destined to become dead cells when the process of xylem differentiation and maturation is completed.
The current study also provides information on the histogenesis of other tissues, such as the differentiation of phloem tissue in both roots and stems. ATG8 was detected in both forms in the root tips of pioneer roots, with the free form of ATG8 being dominant. In developing roots, ATG8 can be involved in vacuole biogenesis in cortical parenchyma cells that develop from meristematic tissue, and in differentiating phloem tissues. The entire volume of cells is occupied by cytoplasm in meristematic tissues. These cytoplasmic components, however, are quickly degraded as cells mature and a large volume of parenchyma cells become occupied by a large central vacuole (Evert 2006a). Constitutive autophagy also plays a critical role in the formation of the central vacuole in maturing plant cells (Zouhar and Rojo 2009). Although the role of autophagy during vacuole biogenesis in plants has been documented (Marty 1999), studies utilizing atg mutants have indicated that autophagy may not be essential for vacuole biogenesis to occur (Doelling et al. 2002). Constitutive autophagy, however, was reported to occur during the differentiation of cortical root cells, along with the formation of an enlarged central vacuole (Yano et al. 2007). Similarly, in Arabidopsis and barley root tips, from meristematic to elongation zones, vacuolar autophagy occurs constitutively in these regions of cells (Inoue et al. 2006).
Mature phloem cells are also poor in organelles, and those that are present are mainly located in a peripheral strand of cytoplasm along the cell wall (Evert 2006b; Knoblauch and Peters 2010; Holbrook and Knoblauch 2018). This suggests that organelles are degraded during the differentiation of primary phloem cells from meristematic tissue. The mechanism underlying and responsible for this degradation is unknown. Selective autophagy, however, may possibly play a key role in this process (Evert 2006b). A selective autophagy-like process, similar to microautophagy, has been reported to be involved in the formation of sieve elements in phloem tissues of wheat (Wang et al. 2008). Detailed studies of autophagy during phloem differentiation and the involvement of autophagy in the development of phloem sieve cells, however, are still lacking. In this case, possible reorganization and reprogramming without cell death is possible, as suggested previously (Papini et al. 2014). In the present study, ATG8 protein was also localized in cambium and developing phloem elements in both of the organs analyzed. A weaker signal was detectable in mature phloem cells, usually along cell walls. A strong signal was localized in and close to differentiating fibers, similar to the signal level observed in xylary fibers. This result is consistent with the expected patterns, as all of these cells do not retain their protoplasts at maturity.
All of the different types of autophagy were observed at the ultrastructural level during the differentiation of xylem tracheary elements and fibers. As previously reported (Bagniewska-Zadworna et al. 2012), micro- and macroautophagy are the first apparent forms of autophagy that are visible in differentiating cells. During microautophagy, invaginations of the tonoplast containing protoplast contents protrude into vacuoles. Such structures were readily observed. During this type of autophagy, the contents within an invagination are typically hard to identify, except for membrane components in multivesicular bodies, which may be considered as a specific type of microautophagy (van Doorn and Papini 2013). Macroautophagy is a much more studied process. It is initiated by a double-membrane structure, a phagophore, which surrounds a large portion of cytoplasm; soon the ends of the phagophore close and the double-membrane structure is now called an autophagosome (Zhuang et al. 2018). Subsequently, the outer membrane of the autophagosome fuses with a vacuole and its contents are digested by hydrolases present in the vacuole. In the current study, autophagosomes were readily observed in most of the differentiating xylem cells that were analyzed, as well as in cells of differentiating root and stem tissues. In our observations of macroautophagy, both ‘tubule-like’ and ‘thin-line’ autophagosome formation structures, as described by van