Abstract
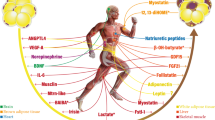
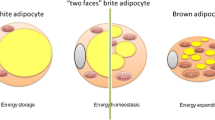
The need for effective and convenient ways of combatting obesity has created great interest in brown adipose tissue (BAT). However, because adult humans have relatively little amounts of BAT, the possibility of browning white adipose tissue (WAT), i.e., switching the metabolism of WAT from an energy storing to energy burning organ, has gained considerable attention. Exercise has countless health benefits, and has consistently been shown to cause browning in rodent white adipose tissue. The purpose of this review is to provide an overview of recent studies examining the effects of exercise and other interventions on the browning of white adipose tissue. The role of various endocrine factors, including catecholamines, interleukin-6, irisin, and meteorin-like in addition to local re-esterification-mediated mechanisms in inducing the browning of WAT will be discussed. The physiological importance of browning will be discussed, as will discrepancies in the literature between human and rodent studies.




Similar content being viewed by others
References
Abdullahi A, Chen P, Stanojcic M, Sadri A-R, Coburn N, Jeschke MG (2017) IL-6 signal from the bone marrow is required for the browning of white adipose tissue post burn injury. Shock 47:33–39. https://doi.org/10.1097/SHK.0000000000000749
Alberti KGM, Zimmet P, Shaw J (2005) The metabolic syndrome—a new worldwide definition. Lancet 366:1059–1062. https://doi.org/10.1016/S0140-6736(05)67402-8
Albrecht E, Norheim F, Thiede B, Holen T, Ohashi T, Schering L, Lee S, Brenmoehl J, Thomas S, Drevon CA, Erickson HP, Maak S (2015) Irisin—a myth rather than an exercise-inducible myokine. Sci Rep 5:8889. https://doi.org/10.1038/srep08889
Atherton PJ, Phillips BE (2013) Greek goddess or Greek myth: the effects of exercise on irisin/FNDC5 in humans. J Physiol 591:5267–5268. https://doi.org/10.1113/jphysiol.2013.265371
Bartelt A, Heeren J (2014) Adipose tissue browning and metabolic health. Nat Rev Endocrinol 10:24–36. https://doi.org/10.1038/nrendo.2013.204
Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, Eychmüller A, Gordts PLSM, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M, Heeren J (2011) Brown adipose tissue activity controls triglyceride clearance. Nat Med 17:200–205. https://doi.org/10.1038/nm.2297
Bartelt A, John C, Schaltenberg N, Berbée JFP, Worthmann A, Cherradi ML, Schlein C, Piepenburg J, Boon MR, Rinninger F, Heine M, Toedter K, Niemeier A, Nilsson SK, Fischer M, Wijers SL, van Marken Lichtenbelt W, Scheja L, Rensen PCN, Heeren J (2017) Thermogenic adipocytes promote HDL turnover and reverse cholesterol transport. Nat Commun 8:15010. https://doi.org/10.1038/ncomms15010
Billington CJ, Briggs JE, Link JG, Levine AS (1991) Glucagon in physiological concentrations stimulates brown fat thermogenesis in vivo. Am J Phys 261:R501–R507. https://doi.org/10.1152/ajpregu.1991.261.2.R501
Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM (2012) A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481:463–468. https://doi.org/10.1038/nature10777
Brooks B, Arch JR, Newsholme EA (1982) Effects of hormones on the rate of the triacylglycerol/fatty acid substrate cycle in adipocytes and epididymal fat pads. FEBS Lett 146:327–330
Buzelle SL, MacPherson REK, Peppler WT, Castellani L, Wright DC (2015) The contribution of IL-6 to beta 3 adrenergic receptor mediated adipose tissue remodeling. Physiol Rep 3:e12312–e12312. https://doi.org/10.14814/phy2.12312
