Abstract
Purpose
This study investigated whether muscle cooling and its associated effects on skeletal muscle oxidative responses, blood gases, and hormonal concentrations influenced energy metabolism during cycling.
Methods
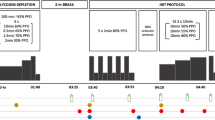
Twelve healthy participants (Males: seven; Females: five) performed two steady-state exercise sessions at 70% of ventilatory threshold on a cycle ergometer. Participants completed one session with pre-exercise leg cooling until muscle temperature (Tm) decreased by 6 °C (LCO), and a separate session without cooling (CON). They exercised until Tm returned to baseline and for an additional 30 min. Cardiovascular, respiratory, metabolic, hemodynamic variables, and skeletal muscle tissue oxidative responses were assessed continuously. Venous blood samples were collected to assess blood gases, and hormones.
Results
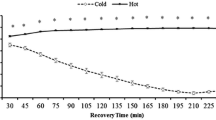
Heart rate, stroke volume, and cardiac output all increased across time but were not different between conditions. V̇O2 was greater in LCO when muscle temperature was restored until the end of exercise (p < 0.05). Cycling in the LCO condition induced lower oxygen availability, tissue oxygenation, blood pH, sO2%, and pO2 (p < 0.05). Insulin concentrations were also higher in LCO vs. CON (p < 0.05). Importantly, stoichiometric equations from respiratory gases indicated no differences in fat and CHO oxidation between conditions.
Conclusion
The present study demonstrated that despite muscle cooling and the associated oxidative and biochemical changes, energy metabolism remained unaltered during cycling. Whether lower local and systemic oxygen availability is counteracted via a cold-induced activation of lipid metabolism pathways needs to be further investigated.







Similar content being viewed by others
Abbreviations
- %TSI:
-
Percentage tissue saturation index
- CHO:
-
Carbohydrates
- CPT-1:
-
Carnitine palmitoyl transferase-I
- diffHb:
-
Difference oxy/deoxygenated hemoglobin
- HHb:
-
Deoxygenated hemoglobin
- NIRS:
-
Near-infrared spectrometry
- O2Hb:
-
Oxygenated hemoglobin
- PCO2 :
-
Partial pressure of carbon dioxide
- PO2 :
-
Partial pressure of oxygen
- RER:
-
Respiratory exchange ratio
- tHb:
-
Total hemoglobin
- T c :
-
Core temperature
- T m :
-
Muscle temperature
- T̅ sk :
-
Mean weighted skin temperature
- V̇E:
-
Minute ventilation
- VL:
-
Vastus lateralis
- VT:
-
Ventilatory threshold
- V t :
-
Tidal volume
- V̇O2peak :
-
Peak oxygen consumption
- V̇O2 :
-
Rate of oxygen consumption
- V̇CO2 :
-
Rate of carbon dioxide release
- W :
-
Watts
References
Alston T (2004) Blood gases and pH during hypothermia: the ‘Stats’. Int Anesth Clin 42(4):73–80
Bacher A (2005) Effect of body temperature on blood gases. Int Care Med 31:24–27
Beelen A, Sargeant AJ (1991) Effect of lowered muscle temperature on the physiology response to exercise in men. Eur J Appl Physiol 63:387–392
Bennett AF (1984) Thermal dependence of muscle function. Am J Physiol Regul Integr Comp Physiol 247(2):R217–R229
Bennett AF (1985) Temperature and muscle. J Exp Biol 115:333–344
Brosek JF, Grande JT, Andersen JT, Keys A (1963) Densiometric analysis of body composition: review of some quantitative assumptions. Ann NY Acad Sci 110:113–140
Bylund-Fellenius AC, Walker PM, Elander A, Holm S, Holm J, Schersten T (1981) Energy metabolism in relation to oxygen partial pressure in human skeletal muscle during exercise. Biochem J 200:247–255
Charloux A, Lonsdorfer-Wolf E, Richard R, Lampert E, Oswald-Mammosser M et al (2000) A new impedance cardiograph device for the non-invasive evaluation of cardiac output at rest and during exercise: comparison with the direct Fick method. Eur J Appl Physiol 82(4):313–320
Chiesa ST, Trangmar SJ, Gonzalez-Alonso J (2016) Temperature and blood flow distribution in the human leg during passive heat stress. J Appl Physiol 120(9):1047–1058
Coggins M, Lindner J, Rattigan S, Jahn L, Fasy E, Kaul S et al (2001) Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes 50(12):2682–2690