Doorn and Papini (2013), were observed and confirmed our previously reported observations in the present and previously described work (Bagniewska-Zadworna et al. 2012; Bagniewska-Zadworna and Arasimowicz-Jelonek 2016). The two types of observed autophagy, micro- and macro-autophagy, appear to play a role in delaying the final death of the cell until a secondary cell wall is sufficiently formed, which is required for the cells to fulfill their mechanical function of providing support while conducting water and nutrients. It is significant, however, that few subsequent phases can be distinguished in dPCD, such as the induction phase, which is dependent on specific inducing signals, the effector phase, during which cell components are degraded, the death phase, when the vacuole collapses and cell death occurs, and post-mortem autolysis (Avci et al. 2008; van Doorn 2011; Bagniewska-Zadworna et al. 2014a; Escamez and Tuominen 2014). A curious aspect of functional autophagy is that the transition between cell survival and cell death cannot be clearly demarcated. The loss of vacuolar integrity and the release of massive amount of hydrolases into the cytoplasm were observed in cells undergoing the last stage of xylogenesis (Avci et al. 2008). Similar observations were made in developing fibers, where autolysis was observed after secondary cell wall formation (Courtois-Moreau et al. 2009). Autophagy plays a role in extending the time needed to complete all of the necessary developmental processes, including lignification of cell walls, before a point of no return is reached. In some cases, however, tracheary elements can continue to lignify post-mortem (Pesquet et al. 2013). In these cases, the genes involved in lignification are expressed in neighboring cells and their encoded proteins, or end products, are transported into adjacent mature xylem cells (Bagniewska-Zadworna et al. 2014a, b). It seems that autophagy plays a dual role in cells, either promoting cell survival via degradation or initiating PCD when the level of degradation is too great and becomes irreversible (Minina et al. 2014). Although it sounds paradoxical, autophagy does not always accelerate cell death, but rather protects cells from death (Liu and Bassham 2012), even if it is only effective for a short period of time and allows cells to complete maturation before cell death is inevitable.
Conclusion
In the present study, the involvement of autophagy in the development of pioneer roots and stems was studied in detail, including the identification and analysis of the expression of ATG genes associated with autophagy. This is the first study to provide a detailed analysis of the level of ATG8 protein and its localization. Our analysis revealed the indispensable contribution of ATG8 to the proper functioning of autophagy during dPCD in both above- and below-ground organs of poplar. Knowledge concerning the patterns and underlying mechanisms of autophagy during xylogenesis can be used to obtain plants and trees with modified wood properties. Despite the tremendous advances being made in developmental research in plants, several key questions pertaining to the role of autophagy in histogenesis still remain to be elucidated. The most important of these questions pertain to the role of autophagy in the process of PCD in plants. Is autophagy only a harbinger of cell death, or can it also be used to postpone the moment of death until cell maturation is completed and a functional cell exists, such as in the maturation of xylem tracheary elements? A dual role for autophagy, as was observed in the present study, is based on the occurrence of different types of autophagy. In the case of xylogenesis, the role of the executioner should only be attributed to mega-autophagy, from which there is no return.
Continued research on plant developmental autophagy will allow us to better understand the functions of autophagy in dPCD, including the involvement of constitutive autophagy in vacuole biogenesis, as well as in the response of plants to changing growth conditions.
Author contributions
ABZ conceived the original concept and research plan, designed the experiments and oversaw the study; NW, IS, KMS, ABZ, and AZN conducted analyses; NW and ABZ analyzed the data; ABZ wrote the manuscript; all authors discussed the results, read, and approved the final version of the manuscript.
