Abstract
There is some evidence for temperature-dependent stimulation of mitochondrial biogenesis; however, the role of elevated muscle temperature during exercise in mitochondrial adaptation to training has not been studied in humans in vivo. The purpose of this study was to determine the role of elevating muscle temperature during exercise in temperate conditions through the application of mild, local heat stress on mitochondrial adaptations to endurance training. Eight endurance-trained males undertook 3 weeks of supervised cycling training, during which mild (~ 40 °C) heat stress was applied locally to the upper-leg musculature of one leg during all training sessions (HEAT), with the contralateral leg serving as the non-heated, exercising control (CON). Vastus lateralis microbiopsies were obtained from both legs before and after the training period. Training-induced increases in complex I (fold-change, 1.24 ± 0.33 vs. 1.01 ± 0.49, P = 0.029) and II (fold-change, 1.24 ± 0.33 vs. 1.01 ± 0.49, P = 0.029) activities were significantly larger in HEAT than CON. No significant effects of training, or interactions between local heat stress application and training, were observed for complex I–V or HSP70 protein expressions. Our data provides partial evidence to support the hypothesis that elevating local muscle temperature during exercise augments training-induced adaptations to mitochondrial enzyme activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exercise training can increase mitochondrial volume density, oxidative capacity, and enzyme content and activity [8, 15, 39]. Physiological stresses induced by exercise transiently increase the expression of proteins coordinating these mitochondrial adaptations, via activation of existing proteins and mRNA expression [14]. Exercise stresses thought to influence signalling related to mitochondrial adaptations include cytosolic adenosine monophosphate (AMP) and Ca2+ accumulation, oxidative stress, and substrate depletion [18, 30, 32, 36, 44]. Increased muscle temperature is a ubiquitous exercise stress [6], but the role of transient exercise-induced elevations in muscle temperature on mitochondrial adaptation in response to chronic endurance training is not known [12].
Temperature-dependent stimulation of mitochondrial biogenesis has been observed in in vitro models [23], animal models of post-exercise passive heat stress [41], and following repeated bouts of passive locally-applied heat stress in humans [10]. In endurance-trained males, we recently reported 3 weeks of endurance training performed under moderate whole-body heat stress (33 °C) increased maximal vastus lateralis citrate synthase activity, whereas matched training in 18 °C did not [28]. It is possible greater muscle temperature during training sessions in 33 °C contributed to the observed adaptive effects [3]. Increased muscle temperature during exercise increases glycogenolysis [5, 40], which may increase 5′AMP-activated protein kinase (AMPK) activation through reduced inhibition of AMPK via the glycogen-binding domain [17, 30]. Similarly, heat stress has been shown to upregulate heat shock protein 70 (HSP70) expression [10, 41], which promotes mitochondrial biogenesis [13] via the role of HSP70 as a molecular chaperone [46]. Indeed, locally applied heat stress during acute exercise promoted mitochondrial transcription factor A (TFAM) mRNA expression, and TFAM is involved in the coordination of mitochondrial biogenesis [35]. Accordingly, undertaking chronic exercise training with heat stress applied locally to working muscle to elevate muscle temperature above typical exercising values may promote mitochondrial biogenesis, but this has not yet been studied.
Therefore, the primary aim of the present investigation was to determine if elevating muscle temperature during exercise through the application of mild, local heat stress would promote adaptations to mitochondrial protein content in response to endurance training. We hypothesised that elevating local muscle temperature during exercise would augment training-induced increases in mitochondrial content, and that this would occur alongside greater skeletal muscle HSP70 accumulation.
Methods
Ethical approval
This study was performed in accordance with the standards of the Declaration of Helsinki, 2013. The Auckland University of Technology Ethics Committee approved all procedures in the human studies (21/170), and all participants provided written informed consent prior to participation. This study was not registered in a database. Data associated with this study are available from the corresponding author upon reasonable request.
Participants
Eight recreationally-trained males regularly engaged in aerobic exercise such as running and/or cycling took part in the study (age, 30 ± 8 years; height, 182 ± 5 cm; body mass, 76.7 ± 9.6 kg; first ventilatory threshold, 198 ± 28 W; second ventilatory threshold, 240 ± 29 W; peak oxygen uptake [V̇O2peak], 4.1 ± 0.5 L.min−1). All participants were healthy, with no recent viral illness or musculoskeletal injury (< 3 months). Male and female participants were eligible to participate; however, only male participants volunteered. The achieved sample size was limited by COVID-19 restrictions. At N = 8, we had 50% power to detect a large magnitude effect (d = 0.8) with a type I error rate of 0.05 using a two-tailed test.