Camera DM, Anderson MJ, Hawley JA, Carey AL (2010) Short-term endurance training does not alter the oxidative capacity of human subcutaneous adipose tissue. Eur J Appl Physiol 109:307–316. https://doi.org/10.1007/s00421-010-1356-3
Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84:277–359. https://doi.org/10.1152/physrev.00015.2003
Cannon B, Nedergaard J (2010) Metabolic consequences of the presence or absence of the thermogenic capacity of brown adipose tissue in mice (and probably in humans). Int J Obes 34(Suppl 1):S7–S16. https://doi.org/10.1038/ijo.2010.177
Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, Wu J, Gunawardana SC, Banks AS, Camporez J-PG, Jurczak MJ, Kajimura S, Piston DW, Mathis D, Cinti S, Shulman GI, Seale P, Spiegelman BM (2014) Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 156:304–316. https://doi.org/10.1016/j.cell.2013.12.021
Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng Y-H, Doria A, Kolodny GM, Kahn CR (2009) Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360:1509–1517. https://doi.org/10.1056/NEJMoa0810780
Douris N, Stevanovic DM, Fisher FM, Cisu TI, Chee MJ, Nguyen NL, Zarebidaki E, Adams AC, Kharitonenkov A, Flier JS, Bartness TJ, Maratos-Flier E (2015) Central fibroblast growth factor 21 browns white fat via sympathetic action in male mice. Endocrinology 156:2470–2481. https://doi.org/10.1210/en.2014-2001
Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS (2007) Regulation of lipolysis in adipocytes. Ann Rev Nutr 27:79–101. https://doi.org/10.1146/annurev.nutr.27.061406.093734
Dutchak PA, Katafuchi T, Bookout AL, Choi JH, Yu RT, Mangelsdorf DJ, Kliewer SA (2012) Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell 148:556–567. https://doi.org/10.1016/j.cell.2011.11.062
Dyck DJ (2009) Adipokines as regulators of muscle metabolism and insulin sensitivity. Appl Physiol Nutr Metab 34:396–402. https://doi.org/10.1139/H09-037
Eaton M, Granata C, Barry J, Safdar A, Bishop D, Little JP (2017) Impact of a single bout of high-intensity interval exercise and short-term interval training on interleukin-6, FNDC5, and METRNL mRNA expression in human skeletal muscle. J Sport Health Sci 7:1–6. https://doi.org/10.1016/j.jshs.2017.01.003
Elattar S, Satyanarayana A (2015) Can brown fat win the battle against white fat? J Cell Physiol 230:2311–2317. https://doi.org/10.1002/jcp.24986
Fischer AW, Csikasz RI, Essen v G, Cannon B, Nedergaard J (2016) No insulating effect of obesity. Am J Physiol Endocrinol Metab 311:E202–E213. https://doi.org/10.1152/ajpendo.00093.2016
Fischer K, Ruiz HH, Jhun K, Finan B, Oberlin DJ, van der Heide V, Kalinovich AV, Petrovic N, Wolf Y, Clemmensen C, Shin AC, Divanovic S, Brombacher F, Glasmacher E, Keipert S, Jastroch M, Nagler J, Schramm K-W, Medrikova D, Collden G, Woods SC, Herzig S, Homann D, Jung S, Nedergaard J, Cannon B, Tschöp MH, Müller TD, Buettner C (2017) Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat Med 23:623–630. https://doi.org/10.1038/nm.4316
Fischer AW, Cannon B, Nedergaard J (2018) Optimal housing temperatures for mice to mimic the thermal environment of humans: an experimental study. Mol Metab 7:161–170. https://doi.org/10.1016/j.molmet.2017.10.009
Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM (2012) FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 26:271–281. https://doi.org/10.1101/gad.177857.111
Foster MT, Pagliassotti MJ (2012) Metabolic alterations following visceral fat removal and expansion: beyond anatomic location. Adipocyte 1:192–199. https://doi.org/10.4161/adip.21756
Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu C-Y, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D’Agostino RB, O’Donnell CJ (2007) Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham heart study. Circulation 116:39–48. https://doi.org/10.1161/CIRCULATIONAHA.106.675355
Furuhashi M, Fucho R, Görgün CZ, Tuncman G, Cao H, Hotamisligil GS (2008) Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J Clin Invest 118:2640–2650. https://doi.org/10.1172/JCI34750
Gaidhu MP, Fediuc S, Anthony NM, So M, Mirpourian M, Perry RLS, Ceddia RB (2009) Prolonged AICAR-induced AMP-kinase activation promotes energy dissipation in white adipocytes: novel mechanisms integrating HSL and ATGL. J Lipid Res 50:704–715. https://doi.org/10.1194/jlr.M800480-JLR200
Gao C-L, Zhu C, Zhao Y-P, Chen X-H, Ji C-B, Zhang C-M, Zhu J-G, Xia Z-K, Tong M-L, Guo X-R (2010) Mitochondrial dysfunction is induced by high levels of glucose and free fatty acids in 3T3-L1 adipocytes. Mol Cell Endocrinol 320:25–33. https://doi.org/10.1016/j.mce.2010.01.039
García-Ruiz E, Reynés B, Díaz-Rúa R, Ceresi E, Oliver P, Palou A (2015) The intake of high-fat diets induces the acquisition of brown adipocyte gene expression features in white adipose tissue. Int J Obes 39:1619–1629. https://doi.org/10.1038/ijo.2015.112
Gaskill BN, Garner JP (2014) Letter-to-the-editor on “not so hot: optimal housing temperatures for mice to mimic the thermal environment of humans”. Mol Metab 3:335–336. https://doi.org/10.1016/j.molmet.2013.05.003
Gauthier MS, Miyoshi H, Souza SC, Cacicedo JM, Saha AK, Greenberg AS, Ruderman NB (2008) AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J Biol Chem 283:16514–16524. https://doi.org/10.1074/jbc.M708177200
Granneman JG (2015) Renaissance of brown adipose tissue research: integrating the old and new. Int J Obes Suppl 5:S7–S10. https://doi.org/10.1038/ijosup.2015.3
Gudiksen A, Schwartz CL, Bertholdt L, Joensen E, Knudsen JG, Pilegaard H (2016) Lack of skeletal muscle IL-6 affects pyruvate dehydrogenase activity at rest and during prolonged exercise. PLoS One 11:e0156460. https://doi.org/10.1371/journal.pone.0156460
Guilherme A, Virbasius JV, Puri V, Czech MP (2008) Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 9:367–377. https://doi.org/10.1038/nrm2391
Hagen JH (1961) Effect of glucagon on the metabolism of adipose tissue. J Biol Chem 236:1023–1027
Hamosh M, Hamosh P, Bar-Maor JA, Cohen H (1963) Fatty-acid metabolism by human adipose tissue. J Clin Invest 42:1648–1652. https://doi.org/10.1172/JCI104850
Hansen JS, Clemmesen JO, Secher NH, Hoene M, Drescher A, Weigert C, Pedersen BK, Plomgaard P (2015) Glucagon-to-insulin ratio is pivotal for splanchnic regulation of FGF-21 in humans. Mol Metab 4:551–560. https://doi.org/10.1016/j.molmet.2015.06.001
Hansen JS, Pedersen BK, Xu G, Lehmann R, Weigert C, Plomgaard P (2016) Exercise-induced secretion of FGF21 and follistatin are blocked by pancreatic clamp and impaired in type 2 diabetes. J Clin Endocrinol Metab 101:2016–1681. https://doi.org/10.1210/jc.2016-1681
Hanssen MJW, Hoeks J, Brans B, van der Lans AAJJ, Schaart G, van den Driessche JJ, Jörgensen JA, Boekschoten MV, Hesselink MKC, Havekes B, Kersten S, Mottaghy FM, van Marken Lichtenbelt WD, Schrauwen P (2015) Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med 21:863–865. https://doi.org/10.1038/nm.3891
Heim T, Hull D (1966) The effect of propranalol on the calorigenic response in brown adipose tissue of new-born rabbits to catecholamines, glucagon, corticotrophin and cold exposure. J Physiol 187:271–283