Abbreviations
- ATG/ATG:
-
Autophagy-related genes/proteins
- dPCD:
-
Developmentally programmed cell death
- TEM:
-
Transmission electron microscope
References
Avci U, Petzold HE, Ismail IO, Beers EP, Haigler CH (2008) Cysteine proteases XCP1 and XCP2 aid micro-autolysis within the intact central vacuole during xylogenesis in Arabidopsis roots. Plant J 56(2):303–315. https://doi.org/10.1111/j.1365-313X.2008.03592.x
Avila-Ospina L, Moison M, Yoshimoto K, Masclaux-Daubresse C (2014) Autophagy, plant senescence, and nutrient recycling. J Exp Bot 65(14):3799–3811. https://doi.org/10.1093/jxb/eru039
Bagniewska-Zadworna A, Arasimowicz-Jelonek M (2016) The mystery of underground death: cell death in roots during ontogeny and in response to environmental factors. Plant Biol 18(2):171–184. https://doi.org/10.1111/plb.12391
Bagniewska-Zadworna A, Stelmasik A (2015) Root heterogeneity and developmental stage determine the pattern of cellulose synthase and cinnamyl alcohol dehydrogenase gene expression profiles during xylogenesis in Populus trichocarpa (Torr. et Gray). Int J Plant Sci 176(5):458–467. https://doi.org/10.1086/681050
Bagniewska-Zadworna A, Byczyk J, Eissenstat DM, Oleksyn J, Zadworny M (2012) Avoiding transport bottlenecks in an expanding root system: xylem vessel development in fibrous and pioneer roots under field conditions. Am J Bot 99(9):1417–1426. https://doi.org/10.3732/ajb.1100552
Bagniewska-Zadworna A, Arasimowicz-Jelonek M, Smoliński D, Stelmasik A (2014a) New insight into pioneer root xylem development—evidence obtained from Populus trichocarpa plants grown under field conditions. Ann Bot 113(7):1235–1247. https://doi.org/10.1093/aob/mcu063
Bagniewska-Zadworna A, Barakat A, Łakomy P, Smoliński DJ, Zadworny M (2014b) Lignin and lignans in plant defence: insight from expression profiling of cinnamyl alcohol dehydrogenase during development following Populus plant infection by fungal pathogens. Plant Sci 229:111–121. https://doi.org/10.1016/j.plantsci.2014.08.015
Bagniewska-Zadworna A, Stelmasik A, Minicka J (2014c) From birth to death—Populus trichocarpa fibrous roots functional anatomy. Biol Plant 58(3):551–560. https://doi.org/10.1007/s10535-014-0433-6
Bozhkov PV (2018) Plant autophagy: mechanisms and functions. J Exp Bot 69(6):1281–1285. https://doi.org/10.1093/jxb/ery070
Bozhkov PV, Filonova LH, Suarez MF (2005) Programmed cell death in plant embryogenesis. In: Schatten GP (ed) Current topics in developmental biology, vol 67. Academic Press, San Diego, pp 135–179. https://doi.org/10.1016/s0070-2153(05)67004-4
Contento AL, Xiong Y, Bassham DC (2005) Visualization of autophagy in Arabidopsis using the fluorescent dye monodansylcadaverine and a GFP-AtATG8e fusion protein. Plant J 42(4):598–608. https://doi.org/10.1111/j.1365-313X.2005.02396.x
Courtois-Moreau CL, Pesquet E, Sjodin A, Muniz L, Bollhoner B, Kaneda M, Samuels L, Jansson S, Tuominen H (2009) A unique program for cell death in xylem fibers of Populus stem. Plant J 58(2):260–274. https://doi.org/10.1111/j.1365-313X.2008.03777.x
Di Berardino J, Marmagne A, Berger A, Yoshimoto K, Cueff G, Chardon F, Masclaux-Daubresse C, Reisdorf-Cren M (2018) Autophagy controls resource allocation and protein storage accumulation in Arabidopsis seeds. J Exp Bot 69(6):1403–1414. https://doi.org/10.1093/jxb/ery012