Study design
This investigation used a longitudinal, contralateral leg design (Fig. 1). Participants initially reported to the laboratory twice during the pre-intervention week, once for an incremental exercise test to determine training zones and once for resting vastus lateralis microbiopsies. Subsequently, participants undertook 3 weeks of supervised exercise training in the laboratory in temperate conditions (~ 18 °C), during which mild local heat stress (~ 40 °C) was applied to the upper-leg musculature of one leg during all training sessions (HEAT), with the contralateral leg serving as the non-heated, exercising control (CON). Participants were randomised and counterbalanced to receive the local heat stress intervention to either the dominant or non-dominant leg. Following the training intervention, the pre-intervention assessments were repeated.
Incremental exercise test
Participants initially reported to the laboratory at ~ 07:00 following a 10-h overnight fast, having refrained from vigorous exercise and alcohol for 24 h. After providing written, informed consent, height and body mass were recorded. Participants subsequently undertook an incremental cycling test on an electromagnetically braked ergometer (Excalibur Sport, Lode, Groningen, NET). A heart rate (HR) monitor was fitted for continuous collection of HR data (TICKR, Wahoo, Taiwan), and expired gases were collected throughout (TrueOne2400, ParvoMedics, Sandy, UT, USA). The test began at 95 W, with the work rate increasing by 35 W every 3 min until the respiratory exchange ratio exceeded 1.0 and attainment of the second ventilatory threshold (VT2) was confirmed, after which the work rate increased by 35 W every minute until task failure. The V̇O2peak was identified as the highest 15-s average V̇O2. The first ventilatory threshold (VT1) was identified as the V̇O2 at which a systematic rise in V̇E.V̇O2−1 occurred, and VT2 was identified as the V̇O2 at which a systematic rise in V̇E.V̇CO2−1 occurred. The V̇O2 at VT1 and VT2 were converted to power output by linear fit of the power output vs. V̇O2 relationship, using the last minute of V̇O2 data from each 3-min stage. The HR associated with VT1 and VT2 was then estimated by linear fit of the power output vs. HR relationship, using the last 30 s of HR data from each 3-min stage. The last minute of expired gas data in each 3-min stage was used to determine whole-body fat oxidation rates through standard calculations [19]. The highest observed rate of whole-body fat oxidation was accepted as the peak fat oxidation rate (PFO), as per our recent work [27].
Resting vastus lateralis microbiopsy
Approximately 48 h following the incremental exercise test, participants returned to the laboratory at ~ 07:00 having recorded their dietary intake for 48 h, including that morning’s breakfast. A resting vastus lateralis sample was obtained from both legs via the microbiopsy technique. Local anaesthesia was applied to the skin and superficial muscle fascia, after which a microbiopsy needle was inserted into the mid-belly of the vastus lateralis to a depth of ~ 2 cm to recover ~ 20 mg of tissue using a spring-loaded mechanism (14G Ultimate Biopsy Needle, Zamar Care, Croatia). Muscle tissue was immediately frozen using dry ice, and stored at − 80 °C until further analysis. The precise location of the biopsy site was recorded relative to the line between the greater trochanter and tibial tuberosity, such that the same site could be used for the post-intervention sample.
Training intervention
Approximately 72 h following the pre-intervention resting vastus lateralis microbiopsy, participants commenced a supervised, 3-week, 15-session, two-legged cycle training intervention, involving five training sessions performed each week (Table 1). During all training sessions, mild local heat stress (~ 40 °C) was applied to the upper-leg muscles of one leg (HEAT) using a wired heat pad with integrated heating element (VXHP-01, Vidawell, Auckland, NZ). The contralateral leg served as the non-heated, exercising control (CON). This method was designed to elevate the vastus lateralis of the heated leg ~ 2 °C above the vastus lateralis of the non-heated leg, whilst exposing the tissues to the same systemic environment and mechanical training stress. Participants were randomised and counterbalanced to receive local heat stress to either their dominant or non-dominant leg, which was determined prior to the first session by asking ‘If you were to shoot a ball at a target, which leg would you use to kick the ball?’ [42]. The dominant leg was considered as the kicking leg.
During training sessions, HR and power output were recorded continuously. Training was undertaken on participants’ own road bicycles mounted to a direct-drive indoor trainer (Kickr v5, Wahoo Fitness, Atlanta, USA). Additionally, the temperature of the vastus lateralis of both legs was estimated using the insulated skin temperature technique [7]. Briefly, a skin temperature thermistor was taped over the vastus lateralis ~ 15 cm above the patella and covered by a 6-mm-thick insulative neoprene layer. Vastus lateralis muscle temperature (Tmus) was subsequently estimated using validated equations [7] (Eq. 1):
where Tins is insulated skin temperature over the vastus lateralis, TinsLag2 = Tins − Tins two min beforehand, etc.