Deban SM, Lappin AK (2011) The effects on the dynamics and motor control of ballistic prey capture in toads: maintaining high performance at low temperature. J Exp Biol 214:1333–1346
DuBois DF (1916) A formula to estimate the approximate surface area if height and body mass be known. Arch Intern Med 17:836–871
Febbraio MA, Snow RJ, Stathis CG, Hargreaves M, Carey M (1996) Blunting the rise in body temperature reduces muscle glycogenolysis during exercise in humans. Exp Physiol 81:685–693
Fink WJ, Costill DL, Van Handel PJ (1975) Leg muscle metabolism during exercise in the heat and cold. Eur J Appl Physiol 34(3):183–190
Frank SM, Higgins MS, Fleisher LA, Sitzmann JV, Raff H, Breslow MJ (1997) Adrenergic, respiratory, and cardiovascular effects of core cooling in humans. Am J Physiol Regul Integr Comp Physiol 272(2):R557–R562
Freedman RR, Sabharwal SC, Moten M, Migaly P (1992) Local temperature modulates α1- and α2-adrenergic vasoconstriction in men. Heart Circ Physiol 32:H1197–1200
Gagnon DD, Rintamäki H, Gagnon SS, Cheung SS, Herzig K-H, Porvari K, Kyröläinen H (2013) Cold exposure enhances fat utilization but not non-esterified fatty acids, glycerol or catecholamines availability during submaximal walking and running. Front Physiol. https://doi.org/10.3389/fphys.2013.00099
Gagnon DD, Gagnon SS, Rintamäki H, Törmäkangas T, Puukka K, Herzig KH, Kyröläinen H (2014) The effects of cold exposure on leukocytes, hormones and cytokines during acute exercise in humans. PLoS ONE 9(10):110774
Gagnon DD, Peltonen JE, Rintamäki H, Gagnon SS, Herzig KH, Kyröläinen H (2017) The effects of skin and core tissue cooling on oxygenation of the vastus lateralis muscle during walking and running. J Sports Sci 35(20):1995–2004
Gagnon DD, Perrier L, Dorman S, Oddson B, Larivière C, Serresse O (2020) Ambient temperature inifluence metabolic substrate oxidation curves during running and cycling in healthy men. Eur J Sport Sci 20(1):90–99. https://doi.org/10.1080/17461391.2019.1612949
Gasparetti AL, de Souza CT, Pereira-da-Silva M, Oliveira RL, Saad MJ, Carneiro EM, Velloso LA (2003) Cold exposure induces tissue-specific modulation of the insulin-signalling pathway in Rattus norvegicus. J Physiol 552(1):149–162
Goodman AH, Einstein R, Granger HJ (1978) Effect of changing metabolic rate on local blood flow control in the canine hind limb. Circ Res 43:769–776
Gordon N, Abbiss C, Maiorana A, Marston K, Peiffer J (2018) Interrater reliability and agreement of the Physioflow bioimpedance cardiography device during rest, moderate and high-intensity exercise. Kinesiology 50:140–149
Granger HJ, Goodman AH, Granger DN (1976) Role of resistance and exchange vessels in local microvascular control of skeletal muscle oxygenation in the dog. Circ Res 38:379–385
Haycock GB, Schwartz GJ, Wisotsky DH (1978) Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediat 93(1):62–66
Heinonen I, Btothers RM, Kemppainen J, Knuuti J, Kalliokoski KK, Crandall GG (2011) Local heating, but not indirect whole body heating, increases human skeletal muscle blood flow. J Appl Physiol 111(3):818–824
Heinonen I, Koga S, Kalliokoski KK, Musch TI, Poole DC (2015) Heterogeneity of muscle blood flow and metabolism: influence of exercise, aging and disease state. Exerc Sport Sci Rev 43(3):117–124
Heinonen I, Saltin B, Hellsten Y, Kalliokoski KK (2017) The effect of nitric oxide synthase inhibition with and without inhibition of prostaglandins on blood flow in different human skeletal muscles. Eur J Appl Physiol 117(6):1175–1180
Hiramatsu K, Yamada T, Katakura M (1984) Acute effects of cold on blood pressure, renin-angiotensin-aldosterone system, catecholamines and adrenal steroids in man. Clin Exp Pharmacol Physiol 11(2):171–179
Honig CR, Odoroff CL, Frierson JL (1980) Capillary recruitment in exercise: rate, extent, uniformity, and relation to blood flow. Am J Physiol 238:H31–H42
Honig CR, Odoroff CL, Frierson JL (1982) Active and passive capillary control in red muscle at rest and in exercise. Am J Physiol 243:H196–H206