Doelling JH, Walker JM, Friedman EM, Thompson AR, Vierstra RD (2002) The APG8/12-activating enzyme APG7 is required for proper nutrient recycling and senescence in Arabidopsis thaliana. J Biol Chem 277(36):33105–33114. https://doi.org/10.1074/jbc.M204630200
Escamez S, Tuominen H (2014) Programmes of cell death and autolysis in tracheary elements: when a suicidal cell arranges its own corpse removal. J Exp Bot 65(5):1313–1321. https://doi.org/10.1093/jxb/eru057
Evert RF (2006a) Parenchyma and collenchyma. In: Esau’s plant anatomy. Meristems, cells and tissues of the plat body: their structure, function and development. Wiley-Interscience, Hoboken, pp 175–190
Evert RF (2006b) Phloem: cell types and developmental aspects. In: Esau’s plant anatomy. Meristems, cells and tissues of the plant body: their structure, function and development. Wiley-Interscience, Hoboken, pp 357–406
Fukuda H (1996) Xylogenesis: initiation, progression, and cell death. Annu Rev Plant Physiol Plant Mol Biol 47:299–325. https://doi.org/10.1146/annurev.arplant.47.1.299
Fukuda H (2000) Programmed cell death of tracheary elements as a paradigm in plants. Plant Mol Biol 44(3):245–253. https://doi.org/10.1023/A:1026532223173
Fukuda H (2004) Signals that control plant vascular cell differentiation. Nat Rev Mol Cell Biol 5(5):379–391. https://doi.org/10.1038/nrm1364
Fukuda H, Yoshimura T, Sato Y, Demura T (1993) In: Molecular mechanism of xylem differentiation. The Botanical Society of Japan, Tokyo, pp 93–107 (J Plant Res Special Issue 3)
Fukuda H, Watanabe Y, Kuriyama H, Aoyagi S, Sugiyama M, Yamamoto R, Demura T, Minami A (1998) Programming of cell death during xylogenesis. J Plant Res 111(1102):253–256
Gunawardena A, Greenwood JS, Dengler NG (2004) Programmed cell death remodels lace plant leaf shape during development. Plant Cell 16(1):60–73. https://doi.org/10.1105/tpc.016188
Han SJ, Yu BJ, Wang Y, Liu YL (2011) Role of plant autophagy in stress response. Protein Cell 2(10):784–791. https://doi.org/10.1007/s13238-011-1104-4
Hanamata S, Kurusu T, Kuchitsu K (2014) Roles of autophagy in male reproductive development in plants. Front Plant Sci 5:457. https://doi.org/10.3389/fpls.2014.00457
Hayward AP, Tsao J, Dinesh-Kumar SP (2009) Autophagy and plant innate immunity: defense through degradation. Semin Cell Dev Biol 20(9):1041–1047. https://doi.org/10.1016/j.semcdb.2009.04.012
Holbrook NM, Knoblauch M (2018) Editorial overview: Physiology and metabolism: Phloem: a supracellular highway for the transport of sugars, signals, and pathogens. Curr Opin Plant Biol 43:3–7. https://doi.org/10.1016/j.pbi.2018.05.013
Inoue Y, Suzuki T, Hattori M, Yoshimoto K, Ohsumi Y, Moriyasu Y (2006) AtATG genes, homologs of yeast autophagy genes, are involved in constitutive autophagy in Arabidopsis root tip cells. Plant Cell Physiol 47(12):1641–1652. https://doi.org/10.1093/pcp/pcl031
Kacprzyk J, Daly CT, McCabe PF (2011) The botanical dance of death: programmed cell death in plants. Adv Bot Res 60:169–261. https://doi.org/10.1016/B978-0-12-385851-1.00004-4
Kellner R, De la Concepcion JC, Maqbool A, Kamoun S, Dagdas YF (2017) ATG8 expansion: a driver of selective autophagy diversification? Trends Plant Sci 22(3):204–214. https://doi.org/10.1016/j.tplants.2016.11.015