Enzyme activities
For analysis of citrate synthase (CS) and complex I–V activities, ~ 10 mg of frozen muscle was cut and rinsed using cold phosphate-buffered saline (PBS) and then suspended to ~ 25 mg.mL−1 in PBS. Samples were then ground manually and thoroughly using a pre-cooled Dounce homogeniser. Homogenate was solubilised in extraction buffer (ab260490, Abcam®) to ~ 5 mg.mL−1 and incubated on ice for 20 min prior to centrifugation at 16,000 g for 10 min at 4 °C. Supernatant was extracted and stored at − 80 °C prior to further analysis. Supernatant was thawed and kinetic immunocapture assays for CS (ab119692, Abcam®; coefficient of variation [CV], 11.4%), complex I (ab109721, Abcam®; CV, 12.1%), complex II (ab228560, Abcam®, CV; 14.1%), complex III (ab287844, Abcam®; CV, 13.4%), complex IV (ab109909, Abcam®; CV, 2.3%), and complex V (ab109714, Abcam®; CV, 5.9%) activities were performed in duplicate. Enzyme activities were expressed relative to sample protein concentration using a Bradford assay (ab102535, Abcam®; CV, 6.1%). Assays were performed on a spectrophotometer (Multiskan GO, ThermoFisher Scientific Inc., Porto Salvo, POR).
Immunoblotting
For analysis of complex I–V and HSP70 protein expression, the remaining ~ 10 mg of frozen muscle was cut and rinsed using cold PBS and then suspended in RIPA buffer (20 µL.mg−1 muscle) made with protease inhibitor cocktail (ab65621, Abcam®, 20 μL.mL−1) and PMSF (ab141032, Abcam®, 5 μL.mL−1), and PBS (20 μL.mg−1). Samples were then ground manually and thoroughly using a pre-cooled Dounce homogeniser. Homogenate was extracted and incubated on ice for 20 min prior to centrifugation at 16,000 g for 10 min at 4 °C. Supernatants was extracted and stored at − 80 °C prior to further analysis.
Total protein concentration was determined using a BCA-protein kit (Pierce BCA protein assay Kit, Thermo Fisher Scientific) with bovine serum albumin (BSA) as a standard. Sample aliquots containing ~ 5 µg protein suspended in 1 × Laemmli buffer (0.5 M Tris/HCl, pH 6.8, 800 mM 2-mercaptoethanol, 2 mM EGTA, 10% glycerol, 2% SDS, 0.25% Bromophenol Blue) were resolved by SDS-PAGE using 12.5% hand cast gels and transferred to PVDF membranes (Trans-Blot Turbo transfer pack, Bio-Rad Laboratories) using a Trans-Blot Turbo transfer system (Bio-Rad Laboratories). Membranes were blocked using 2% fish gelatine in Tris buffered saline-0.1% Tween-20 (TBST) for 2 h, then washed in TBST for 15 min, and incubated overnight at 4 °C in primary antibodies diluted in TBST with 3% w/v BSA. Membranes were probed for HSP70 (1:500, H5147, Sigma-Aldrich) and the five oxidative phosphorylation complex subunits using an antibody cocktail (1:2000, ab110411, Abcam). After the removal of primary antibody, the membranes were washed in TBST for 15 min and then incubated for 2 h in horseradish peroxidase-conjugated secondary antibody diluted in TBST with 2% fish gelatine. A ChemiDoc MP Imaging System (Bio-Rad Laboratories) was used to capture the images in the presence of chemiluminescent substrate (Clarity Western Substrate; Bio-Rad Laboratories). Bands were quantified using ImageLab 6.1 software (Bio-Rad Laboratories). To adjust for protein loading, bands of interest were standardised by probing for glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:5000, ab9485, Abcam). To control for variation between gels, a pooled sample was loaded in every gel and all target proteins are quantified relative to the pooled sample.
Statistical analysis
Data is expressed as mean ± standard deviation. Statistical analysis was performed with GraphPad Prism Version 9.3.1 (GraphPad Software, San Diego, CA, USA). Normality of datasets was assessed using the Shapiro–Wilk test. Pre- and post-intervention V̇O2peak, VT1, VT2, and PFO were compared using paired t-tests. The effect of local heat stress application during the first of each training session type on estimated Tmus was assessed using a two-way analysis of variance with repeated measures. The effect of local heat stress application during training on skeletal muscle variables was assessed using two-way analyses of variance with repeated measures. Training-induced changes in skeletal muscle variables are expressed as fold changes and compared between legs using two-tailed paired t-tests (or non-parametric equivalents). Significance was accepted when P ≤ 0.05.
Results
Mean estimated Tmus of CON and HEAT was calculated for the first of each session type (Fig. 2a). A main effect of local heat stress application was observed, whereby mean estimated Tmus was greater in HEAT than CON (38.3 ± 0.4 vs. 35.7 ± 0.9 °C, mean difference 2.5 ± 0.8 °C, P < 0.0001), with no main effect of session type (P = 0.562) or interaction between local heat stress application and session type (P = 0.148). Increased V̇O2peak (53 ± 7 vs. 55 ± 7 mL.kg−1.min−1, P = 0.01, Fig. 2b), VT1 (192 ± 28 vs. 217 ± 27 W, P = 0.0001, Fig. 2c), and VT2 (240 ± 29 vs. 260 ± 29 W, P = 0.003, Fig. 2d) was observed post-intervention, with no effect on PFO (0.40 ± 0.16 vs. 0.44 ± 0.09 g.min−1, P = 0.615, Fig. 2e). Body mass was unchanged post-intervention (76.7 ± 9.6 vs. 76.4 ± 9.2 kg, P = 0.507).