Horowitz JF (2003) Fatty acid mobilization from adipose tissue during exercise. Trends Endocrinol Metab 14:386–392
Hotamisligil GS (2006) Inflammation and metabolic disorders. Nature 444:860–867. https://doi.org/10.1038/nature05485
Hotamisligil GS, Shargill NS, Spiegelman BM (1993) Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259:87–91
Jay O, Raubenheimer D (2016) Some problems with translating the insulating effect of obesity from mice to men. Am J Physiol Endocrinol Metab 311:E638–E638. https://doi.org/10.1152/ajpendo.00265.2016
Kinoshita K, Ozaki N, Takagi Y, Murata Y, Oshida Y, Hayashi Y (2014) Glucagon is essential for adaptive thermogenesis in brown adipose tissue. Endocrinology 155:3484–3492. https://doi.org/10.1210/en.2014-1175
Kleiner S, Mepani RJ, Laznik D, Ye L, Jurczak MJ, Jornayvaz FR, Estall JL, Chatterjee Bhowmick D, Shulman GI, Spiegelman BM (2012) Development of insulin resistance in mice lacking PGC-1α in adipose tissues. Proc Natl Acad Sci U S A 109:9635–9640. https://doi.org/10.1073/pnas.1207287109
Knudsen JG, Murholm M, Carey AL, Biensø RS, Basse AL, Allen TL, Hidalgo J, Kingwell BA, Febbraio MA, Hansen JB, Pilegaard H (2014) Role of IL-6 in exercise training- and cold-induced UCP1 expression in subcutaneous white adipose tissue. PLoS One 9:e84910. https://doi.org/10.1371/journal.pone.0084910
Krief S, Lönnqvist F, Raimbault S, Baude B, Van Spronsen A, Arner P, Strosberg AD, Ricquier D, Emorine LJ (1993) Tissue distribution of beta 3-adrenergic receptor mRNA in man. J Clin Invest 91:344–349. https://doi.org/10.1172/JCI116191
Labbé SM, Caron A, Chechi K, Laplante M, Lecomte R, Richard D (2016) Metabolic activity of brown, “beige,” and white adipose tissues in response to chronic adrenergic stimulation in male mice. Am J Physiol Endocrinol Metab 311:E260–E268. https://doi.org/10.1152/ajpendo.00545.2015
Lee P, Brychta RJ, Linderman J, Smith S, Chen KY, Celi FS (2013) Mild cold exposure modulates fibroblast growth factor 21 (FGF21) diurnal rhythm in humans: relationship between FGF21 levels, lipolysis, and cold-induced thermogenesis. J Clin Endocrinol Metab 98:E98–E102. https://doi.org/10.1210/jc.2012-3107
Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, Perron RM, Werner CD, Phan GQ, Kammula US, Kebebew E, Pacak K, Chen KY, Celi FS (2014) Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab 19:302–309. https://doi.org/10.1016/j.cmet.2013.12.017
MacPherson REK, Dragos SM, Ramos S, Sutton C, Frendo-Cumbo S, Castellani L, Watt MJ, Perry CGR, Mutch DM, Wright DC (2016) Reduced ATGL-mediated lipolysis attenuates β-adrenergic-induced AMPK signaling, but not the induction of PKA-targeted genes, in adipocytes and adipose tissue. Am J Phys Cell Phys 311:C269–C276. https://doi.org/10.1152/ajpcell.00126.2016
Mottillo EP, Granneman JG (2011) Intracellular fatty acids suppress β-adrenergic induction of PKA-targeted gene expression in white adipocytes. Am J Physiol Endocrinol Metab 301:E122–E131. https://doi.org/10.1152/ajpendo.00039.2011
Mottillo EP, Bloch AE, Leff T, Granneman JG (2012) Lipolytic products activate peroxisome proliferator-activated receptor (PPAR) α and δ in brown adipocytes to match fatty acid oxidation with supply. J Biol Chem 287:25038–25048. https://doi.org/10.1074/jbc.M112.374041
Mottillo EP, Balasubramanian P, Lee Y-H, Weng C, Kershaw EE, Granneman JG (2014) Coupling of lipolysis and de novo lipogenesis in brown, beige, and white adipose tissues during chronic β3-adrenergic receptor activation. J Lipid Res 55:2276–2286. https://doi.org/10.1194/jlr.M050005