Knoblauch M, Peters WS (2010) Munch, morphology, microfluidics—our structural problem with the phloem. Plant, Cell Environ 33(9):1439–1452. https://doi.org/10.1111/j.1365-3040.2010.02177.x
Kuriyama F, Fukuda H (2006) Analysis of mechanisms of xylem cell differentiation by transgene expression in Zinnia elegans L. cultured cells. Plant Cell Physiol 47:S247–S247
Kwon SI, Park O (2008) Autophagy in plants. J Plant Biol 51(5):313–320. https://doi.org/10.1007/BF03036132
Kwon SI, Cho HJ, Jung JH, Yoshimoto K, Shirasu K, Park OK (2010) The Rab GTPase RabG3b functions in autophagy and contributes to tracheary element differentiation in Arabidopsis. Plant J 64(1):151–164. https://doi.org/10.1111/j.1365-313X.2010.04315.x
Lenz HD, Haller E, Melzer E, Kober K, Wurster K, Stahl M, Bassham DC, Vierstra RD, Parker JE, Bautor J, Molina A, Escudero V, Shindo T, van der Hoorn RAL, Gust AA, Nuernberger T (2011a) Autophagy differentially controls plant basal immunity to biotrophic and necrotrophic pathogens. Plant J 66(5):818–830. https://doi.org/10.1111/j.1365-313X.2011.04546.x
Lenz HD, Vierstra RD, Nürnberger T, Gust AA (2011b) ATG7 contributes to plant basal immunity towards fungal infection. Plant Signal Behav 6(7):1040–1042. https://doi.org/10.4161/psb.6.7.15605
Li FQ, Vierstra RD (2012) Autophagy: a multifaceted intracellular system for bulk and selective recycling. Trends Plant Sci 17(9):526–537. https://doi.org/10.1016/j.tplants.2012.05.006
Li F, Chung T, Vierstra RD (2014) AUTOPHAGY-RELATED11 plays a critical role in general autophagy- and senescence-induced mitophagy in Arabidopsis. Plant Cell 26(2):788–807. https://doi.org/10.1105/tpc.113.120014
Li KX, Liu YN, Yu BJ, Yang WW, Yue JY, Wang HZ (2018) Monitoring autophagy in wheat living cells by visualization of fluorescence protein-tagged ATG8. Plant Cell Tiss Org Cult 134(3):481–489. https://doi.org/10.1007/s11240-018-1437-2
Liu YM, Bassham DC (2012) Autophagy: pathways for self-eating in plant cells. Annu Rev Plant Biol 63:215–237. https://doi.org/10.1146/annurev-arplant-042811-105441
Liu Y, Schiff M, Czymmek K, Talloczy Z, Levine B, Dinesh-Kumar SP (2005) Autophagy regulates programmed cell death during the plant innate immune response. Cell 121(4):567–577. https://doi.org/10.1016/j.cell.2005.03.007
Luo LM, Zhang PP, Zhu RH, Fu J, Su J, Zheng J, Wang ZY, Wang D, Gong QQ (2017) Autophagy is rapidly induced by salt stress and is required for salt tolerance in Arabidopsis. Front Plant Sci 8:1459. https://doi.org/10.3389/fpls.2017.01459
Marty F (1999) Plant vacuoles. Plant Cell 11(4):587–599. https://doi.org/10.1105/tpc.11.4.587
Masclaux-Daubresse C (2016) Autophagy controls carbon, nitrogen, and redox homeostasis in plants. Autophagy 12(5):896–897. https://doi.org/10.4161/auto.36261
Masclaux-Daubresse C, Chen QW, Have M (2017) Regulation of nutrient recycling via autophagy. Curr Opin Plant Biol 39:8–17. https://doi.org/10.1016/j.pbi.2017.05.001
Minina EA, Bozhkov PV, Hofius D (2014) Autophagy as initiator or executioner of cell death. Trends Plant Sci 19(11):692–697. https://doi.org/10.1016/j.tplants.2014.07.007
Nair U, Yen W-L, Mari M, Cao Y, Xie Z, Baba M, Reggiori F, Klionsky DJ (2012) A role for Atg8–PE deconjugation in autophagosome biogenesis. Autophagy 8(5):780–793. https://doi.org/10.4161/auto.19385