Verification of the effectiveness of the local heat stress and training intervention. a Mean estimated vastus lateralis temperature (Tmus) in the control (CON) and heated (HEAT) leg during the first threshold, moderate, heavy, and severe-intensity training session. b Peak oxygen uptake (V̇O2peak), c first ventilatory threshold (VT1), d second ventilatory threshold (VT2), and e peak fat oxidation rate (PFO) before (PRE, week 1) and after (POST, week 5) the training intervention. One post-intervention datapoint for PFO was missed due to a technical error. The symbol ‘**’ indicates P < 0.01, and the symbol ‘****’ indicates P < 0.0001
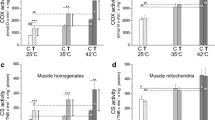
No significant between-limb differences in enzyme activity were observed pre-intervention (in all cases, P > 0.100). An interaction between local heat stress application and training was not observed for CS activity (P = 0.059). The training-induced increase in CS activity was not significantly larger in HEAT than CON (fold-change, 1.47 ± 0.62 vs. 1.23 ± 0.45, P = 0.068, Fig. 3a). An interaction between local heat stress application and training for complex I activity was observed (P = 0.012). The training-induced increase in complex I activity was larger in HEAT than CON (fold-change, 1.24 ± 0.33 vs. 1.01 ± 0.49, P = 0.029, Fig. 3b). An interaction between local heat stress application and training was not observed for complex II activity (P = 0.222). However, the training-induced increase in complex II activity was greater in HEAT vs. CON (fold-change, 1.73 ± 0.73 vs. 1.23 ± 0.59, P = 0.042, Fig. 3c). Interactions between local heat stress application and training, and differences in training-induced increases between CON and HEAT, were not observed for complex III, IV, or V activity (Fig. 3d–f). No significant effects of training, or interactions between local heat stress application and training, were observed for complex I–V or HSP70 protein expressions (Fig. 4).
Mitochondrial enzyme activity adaptations to three weeks of bilateral cycling training in the vastus lateralis of a control leg (CON) and a leg exposed to mild, local heat stress during all training sessions (HEAT). a Citrate synthase, b complex I, c complex II, d complex III, e complex IV, f complex V. Bars indicate means and dots indicate individual values, linked for each participant. The symbol ‘*’ denotes P ≤ 0.05
Mitochondrial protein expression adaptations to three weeks of bilateral cycling training in the vastus lateralis of a control leg (CON) and a leg exposed to mild, local heat stress during all training sessions (HEAT). a complex I, b complex II, c complex III, d complex IV, e complex V, and f heat shock protein 70 (HSP70). Bars indicate means and dots indicate individual values, linked for each participant. g Representative immunoblot
Discussion
The primary aim of the present investigation was to determine the effect of elevating muscle temperature during exercise through application of mild, local heat stress on adaptations to mitochondrial protein content in response to endurance training. Our main observations were that the application of mild, local heat stress during cycling training: (i) elicited significantly greater adaptations to complex I and II activities, but not complex III-V or citrate synthase activity, (ii) did not impact mitochondrial protein expression, and (iii) did not impact HSP70 expression. Our data therefore provide partial support for our hypothesis that elevating muscle temperature during exercise augments training-induced adaptations to mitochondrial enzyme activity, although more work is necessary to explore this effect.
The effectiveness of our experimental model for studying the impact of elevating muscle temperature during exercise on mitochondrial adaptations is evidenced by improvements in physiological parameters relevant to endurance, and the consistently greater estimated Tmus in the heated compared to control limb (Fig. 2). Specifically, VT1 (10 ± 4%), VT2 (8 ± 6%), and V̇O2peak (3 ± 2%) significantly increased from pre-to-post training, which are evidence that our training programme was effective at stimulating endurance-related adaptations [27, 29, 31], and therefore allowed us to delineate the effect of local heat application on mitochondrial biogenesis. Whilst we cannot ascribe these improvements solely to mitochondrial factors given the myriad adaptations that occur in response to endurance exercise training, the timeframe of the intervention in the present study [28, 39] has been adequate to increase mitochondrial enzyme activity and protein expression in previous studies [4, 20, 28, 34]. The ~ 2 °C between-limb difference in estimated Tmus is similar to that which we observed between 20 min of moderate-intensity exercise in 18 vs. 40 °C [26], and to a study of 40 min of moderate-intensity exercise in 20 vs. 40 °C measuring muscle temperature directly [6], although lower than studies observing mitochondrial biogenesis following passively applied local heat stress using short-wave diathermy (~ 3.9 °C) [10]. Studies of passive exposure are an inherently different model, as the control limb remains at resting temperatures; muscle temperature does rise above resting temperatures during exercise in temperate conditions [6]. As the estimated muscle temperature technique has not been validated with concomitant local heat stress application, direct assessment of indwelling skeletal muscle temperature would have strengthened the present work. However, we are confident that the intervention sufficiently manipulated working skeletal muscle temperature to allow us to test our hypothesis.