Hoy A, McLennan PL, Peoples GE (2009) The effect of vasoconstrictors on oxygen consumption in resting and contracting skeletal muscle of the autologous pump-perfused rat hindlimb. J Physiol Pharma 60(3):155
Hudlicka O (1985) Regulation of muscle blood flow. Clin Physiol 5:201–229
Idström JP, Harihara Subramanian V, Chance B, Schersten T, Bylund-Fellenius AC (1985) Oxygen dependence of energy metabolism in contracting and recovering rat skeletal muscle. Heart Circ Physiol 17:H40–H48
Ihsan M, Watson G, Lipski M, Abbiss CR (2013) Influence of postexercise cooling on muscle oxygenation and blood volume changes. Med Sci Sports Exerc 45(5):876–882
Jeacocke NA, Burke LM (2010) Methods to standardize dietary intake before performance testing. Int J Sport Nutr Exerc Metab 20:87–103
Jeukendrup AE, Wallis GA (2005) Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int J Sports Med 28:528–537
Johnson JM, Kellog DL (2010) Local thermal control of the human cutaneous circulation. J Appl Physiol 109:1229–1238
Johnson DG, Hayward JS, Jacobs TP, Collis ML, Eckerson JD, Williams RH (1977) Plasma norepinephrine responses of man in cold water. J Appl Physiol 43(2):216–220
Johnson JM, Minson CT, Kellog DL (2014) Cutaneous vasodilation and vasoconstrictor mechanism in temperature regulation. Comp Physiol 4(1):33–89
Kamariah Binti Md I, Kawasaki N, Ueyama K, Sumii T, Kodu S (2011) Effects of cold exposure and shear stress on endothelial nitric oxide synthase activation. Biochem Biophys Res Comm 412:318–322
Krogh A (1919) The supply of oxygen to the tissues and the regulation of the capillary circulation. J Physiol 52(6):457–474
Loenneke JP, Kim D, Fahs CA, Thiebaud RS, Abe T, Larson RD, Bemben DA, Bemben MG (2017) The influence of exercise load with and without different levels of blood flow restriction on acute changes in muscle thickness and lactate. Clin Physiol Funct Imaging 37(6):734–740
Mairbäurl H, Humpeler E (1980) Diminution of the temperature effects on the oxygen affinity of hemoglobin after prolonged hypothermia. Pflug Arch 383(3):209–213
Mancini DM, Bolinger L, Li H, Kendrick K, Chance B, Wilson JR (1994) Validation of near-infrared spectroscopy in humans. J Appl Physiol 77:2740–2747
McCully KK, Smith S, Rajaei S, Leigh JS Jr, Natelson BH (2004) Muscle metabolism with blood flow restriction in chronic fatigue syndrome. J Appl Physiol 96:871–878
Murias JM, Spencer MD, Keir DA, Paterson DH (2013) Systemic and vastus lateralis muscle blood flow and O2 extraction during ramp incremental cycle exercise. AJP Regul Integr Comp Physiol 304(9):R720–R725
O’Malley BP, Cook N, Richardson A, Barnett DB, Rosenthal FD (1984) Circulating catecholamines, thyrotrophin, thryroind hormone and prolactin responses of normal subjects to acute cold exposure. Clin Endocrinol 21:285–291
Oksa J, Kaikkonen H, Sorvisto P, Vaappo M, Martikkala V, Rintamaki H (2004) Changes in maximal cardiorespiratory capacity and submaximal strain while exercising in the cold. J Therm Biol 29:815–818
Orava J, Muutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T et al (2011) Different metabolic responses of human brown adipose tissue to activation of cold and insulin. Cell Metab 14:272–279
Palmes ED, Park CR (1948) An improved mounting for thermocouples for the measurement of the surface temperature of the body. J Lab Clin Med 33(8):1044–1046
Pearson J, Low DA, Stöhr E, Kalsi K, Ali L, Barker H, Gonzalez-Alonso J (2011) Hemodynamic responses to heat stress in the resting and exercising human leg: insight on the effect of temperature on skeletal muscle blood flow. Am J Physiol Regul Integr Comp Physiol 300(3):R663–R673
Richard R, Lonsdorfer-Wolf E, Charloux A, Doutreleau S, Buchheit M, Oswald-Mammosser M et al (2001) Non-invasive cardiac output evaluation during a maximal progressive exercise test, using a new impedance cardiograph device. Eur J Appl Physiol 85(3–4):202–207
Roberts CK, Barnard RJ, Jasman A, Balon TW (1999) Acute exercise increases nitric oxide synthase activity in skeletal muscle. Endocrinol Metab 40:E-390–E-394