Mottillo EP, Desjardins EM, Crane JD, Smith BK, Green AE, Ducommun S, Henriksen TI, Rebalka IA, Razi A, Sakamoto K, Scheele C, Kemp BE, Hawke TJ, Ortega J, Granneman JG, Steinberg GR (2016) Lack of adipocyte AMPK exacerbates insulin resistance and hepatic steatosis through brown and beige adipose tissue function. Cell Metab 24:118–129. https://doi.org/10.1016/j.cmet.2016.06.006
Mottillo EP, Desjardins EM, Fritzen AM, Zou VZ, Crane JD, Yabut JM, Kiens B, Erion DM, Lanba A, Granneman JG, Talukdar S, Steinberg GR (2017) FGF21 does not require adipocyte AMP-activated protein kinase (AMPK) or the phosphorylation of acetyl-CoA carboxylase (ACC) to mediate improvements in whole-body glucose homeostasis. Mol Metab 6:471–481. https://doi.org/10.1016/j.molmet.2017.04.001
Muzik O, Mangner TJ, Granneman JG (2012) Assessment of oxidative metabolism in brown fat using PET imaging. Front Endocrinol 3:15. https://doi.org/10.3389/fendo.2012.00015
Muzik O, Mangner TJ, Leonard WR, Kumar A, Janisse J, Granneman JG (2013) 15O PET measurement of blood flow and oxygen consumption in cold-activated human brown fat. J Nucl Med 54:523–531. https://doi.org/10.2967/jnumed.112.111336
Nakhuda A, Josse AR, Gburcik V, Crossland H, Raymond F, Metairon S, Good L, Atherton PJ, Phillips SM, Timmons JA (2016) Biomarkers of browning of white adipose tissue and their regulation during exercise- and diet-induced weight loss. Am J Clin Nutr 104:557–565. https://doi.org/10.3945/ajcn.116.132563
Nedergaard J, Cannon B (2014) The browning of white adipose tissue: some burning issues. Cell Metab 20:396–407. https://doi.org/10.1016/j.cmet.2014.07.005
Nguyen KD, Qiu Y, Cui X, Goh YPS, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A (2011) Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480:104–108. https://doi.org/10.1038/nature10653
Norheim F, Langleite TM, Hjorth M, Holen T, Kielland A, Stadheim HK, Gulseth HL, Birkeland KI, Jensen J, Drevon CA (2014) The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J 281:739–749. https://doi.org/10.1111/febs.12619
Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, Scheinin M, Taittonen M, Niemi T, Enerbäck S, Virtanen KA (2011) Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab 14:272–279. https://doi.org/10.1016/j.cmet.2011.06.012
Orava J, Nuutila P, Noponen T, Parkkola R, Viljanen T, Enerbäck S, Rissanen A, Pietiläinen KH, Virtanen KA (2013) Blunted metabolic responses to cold and insulin stimulation in brown adipose tissue of obese humans. Obesity (Silver Spring) 21:2279–2287. https://doi.org/10.1002/oby.20456
Ostman J, Arner P, Engfeldt P, Kager L (1979) Regional differences in the control of lipolysis in human adipose tissue. Metabolism 28:1198–1205
Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK (1998) Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol 508:949–953. https://doi.org/10.1111/j.1469-7793.1998.949bp.x
Patsouris D, Qi P, Abdullahi A, Stanojcic M, Chen P, Parousis A, Amini-Nik S, Jeschke MG (2015) Burn induces browning of the subcutaneous white adipose tissue in mice and humans. Cell Rep 13:1538–1544. https://doi.org/10.1016/j.celrep.2015.10.028
Pedersen BK, Saltin B (2015) Exercise as medicine—evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports 25:1–72. https://doi.org/10.1111/sms.12581