Our data provides partial support for the hypothesis that elevating muscle temperature during exercise augments adaptations to mitochondrial enzyme activity in response to endurance training. Specifically, we report greater training-induced increases in complex I and II enzyme activities in HEAT vs. TEMP (Fig. 3bc). Increases in mitochondrial enzyme activities following training interventions have typically been interpreted as evidence of mitochondrial biogenesis [2], given previously-observed relationships with total mitochondrial content measured using transmission electron microscopy [22]. The absence of significant effects on citrate synthase activity, or in complex III-V activities, may be related to normal variability in the assays themselves (CV, 2.3–13.4%), small differences in the location of pre- and post-training biopsy sites [16], and the small sample size. We mitigated the effect of these error sources by measuring the pre-training biopsy sites for replication post-training, and by use of the highly-controlled contralateral leg design. It remains possible that real effects were missed due to having small magnitudes relative to the typical variability in the measurements, or that real effects were only observed for complex I and II, which emphasises the need for further studies with greater statistical power. Overall, our data provides some initial suggestion that elevated muscle temperature during exercise may positively affect some mitochondrial enzyme activity adaptations, although more work is needed to elucidate this effect.
Opposed to this conclusion is our mitochondrial protein expression data, derived from immunoblotting (Fig. 4). We observed no significant main effects of training on complex I–V expression, or interactions between training and application of mild, local heat stress. When considering the individual datasets, the only significant effect of training was an increase in complex IV protein expression observed in HEAT (fold-change, 1.17 ± 0.19, P = 0.013). Why increased mitochondrial enzyme activity would be observed without concomitant increases in mitochondrial protein expression is not clear and may relate to technical challenges and variability associated with immunoblotting [1], alongside the methodological aspects cited above. It is also possible that mitochondrial enzyme activity was enhanced independently of enzyme content, perhaps due to adaptations to co-factors. Regardless, whilst not conclusive, our data supports further research in larger studies with measures of mitochondrial content and respiratory activity to assess if elevating muscle temperature during exercise promotes mitochondrial adaptation and is therefore a driver of mitochondrial biogenesis.
Contrary to our hypothesis, application of mild, local heat stress during endurance training did not promote a detectable increase in HSP70 protein expression (Fig. 4f). In fact, a main effect of training on HSP70 protein expression was not observed. Previous work has reported increased HSP70 expression following endurance training [24, 25], although this has not always been observed [33]. We hypothesised that greater increases in HSP70 protein expression would be observed following HEAT based on recent work with passive heat stress using short-wave diathermy [9, 10], and that greater HSP70 accumulation would contribute to greater mitochondrial adaptations in HEAT [13, 46]. It is possible the training programme used in the present study, along with the magnitude of the thermal stress in HEAT, was insufficient to stimulate HSP70 accumulation. It is also possible the timing of post-exercise biopsies (~ 4–6 days following the last intervention session) meant changes to HSP70 expression were missed. Nevertheless, our data do not support the hypothesis that elevated muscle temperature during exercise training promotes HSP70 accumulation, although more work is warranted.
The mechanism behind any effect of elevated muscle temperature during exercise on adaptations to mitochondrial enzyme activity is not clear but could plausibly be related to greater acute increases in AMPK activation in response to individual training sessions in HEAT, via greater muscle glycogenolysis. Exercise with elevated skeletal muscle temperature has been shown to increase muscle glycogen utilisation [5, 40], and glycogen-AMPK binding inhibits AMPK activity [30]. Therefore, greater muscle glycogen utilisation in a limb exposed to mild, local heat stress during training might be expected to increase AMPK activation. This contention is supported by observations of an inverse relationship between muscle glycogen content and AMPK activity [37, 38] and observed positive effects of low glycogen exercise on AMPK activity [43]. Greater AMPK activation is thought to induce PGC-1α phosphorylation [18], a key regulator of mitochondrial biogenesis [11, 45]. As the purpose of the present study was to assess how application of local heat stress during exercise impacts training-induced adaptations to mitochondrial enzyme activity and protein expression, we obtained resting muscle samples before and after the intervention period, with the post-intervention samples obtained several days following the last intervention session. Given the time-course of signalling responses to exercise [21], our study design is not appropriate for assessing signalling-related responses to exercise performed with application of local heat stress. Accordingly, further research into the effect of elevating muscle temperature during exercise on signalling responses, including AMPK activation, is warranted.