Rosenmeier JB, Hansen J, Gonzalez-Alonso J (2004) Circulating ATP-induced vasodilation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol 558(1):351–365
Sasaki Y, Takahashi H, Aso H, Ohneda A, Weekes TE (1982) Effects of cold exposure on insulin and glucagon secretion in sheep. Endocrinology 111(6):2070–2076
Segal S (2005) Regulation of blood flow in the microcirculation. Microcirculation 12:33–45
Sheeren TWL, Schober P, Schwarte LA (2012) Monitoring tissue oxygenation by near infrared spectroscopy (NIRS): background and current applications. J Clin Monit Comput 26:279–287
Sidell BD (1998) Intracellular oxygen diffusion: the roles of myoglobin and lipid at cold body temperature. J Exp Biol 201:1118–1127
Siggaard-Andersen O, Wimberley PD, Göthgen I, Siggaard-Andersen M (1984) A mathematical model of the hemoglobin-oxygen dissociation curve of human blood and of the oxygen partial pressure as a function of temperature. Clin Chem 30(10):1646–1651
Starkie RL, Hargreaves M, Lambert DL, Proietto J, Febbraio M (1999) Effect of temperature on muscle metabolism during submaximal exercise in humans. Exp Physiol 84(4):775–784
Thomas GD, Segal SS (2004) Neural control of muscle blood flow during exercise. J Appl Physiol 97:731–738
Thompson-Torgerson CS, Holowatz LA, Flavahan NA, Kenney WL (2007) Cold-induced cutaneous vasoconstriction is mediated by Rho kinase in vivo in human skin. Am J Physiol Heart Circ Physiol 292:H1700–H1705
Thorsson O, Lilja B, Ahlgren LARS, Hemdal BENGT, Westlin NILS (1985) The effect of local cold application on intramuscular blood flow at rest and after running. Med Sci Sports Exerc 17(6):710–713
Vaile J, O’Hagan C, Stefanovic B, Walker M, Gill N, Askew CD (2011) Effect of cold-water immersion on repeated cycling performance and limb blood flow. Br J Sports Med 45(10):825–829
Vallerand AL, Lupien J, Bukowiecki LJ (1983) Interactions of cold exposure and starvation on glucose tolerance and insulin response. Am J Physiol 245:E575–E581
Vallerand AL, Perusse F, Bukowiecki LJ (1987) Cold exposure potentiates the effect of insulin on in vivo glucose uptake. Am J Physiol 253:E179–E186
van Loon LJC, Greenhaff PL, Constantin-Teodosiu D, Saris WHM, Wagenmakers AJM (2001) The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol 536(1):295–304
Wilson TE, Sauder CL, Kearney ML, Kuipers NT, Leuenberger UA, Monahan KD, Ray CS (2007) Skin-surface cooling elicits peripheral and visceral vasoconstriction in humans. J Appl Physiol 103:1257–1262
Yanagisawa O, Homma T, Okuwaki T, Shimao D, Takahashi H (2007) Effects of cooling on human skin and skeletal muscle. Eur J Appl Physiol 100:737–745
Zhang L, Vincent MA, Richards SM, Clerk LH, Rattigan S, Clark MG et al (2004) Insulin sensitivity of muscle capillary recruitment in vivo. Diabetes 53(2):447–453
Acknowledgements
We want to thank all participants for taking part in this study. Dr. Gagnon is supported by a Natural Sciences and Engineering Research Council of Canada Discovery Grant (NSERC#2016-060883).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation was performed by DDG, CH, AM, JG, and NB-B. Data collection DDG, CH, AM, DM, LW, SM, and NB-B. Data reduction and analysis were performed by DDG, LW, JG, and SM. The first draft of the manuscript was written by DDG and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Laurentian University Research Ethics Board; LUREB #6014986) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
All participants who took part in the present work provided written informed consent. Their data remained protected, confidential, and private.
Additional information
Communicated by George Havenith.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gagnon, D.D., Hancock, C., McCue, A. et al. Muscle cooling modulates tissue oxidative and biochemical responses but not energy metabolism during exercise. Eur J Appl Physiol 120, 1761–1775 (2020). https://doi.org/10.1007/s00421-020-04407-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-020-04407-4