Papini A, Mosti S, van Doorn WG (2014) Classical macroautophagy in Lobivia rauschii (Cactaceae) and possible plastidial autophagy in Tillandsia albida (Bromeliaceae) tapetum cells. Protoplasma 251(3):719–725. https://doi.org/10.1007/s00709-013-0567-y
Pesquet E, Pichon M, Pineau C, Ranocha P, Digonnet C, Jauneau A, Boudet AM, Fukuda H, Demura T, Goffner D (2001) Xylem formation and lignification in trees and model species. In: Morohoshi N, Komamine A (eds) Molecular breeding of woody plants, Proceedings, vol 18. Progress Biotechnology. Elsevier Science, Amsterdam, pp 11–18
Pesquet E, Zhang B, Gorzsas A, Puhakainen T, Serk H, Escamez S, Barbier O, Gerber L, Courtois-Moreau C, Alatalo E, Paulin L, Kangasjarvi J, Sundberg B, Goffner D, Tuominen H (2013) Non-cell-autonomous postmortem lignification of tracheary elements in Zinnia elegans. Plant Cell 25(4):1314–1328. https://doi.org/10.1105/tpc.113.110593
Pillajo JOQ, Chapin LJ, Jones ML (2018) Senescence and abiotic stress induce expression of autophagy-related genes in Petunia. J Am Soc Hort Sci 143(2):154–163. https://doi.org/10.21273/jashs04349-18
Rockenfeller P, Koska M, Pietrocola F, Minois N, Knittelfelder O, Sica V, Franz J, Carmona-Gutierrez D, Kroemer G, Madeo F (2015) Phosphatidylethanolamine positively regulates autophagy and longevity. Cell Death Differ 22:499. https://doi.org/10.1038/cdd.2014.219
Ryabovol VV, Minibayeva FV (2016) Molecular mechanisms of autophagy in plants: role of ATG8 proteins in formation and functioning of autophagosomes. Biochemistry (Moscow) 81(4):348–363. https://doi.org/10.1134/S0006297916040052
Shangguan LF, Fang X, Chen LD, Cui LW, Fang JG (2018) Genome-wide analysis of autophagy-related genes (ARGs) in grapevine and plant tolerance to copper stress. Planta 247(6):1449–1463. https://doi.org/10.1007/s00425-018-2864-3
Sobieszczuk-Nowicka E, Wrzesiński T, Bagniewska-Zadworna A, Kubala S, Rucińska-Sobkowiak R, Polcyn W, Misztal L, Mattoo AK (2018) Physio-genetic dissection of dark-induced leaf senescence and timing its reversal in barley. Plant Physiol 178:654–671. https://doi.org/10.1104/pp.18.00516
Suzuki K, Kubota Y, Sekito T, Ohsumi Y (2007) Hierarchy of Atg proteins in pre-autophagosomal structure organization. Genes Cells 12(2):209–218. https://doi.org/10.1111/j.1365-2443.2007.01050.x
van Doorn WG (2011) Classes of programmed cell death in plants, compared to those in animals. J Exp Bot 62(14):4749–4761. https://doi.org/10.1093/jxb/err196
van Doorn WG, Papini A (2013) Ultrastructure of autophagy in plant cells: a review. Autophagy 9(12):1922–1936. https://doi.org/10.4161/auto.26275
van Doorn WG, Woltering EJ (2005) Many ways to exit? Cell death categories in plants. Trends Plant Sci 10(3):117–122. https://doi.org/10.1016/j.tplants.2005.01.006
van Doorn WG, Woltering EJ (2010) What about the role of autophagy in PCD? Trends Plant Sci 15(7):361–362. https://doi.org/10.1016/j.tplants.2010.04.009
Wang L, Zhou Z, Song X, Li J, Deng X, Mei F (2008) Evidence of ceased programmed cell death in metaphloem sieve elements in the developing caryopsis of Triticum aestivum L. Protoplasma 234(1–4):87–96. https://doi.org/10.1007/s00709-008-0023-6