In conclusion, this study suggests that elevated muscle temperature during exercise might enhance certain mitochondrial enzyme activities (complex I and II). However, further research is required to fully understand this effect due to variations in mitochondrial enzyme activity responses and the absence of changes in mitochondrial protein expression. We consider these findings as a foundation for conducting more comprehensive investigations into this hypothesis, involving larger-scale studies with extended exercise training durations.
Data availability
Data is available from the corresponding author upon reasonable request.
Code availability
None used.
Abbreviations
- AMP:
-
Adenosine monophosphate
- AMPK:
-
5′AMP-activated protein kinase
- CON:
-
Control leg
- CS:
-
Citrate synthase
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- HEAT:
-
Heated leg
- HR:
-
Heart rate
- HSP70:
-
Heat shock protein 70
- PFO:
-
Peak fat oxidation rate
- T mus :
-
Muscle temperature
- V̇CO2 :
-
Rate of carbon dioxide production
- V̇O2 :
-
Rate of oxygen consumption
- V̇O2peak:
-
Peak rate of oxygen consumption
- VT1 :
-
First ventilatory threshold
- VT2 :
-
Second ventilatory threshold
References
Bass JJ, Wilkinson DJ, Rankin D, Phillips BE, Szewczyk NJ, Smith K, Atherton PJ (2017) An overview of technical considerations for Western blotting applications to physiological research. Scand J Med Sci Sports 27:4–25. https://doi.org/10.1111/sms.12702
Bishop DJ, Botella J, Genders AJ, Lee MJ-C, Saner NJ, Kuang J, Yan X, Granata C (2019) High-intensity exercise and mitochondrial biogenesis: current controversies and future research directions. Physiology 34:56–70. https://doi.org/10.1152/physiol.00038.2018
Charoensap T, Kilding AE, Maunder E (2023) Carbohydrate, but not fat, oxidation is reduced during moderate-intensity exercise performed in 33 vs. 18 °C atmatched heart rates. Eur J Appl Physiol 123(9):2073–2085. https://doi.org/10.1007/s00421-023-05225-0
Egan B, O’Connor PL, Zierath JR, O’Gorman DJ (2013) Time course analysis reveals gene-specific transcript and protein kinetics of adaptation to short-term aerobic exercise training inhuman skeletal muscle. PLoS ONE 8:e74098. https://doi.org/10.1371/journal.pone.0074098
Febbraio MA, Carey MF, Snow RJ, Stathis CG, Hargreaves M (1996) Influence of elevated muscle temperature on metabolism during intense, dynamic exercise. Am J Physiol Regul Integr Comp Physiol 271:R1251–R1255. https://doi.org/10.1111/j.1748-1716.1984.tb00128.x
Febbraio MA, Snow RJ, Stathis CG, Hargreaves M, Carey MF (1994) Effect of heat stress on muscle energy metabolism during exercise. J Appl Physiol 77:2827–2831
Flouris AD, Webb P, Kenny GP (2015) Non-invasive assessment of muscle temperature during rest, exercise, and post-exercise recovery in different environments. J Appl Physiol 118:1310–1320. https://doi.org/10.1152/japplphysiol.00932.2014
Granata C, Oliveira RSF, Little JP, Renner K, Bishop DJ (2016) Training intensity modulates changes in PGC-1α and p53 protein content and mitochondrial respiration, but not markers of mitochondrial content in human skeletal muscle. FASEB J 30:959–970. https://doi.org/10.1096/fj.15-276907
Hafen PS, Abbott K, Bowden J, Lopiano R, Hancock CR, Hyldahl RD (2019) Daily heat treatment maintains mitochondrial function and attenuates atrophy in human skeletal muscle subjected to immobilization. J Appl Physiol 127:47–57. https://doi.org/10.1152/japplphysiol.01098.2018
Hafen PS, Preece CN, Sorensen JR, Hancock CR, Robert D (2018) Repeated exposure to heat stress induces mitochondrial adaptation in human skeletal muscle. J Appl Physiol 125:1447–1455. https://doi.org/10.1152/japplphysiol.00383.2018
Handschin C, Spiegelman BM (2008) The role of exercise and PGC1α in inflammation and chronic disease. Nature 454:463–469. https://doi.org/10.1038/nature07206
Hawley JA, Lundby C, Cotter JD, Burke LM (2018) Maximizing cellular adaptation to endurance exercise in skeletal muscle. Cell Metab 27:962–976. https://doi.org/10.1016/j.cmet.2018.04.014
Henstridge DC, Bruce CR, Drew BG, Tory K, Kolonics A, Estevez E, Chung J, Watson N, Gardner T, Lee-Young RS, Connor T, Watt MJ, Carpenter K, Hargreaves M, McGee SL, Hevener AL, Febbraio MA (2014) Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes 63:1881–1894. https://doi.org/10.2337/db13-0967
Holloszy JO (2011) Regulation of mitochondrial biogenesis and GLUT4 expression by exercise. Compr Physiol 1:921–940
Hoppeler H, Howald H, Conley K, Lindstedt SL, Claassen H, Vock P, Weibel ER (1985) Endurance training in humans: aerobic capacity and structure of skeletal muscle. J Appl Physiol 59:320–327