Pekkala S, Wiklund PK, Hulmi JJ, Ahtiainen JP, Horttanainen M, Pöllänen E, Mäkelä KA, Kainulainen H, Häkkinen K, Nyman K, Alén M, Herzig K-H, Cheng S (2013) Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J Physiol 591:5393–5400. https://doi.org/10.1113/jphysiol.2013.263707
Peppler WT, Anderson ZG, McCrae LM, MacPherson REK, Wright DC (2016) Habitual physical activity protects against lipopolysaccharide-induced inflammation in mouse adipose tissue. Adipocyte:1–11
Peppler WT, Townsend LK, Knuth CM, Foster MT, Wright DC (2017) Subcutaneous inguinal white adipose tissue is responsive to, but dispensable for, the metabolic health benefits of exercise. Am J Physiol Endocrinol Metab. https://doi.org/10.1152/ajpendo.00226.2017
Perry RJ, Samuel VT, Petersen KF, Shulman GI (2014) The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 510:84–91. https://doi.org/10.1038/nature13478
Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, Swarbrick M, Rose-John S, Rincon M, Robertson G, Zechner R, Wagner EF (2014) A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab 20:433–447. https://doi.org/10.1016/j.cmet.2014.06.011
Pino MF, Parsons SA, Smith SR, Sparks LM (2016) Active individuals have high mitochondrial content and oxidative markers in their abdominal subcutaneous adipose tissue. Obesity (Silver Spring) 24:2467–2470. https://doi.org/10.1002/oby.21669
Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM (1998) A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92:829–839
Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, Camera DM, Lachey J, Gygi S, Seehra J, Hawley JA, Spiegelman BM (2014) Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 157:1279–1291. https://doi.org/10.1016/j.cell.2014.03.065
Reilly SM, Saltiel AR (2017) Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol 13:633–643. https://doi.org/10.1038/nrendo.2017.90
Reshef L, Hanson RW, Ballard FJ (1970) A possible physiological role for glyceroneogenesis in rat adipose tissue. J Biol Chem 245:5979–5984
Richard JE, López-Ferreras L, Chanclón B, Eerola K, Micallef P, Skibicka KP, Wernstedt Asterholm I (2017) CNS β3-adrenergic receptor activation regulates feeding behavior, white fat browning, and body weight. Am J Physiol Endocrinol Metab 313:E344–E358. https://doi.org/10.1152/ajpendo.00418.2016
Rohas LM, St-Pierre J, Uldry M, Jäger S, Handschin C, Spiegelman BM (2007) A fundamental system of cellular energy homeostasis regulated by PGC-1alpha. Proc Natl Acad Sci U S A 104:7933–7938. https://doi.org/10.1073/pnas.0702683104
Rosen ED, Spiegelman BM (2014) What we talk about when we talk about fat. Cell 156:20–44. https://doi.org/10.1016/j.cell.2013.12.012
Safdar A, Little JP, Stokl AJ, Hettinga BP, Akhtar M, Tarnopolsky MA (2011) Exercise increases mitochondrial PGC-1alpha content and promotes nuclear-mitochondrial cross-talk to coordinate mitochondrial biogenesis. J Biol Chem 286:10605–10617. https://doi.org/10.1074/jbc.M110.211466
Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M (2009) High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58:1526–1531. https://doi.org/10.2337/db09-0530
Salem V, Izzi-Engbeaya C, Coello C, Thomas DB, Chambers ES, Comninos AN, Buckley A, Win Z, Al-Nahhas A, Rabiner EA, Gunn RN, Budge H, Symonds ME, Bloom SR, Tan TM, Dhillo WS (2016) Glucagon increases energy expenditure independently of brown adipose tissue activation in humans. Diabetes Obes Metab 18:72–81. https://doi.org/10.1111/dom.12585
Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM (2008) PRDM16 controls a brown fat/skeletal muscle switch. Nature 454:961–967. https://doi.org/10.1038/nature07182
Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM (2011) Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 121:96–105. https://doi.org/10.1172/JCI44271
Sepa-Kishi DM, Ceddia RB (2016) Exercise-mediated effects on white and brown adipose tissue plasticity and metabolism. Exerc Sport Sci Rev 44:37–44. https://doi.org/10.1249/JES.0000000000000068
Sidossis LS, Porter C, Saraf MK, Børsheim E, Radhakrishnan RS, Chao T, Ali A, Chondronikola M, Mlcak R, Finnerty CC, Hawkins HK, Toliver-Kinsky T, Herndon DN (2015) Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Cell Metab 22:219–227. https://doi.org/10.1016/j.cmet.2015.06.022
Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Näslund E, Britton T, Concha H, Hassan M, Rydén M, Frisén J, Arner P (2008) Dynamics of fat cell turnover in humans. Nature 453:783–787. https://doi.org/10.1038/nature06902
Speakman JR, Keijer J (2012) Not so hot: optimal housing temperatures for mice to mimic the thermal environment of humans. Mol Metab 2:5–9. https://doi.org/10.1016/j.molmet.2012.10.002
Stallknecht B, Vinten J, Ploug T, Galbo H (1991) Increased activities of mitochondrial enzymes in white adipose tissue in trained rats. Am J Physiol Endocrinol Metab 261:E410–E414
Stanford KI, Middelbeek RJW, Townsend KL, Lee M-Y, Takahashi H, So K, Hitchcox KM, Markan KR, Hellbach K, Hirshman MF, Tseng Y-H, Goodyear LJ (2015) A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes 64:2002–2014. https://doi.org/10.2337/db14-0704
Sutherland LN, Bomhof MR, Capozzi LC, Basaraba SAU, Wright DC (2009) Exercise and adrenaline increase PGC-1alpha mRNA expression in rat adipose tissue. J Physiol 587:1607–1617. https://doi.org/10.1113/jphysiol.2008.165464
Thyfault JP, Wright DC (2016) “Weighing” the effects of exercise and intrinsic aerobic capacity: are there beneficial effects independent of changes in weight? Appl Physiol Nutr Metab 41:911–916. https://doi.org/10.1139/apnm-2016-0122
Timmons JA, Baar K, Davidsen PK, Atherton PJ (2012) Is irisin a human exercise gene? Nature 488:E9–E10– discussion E10–1. https://doi.org/10.1038/nature11364
Townsend LK, Knuth CM, Wright DC (2017) Cycling our way to fit fat. Physiol Rep 5:e13247. https://doi.org/10.14814/phy2.13247
Tran TT, Yamamoto Y, Gesta S, Kahn CR (2008) Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab 7:410–420. https://doi.org/10.1016/j.cmet.2008.04.004
Tsiloulis T, Carey AL, Bayliss J, Canny B, Meex RCR, Watt MJ (2017) No evidence of white adipocyte browning after endurance exercise training in obese men. Int J Obes 42:721–727. https://doi.org/10.1038/ijo.2017.295
van der Lans AAJJ, Hoeks J, Brans B, Vijgen GHEJ, Visser MGW, Vosselman MJ, Hansen J, Jörgensen JA, Wu J, Mottaghy FM, Schrauwen P, van Marken Lichtenbelt WD (2013) Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest 123:3395–3403. https://doi.org/10.1172/JCI68993
van Hall G, Steensberg A, Sacchetti M, Fischer C, Keller C, Schjerling P, Hiscock N, Møller K, Saltin B, Febbraio MA, Pedersen BK (2003) Interleukin-6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab 88:3005–3010. https://doi.org/10.1210/jc.2002-021687
Vaughan M (1962) The production and release of glycerol by adipose tissue incubated in vitro. J Biol Chem 237:3354–3358
Véniant MM, Sivits G, Helmering J, Komorowski R, Lee J, Fan W, Moyer C, Lloyd DJ (2015) Pharmacologic effects of FGF21 are independent of the “Browning” of white adipose tissue. Cell Metab 21:731–738. https://doi.org/10.1016/j.cmet.2015.04.019