Wang W, Xu M, Wang G, Galili G (2016) Autophagy: an important biological process that protects plants from stressful environments. Front Plant Sci 7:2030. https://doi.org/10.3389/fpls.2016.02030
Wertman J, Lord CEN, Dauphinee AN, Gunawardena A (2012) The pathway of cell dismantling during programmed cell death in lace plant (Aponogeton madagascariensis) leaves. BMC Plant Biol 12:16. https://doi.org/10.1186/1471-2229-12-115
Wojciechowska N, Marzec-Schmidt K, Kalemba EM, Zarzyńska-Nowak A, Jagodziński AM, Bagniewska-Zadworna A (2018) Autophagy counteracts instantaneous cell death during seasonal senescence of the fine roots and leaves in Populus trichocarpa. BMC Plant Biol 18(1):260. https://doi.org/10.1186/s12870-018-1439-6
Xie ZP, Klionsky DJ (2007) Autophagosome formation: core machinery and adaptations. Nat Cell Biol 9(10):1102–1109. https://doi.org/10.1038/ncb1007-1102
Xiong Y, Contento AL, Bassham DC (2005) AtATG18a is required for the formation of autophagosomes during nutrient stress and senescence in Arabidopsis thaliana. Plant J 42(4):535–546. https://doi.org/10.1111/j.1365-313X.2005.02397.x
Xiong Y, Contento AL, Nguyen PQ, Bassham DC (2007) Degradation of oxidized proteins by autophagy during oxidative stress in Arabidopsis. Plant Physiol 143(1):291–299. https://doi.org/10.1104/pp.106.092106
Yang W, Cai J, Zhou Z, Zhou G, Mei F, Wang L (2015) Microautophagy involves programmed cell semi-death of sieve elements in developing caryopsis of Triticum aestivum L. Cell Biol Int 39:1364–1375. https://doi.org/10.1002/cbin.10512
Yano K, Suzuki T, Moriyasu Y (2007) Constitutive autophagy in plant root cells. Autophagy 3(4):360–362
Yorimitsu T, Klionsky DJ (2005) Atg11 links cargo to the vesicle-forming machinery in the cytoplasm to vacuole targeting pathway. Mol Biol Cell 16(4):1593–1605. https://doi.org/10.1091/mbc.e04-11-1035
Yoshimoto K (2012) Beginning to understand autophagy, an intracellular self-degradation system in plants. Plant Cell Physiol 53(8):1355–1365. https://doi.org/10.1093/pcp/pcs099
Yoshimoto K, Ohsumi Y (2018) Unveiling the molecular mechanisms of plant autophagy-from autophagosomes to vacuoles in plants. Plant Cell Physiol 59(7):1337–1344. https://doi.org/10.1093/pcp/pcy112
Zhou J, Yu J-Q, Chen Z (2014) The perplexing role of autophagy in plant innate immune responses. Mol Plant Pathol 15(6):637–645. https://doi.org/10.1111/mpp.12118
Zhuang XH, Chung KP, Luo MQ, Jiang LW (2018) Autophagosome biogenesis and the endoplasmic reticulum: a plant perspective. Trends Plant Sci 23(8):677–692. https://doi.org/10.1016/j.tplants.2018.05.002
Zouhar J, Rojo E (2009) Plant vacuoles: where did they come from and where are they heading? Curr Opin Plant Biol 12(6):677–684. https://doi.org/10.1016/j.pbi.2009.08.004
Acknowledgements
This work was supported by the Grant no. 2012/07/E/NZ9/00194 from the National Science Centre, Poland. The Authors would like to thank Dr. Marcin Zadworny for help with confocal microscopy.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wojciechowska, N., Smugarzewska, I., Marzec-Schmidt, K. et al. Occurrence of autophagy during pioneer root and stem development in Populus trichocarpa. Planta 250, 1789–1801 (2019). https://doi.org/10.1007/s00425-019-03265-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-019-03265-5