Horwarth O, Envall H, Röja J, Emanuelsson EB, Sanz G, Ekblom B, Apró W, Moberg M (2021) Variability in vastus lateralis fiber type distribution, fiber size, and myonuclear content along and between the legs. J Appl Physiol 131:158–173. https://doi.org/10.1152/japplphysiol.00053.2021
Hudson ER, Pan DA, James J, Lucocq JM, Hawley SA, Green KA, Baba O, Terashima T, Hardie DG (2003) A novel domain in AMP-activated protein kinase causes glycogen storage bodies similar to those seen in hereditary cardiac arrhythmias. Curr Biol 13:861–866. https://doi.org/10.1016/s0960-9822(03)00249-5
Jäger SS, Handschin CC, St-Pierre JJ, Spiegelman BM (2007) AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A 104:12017–12022. https://doi.org/10.1073/pnas.0705070104
Jeukendrup AE, Wallis GA (2005) Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int J Sports Med 26:S28–S37. https://doi.org/10.1055/s-2004-830512
Kraniou GN, Cameron-Smith D, Hargreaves M (2004) Effect of short-term training on GLUT-4 mRNA and protein expression in human skeletal muscle. Exp Physiol 89:559–563. https://doi.org/10.1113/expphysiol.2004.027409
Kuang J, McGinley C, Lee MJC, Saner NJ, Garnham A, Bishop DJ (2022) Interpretation of exercise-induced changes in human skeletal muscle mRNA expression depends on the timing of the post-exercise biopsies. PeerJ 10:e12856. https://doi.org/10.7717/peerj.12856
Larsen S, Nielsen J, Hansen CN, Nielsen LB, Wibrand F, Stride N, Schroder HD, Boushel R, Helge JW, Dela F, Hey-Mogensen M (2012) Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol 590:3349–3360. https://doi.org/10.1113/jphysiol.2012.230185
Liu CT, Brooks GA (2012) Mild heat stress induces mitochondrial biogenesis in C2C12 myotubes. J Appl Physiol 112:354–361. https://doi.org/10.1152/japplphysiol.00989.2011
Liu Y, Lormes W, Baur C, Opitz-Gress A, Altenburg D, Lehmann M, Steinacker JM (2000) Human skeletal muscle HSP70 response to physical training depends on exercise intensity. Int J Sports Med 21:351–355
Liu Y, Mayr S, Opitz-Gress A, Zeller C, Lormes W, Baur S, Lehmann M, Steinacker JM (1999) Human skeletal muscle HSP70 response to training in highly trained rowers. J Appl Physiol 86:101–104
Maunder E, Plews DJ, Merien F, Kilding AE (2020) Exercise intensity regulates the effect of heat stress on substrate oxidation rates during exercise. Eur J Sport Sci 20:935–943
Maunder E, Plews DJ, Wallis GA, Brick MJ, Leigh WB, Chang WL, Stewart T, Watkins CM, Kilding AE (2022) Peak fat oxidation is positively associated with vastus lateralis CD36 content, fed-state exercise fat oxidation, and endurance performance in trained males. Eur J Appl Physiol 122:93–102. https://doi.org/10.1007/s00421-021-04820-3
Maunder E, Plews DJ, Wallis GA, Brick MJ, Leigh WB, Chang WL, Watkins CM, Kilding AE (2021) Temperate performance and metabolic adaptations following endurance training performed under environmental heat stress. Physiol Rep 9:e14849. https://doi.org/10.14814/phy2.14849
Maunder E, Seiler S, Mildenhall MJ, Kilding AE, Plews DJ (2021) The importance of ‘durability’ in the physiological profiling of endurance athletes. Sports Med 51:1619–1628. https://doi.org/10.1007/s40279-021-01459-0
McBride A, Ghilagaber S, Nikolaev A, Hardie DG (2009) The glycogen-binding domain on the AMPK β subunit allows the kinase to act as a glycogen sensor. Cell Metab 9:23–34. https://doi.org/10.1016/j.cmet.2008.11.008
McLaughlin JE, Howley ET, Bassett DR Jr, Thompson DL, Fitzhugh EC (2010) Test of the classic model for predicting endurance running performance. Med Sci Sports Exerc 42:991–997
Merry TL, Ristow M (2016) Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and the anti-oxidant response in mice. J Physiol 594:5195–5207. https://doi.org/10.1113/JP271957
Morton JP, Holloway K, Woods P, Cable NT, Burniston J, Evans L, Kayani AC, McArdle A (2009) Exercise training-induced gender-specific heat shock protein adaptations in human skeletal muscle. Muscle Nerve 39:230–233. https://doi.org/10.1002/mus.21182
Murias JM, Kowalchuk JM, Ritchie D, Hepple RT, Doherty TJ, Paterson DH (2011) Adaptations in capillarization and citrate synthase activity in response to endurance training in older and young men. J Gerontol - Series A Biol Sci Med Sci 66(A):957–964. https://doi.org/10.1093/gerona/glr096
O’Reilly N, Collins C, McGlynn ML, Slivka DR (2021) Effect of local heat application during exercise on gene expression related to mitochondrial homeostasis. Appl Physiol Nutr Metab 46:1545–1551. https://doi.org/10.1139/apnm-2021-0346