Vosselman MJ, Hoeks J, Brans B, Pallubinsky H, Nascimento EBM, van der Lans AAJJ, Broeders EPM, Mottaghy FM, Schrauwen P, van Marken Lichtenbelt WD (2015) Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. Int J Obes 39:1696–1702. https://doi.org/10.1038/ijo.2015.130
Wan Z, Perry CGR, Macdonald T, Chan CB, Holloway GP, Wright DC (2012) IL-6 is not necessary for the regulation of adipose tissue mitochondrial content. PLoS One 7:e51233. https://doi.org/10.1371/journal.pone.0051233
Wan Z, Ritchie I, Beaudoin M-S, Castellani L, Chan CB, Wright DC (2012) IL-6 indirectly modulates the induction of glyceroneogenic enzymes in adipose tissue during exercise. PLoS One 7:e41719. https://doi.org/10.1371/journal.pone.0041719
Wan Z, Root-Mccaig J, Castellani L, Kemp BE, Steinberg GR, Wright DC (2014) Evidence for the role of AMPK in regulating PGC-1 alpha expression and mitochondrial proteins in mouse epididymal adipose tissue. Obesity (Silver Spring) 22:730–738. https://doi.org/10.1002/oby.20605
Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang A-H, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerbäck S, Schrauwen P, Spiegelman BM (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150:366–376. https://doi.org/10.1016/j.cell.2012.05.016
Wu MV, Bikopoulos G, Hung S, Ceddia RB (2014) Thermogenic capacity is antagonistically regulated in classical brown and white subcutaneous fat depots by high fat diet and endurance training in rats: impact on whole-body energy expenditure. J Biol Chem 289:34129–34140. https://doi.org/10.1074/jbc.M114.591008
Xu X, Ying Z, Cai M, Xu Z, Li Y, Jiang SY, Tzan K, Wang A, Parthasarathy S, He G, Rajagopalan S, Sun Q (2011) Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue. Am J Phys Reg Integr Comp Phys 300:R1115–R1125. https://doi.org/10.1152/ajpregu.00806.2010
Yamashita H, Yamamoto M, Sato Y, Izawa T, Komabayashi T, Saito D, Ohno H (1993) Effect of running training on uncoupling protein mRNA expression in rat brown adipose tissue. Int J Biometeorol 37:61–64
Ye L, Wu J, Cohen P, Kazak L, Khandekar MJ, Jedrychowski MP, Zeng X, Gygi SP, Spiegelman BM (2013) Fat cells directly sense temperature to activate thermogenesis. Proc Natl Acad Sci U S A 110:12480–12485. https://doi.org/10.1073/pnas.1310261110
Young P, Arch JR, Ashwell M (1984) Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett 167:10–14
Zuriaga MA, Fuster JJ, Gokce N, Walsh K (2017) Humans and mice display opposing patterns of “Browning” gene expression in visceral and subcutaneous white adipose tissue depots. Front Cardiovasc Med 4:27. https://doi.org/10.3389/fcvm.2017.00027
Zwick RK, Guerrero-Juarez CF, Horsley V, Plikus MV (2018) Anatomical, physiological, and functional diversity of adipose tissue. Cell Metab 27:68–83. https://doi.org/10.1016/j.cmet.2017.12.002
Funding
DCW is funded by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery grant and is a Tier II Canada Research Chair in Lipids, Metabolism, and Health. LKT is supported by an Ontario Graduate Scholarship, Dairy Farmers of Ontario Doctoral Research Scholarship, and NSERC Post-Graduate Scholarship.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the special issue on Exercise Physiology: future opportunities and challenges in Pflügers Archiv—European Journal of Physiology
Rights and permissions
About this article
Cite this article
Townsend, L.K., Wright, D.C. Looking on the “brite” side exercise-induced browning of white adipose tissue. Pflugers Arch - Eur J Physiol 471, 455–465 (2019). https://doi.org/10.1007/s00424-018-2177-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-018-2177-1