Ojuka EO, Jones TE, Han DH, Chen M, Holloszy JO (2003) Raising Ca2+ in L6 myotubes mimics effects of exercise on mitochondrial biogenesis in muscle. FASEB J 17:675–681. https://doi.org/10.1096/fj.02-0951com
Philp A, Hargreaves M, Baar K (2012) More than a store: regulatory roles for glycogen in skeletal muscle adaptation to exercise. Am J Physiol Endocrinol Metab 302:E1343–E1351. https://doi.org/10.1152/ajpendo.00004.2012
Rothschild JA, Islam H, Bishop DJ, Kilding AE, Stewart T, Plews DJ (2022) Factors influencing AMPK activation during cycling exercise: a pooled analysis and meta-regression. Sports Med 52:1273–1294. https://doi.org/10.1007/s40279-021-01610-x
Spina RJ, Chi MM, Hopkins MG, Nemeth PM, Lowry OH, Holloszy JO (1996) Mitochondrial enzymes increase in muscle in response to 7–10 days of cycle exercise. J Appl Physiol 80:2250–2254. https://doi.org/10.1007/S004210000223
Starkie RL, Hargreaves M, Lambert DL, Proietto J, Febbraio MA (1999) Effects of temperature on muscle metabolism during submaximal exercise in humans. Exp Physiol 84:775–784
Tamura Y, Matsunaga Y, Masuda H, Takahashi Y, Terada S, Hoshino D, Hatta H (2014) Postexercise whole body heat stress additively enhances endurance training-induced mitochondrial adaptations in mouse skeletal muscle. Am J Physiol Regul Integr Comp Physiol 307:R931–R943. https://doi.org/10.1152/ajpregu.00525.2013
van Melick N, Meddeler BM, Hoogeboom TJ, Nijhuis-van der Sanden MWG, van Cingel REH (2017) How to determine leg dominance: the agreement between self-reported and observed performance in healthy adults. PLoS One 12:e0189876
Wojtaszewski JFP, MacDonald C, Nielsen JN, Hellsten Y, Hardie DG, Kemp BE, Kiens B, Richter EA (2003) Regulation of 5’AMP-activated protein kinase activity and substrate utilization in exercising human skeletal muscle. Am J Physiol Endocrinol Metab 284:E813–E822. https://doi.org/10.1152/ajpendo.00436.2002
Wu H, Kanatous SB, Thurmond FA, Gallardo T, Isotani E, Bassel-Duby R, Williams RS (1979) Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science 296(349–352):2002. https://doi.org/10.1126/science.1071163/r296/5566/349[pii]
Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM (1999) Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98:115–124. https://doi.org/10.1016/S0092-8674(00)80611-X
Young JC, Hoogenraad NJ, Hartl FU (2003) Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell 112:41–50. https://doi.org/10.1016/S0968-0004(05)00043-5
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This work was supported by an award from the Faculty of Health and Environmental Sciences Research Development Fund, Auckland University of Technology.
Author information
Authors and Affiliations
Contributions
E.M., T.L.M., and A.E.K. conceived and designed the study. E.M., J.A.R., A.K., M.J.B., and W.B.L. undertook the experiments. E.M., C.P.H., and A.K. analysed samples. E.M. completed data analyses. E.M. drafted the manuscript. All authors revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The Auckland University of Technology Ethics Committee approved all procedures (21/170).
Consent to participate
All participants provided written informed consent.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• Increased muscle temperature is a ubiquitous exercise stress, and there is some evidence for temperature-dependent stimulation of skeletal muscle mitochondrial biogenesis.
• The role of elevated muscle temperature during exercise in promoting mitochondrial adaptation to training has not previously been studied directly in humans in vivo.
• Mild heat stress was applied locally to the quadriceps of one leg during 3 weeks of two-legged cycling training. Evidence of greater adaptations to mitochondrial enzyme activities in the heated vs. control leg was observed but no differences were observed in mitochondrial protein expression.
• These data provide partial support for the hypothesis that increased muscle temperature during exercise promotes adaptations to mitochondrial enzyme activity in response to endurance training and provide the basis for future research.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maunder, E., King, A., Rothschild, J.A. et al. Locally applied heat stress during exercise training may promote adaptations to mitochondrial enzyme activities in skeletal muscle. Pflugers Arch - Eur J Physiol 476, 939–948 (2024). https://doi.org/10.1007/s00424-024-02939-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-024-02939-8