Abstract
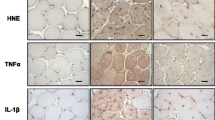
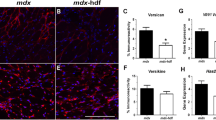
Duchenne muscular dystrophy (DMD) is a human genetic disease characterized by fibrosis and severe muscle weakness. Currently, there is no effective treatment available to prevent progressive fibrosis in skeletal muscles. The serum- and glucocorticoid-inducible kinase SGK1 regulates a variety of physiological functions and participates in fibrosis stimulation. Here, we investigated whether SGK1 influences structure, function and/or fibrosis of the muscles from the mdx mouse, an animal model for DMD. As expected, mdx muscles showed the typical pathological features of muscular dystrophy including fiber size variations, central nuclei of muscle fibers, fibrosis in the diaphragm, and force reduction by 30–50 %. Muscles from sgk1 -/- mice were histologically overall intact and specific force was only slightly reduced compared to wild-type muscles. Surprisingly, soleus and diaphragm muscles of mdx/sgk1 -/- mice displayed forces close to wild-type levels. Most muscle fibers of the double mutants contained central nuclei, but fibrosis was not observed in any of the tested limb and diaphragm muscles. We conclude that the sole lack of SGK1 in mouse muscle does not lead to pronounced changes in muscle structure and function. However, dystrophin-deficient mdx muscle seems to benefit from SGK1 deficiency. SGK1 appears to be an important enzyme in the process of fibrotic remodeling and subsequent weakness of dystrophin-deficient mouse muscle.






Similar content being viewed by others
References
Anderson JE (1991) Dystrophic changes in mdx muscle regenerating from denervation and devascularization. Muscle Nerve 14(3):268–279
Andres-Mateos E, Brinkmeier H, Burks TN, Mejias R, Files DC, Steinberger M, Soleimani A, Marx R, Simmers JL, Lin B, Finanger Hedderick E, Marr TG, Lin BM, Hourde C, Leinwand LA, Kuhl D, Föller M, Vogelsang S, Hernandez-Diaz I, Vaughan DK, Alvarez de la Rosa D, Lang F, Cohn RD (2013) Activation of serum/glucocorticoid-induced kinase 1 (SGK1) is important to maintain skeletal muscle homeostasis and prevent atrophy. EMBO Mol Med 5(1):80–91
Babalola O, Mamalis A, Lev-Tov H, Jagdeo J (2014) NADPH oxidase enzymes in skin fibrosis: molecular targets and therapeutic agents. Arch Dermatol Res 306(4):313–330
Barton ER, Morris L, Kawana M, Bish LT, Toursel T (2005) Systemic administration of l-arginine benefits mdx skeletal muscle function. Muscle Nerve 32(6):751–760
Berchtold MW, Brinkmeier H, Müntener M (2000) Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol Rev 80(3):1215–1265
Bernasconi P, Torchiana E, Confalonieri P, Brugnoni R, Barresi R, Mora M, Cornelio F, Morandi L, Mantegazza R (1995) Expression of transforming growth factor-beta 1 in dystrophic patient muscles correlates with fibrosis. Pathogenetic role of a fibrogenic cytokine. J Clin Invest 96(2):1137–1144
Blaauw B, Mammucari C, Toniolo L, Agatea L, Abraham R, Sandri M, Reggiani C, Schiaffino S (2008) Akt activation prevents the force drop induced by eccentric contractions in dystrophin-deficient skeletal muscle. Hum Mol Genet 17(23):3686–3696
Bo Li Z, Zhang J, Wagner KR (2012) Inhibition of myostatin reverses muscle fibrosis through apoptosis. J Cell Sci 125(Pt 17):3957–3965
Boini KM, Hennige AM, Huang DY, Friedrich B, Palmada M, Boehmer C, Grahammer F, Artunc F, Ullrich S, Avram D, Osswald H, Wulff P, Kuhl D, Vallon V, Haring HU, Lang F (2006) Serum- and glucocorticoid-inducible kinase 1 mediates salt sensitivity of glucose tolerance. Diabetes 55(7):2059–2066
Brinkmeier H (2011) TRP channels in skeletal muscle: gene expression, function and implications for disease. Adv Exp Med Biol 704:749–758
Brisson BK, Spinazzola J, Park S, Barton ER (2014) Viral expression of insulin-like growth factor I E-peptides increases skeletal muscle mass but at the expense of strength. Am J Physiol Endocrinol Metab 306(8):E965–974
Brooks SV, Faulkner JA (1988) Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol 404:71–82
Carberry S, Brinkmeier H, Zhang Y, Winkler CK, Ohlendieck K (2013) Comparative proteomic profiling of soleus, extensor digitorum longus, flexor digitorum brevis and interosseus muscles from the mdx mouse model of Duchenne muscular dystrophy. Int J Mol Med 32(3):544–556
Cohn RD, Liang HY, Shetty R, Abraham T, Wagner KR (2007) Myostatin does not regulate cardiac hypertrophy or fibrosis. Neuromuscul Disord 17(4):290–296
Coirault C, Pignol B, Cooper RN, Butler-Browne G, Chabrier PE, Lecarpentier Y (2003) Severe muscle dysfunction precedes collagen tissue proliferation in mdx mouse diaphragm. J Appl Physiol 94(5):1744–1750
Coulton GR, Morgan JE, Partridge TA, Sloper JC (1988) The mdx mouse skeletal muscle myopathy: I. A histological, morphometric and biochemical investigation. Neuropathol Appl Neurobiol 14(1):53–70
Decary S, Hamida CB, Mouly V, Barbet JP, Hentati F, Butler-Browne GS (2000) Shorter telomeres in dystrophic muscle consistent with extensive regeneration in young children. Neuromuscul Disord 10(2):113–120
DiMario JX, Uzman A, Strohman RC (1991) Fiber regeneration is not persistent in dystrophic (MDX) mouse skeletal muscle. Dev Biol 148(1):314–321
Farini A, Meregalli M, Belicchi M, Battistelli M, Parolini D, D'Antona G, Gavina M, Ottoboni L, Constantin G, Bottinelli R, Torrente Y (2007) T and B lymphocyte depletion has a marked effect on the fibrosis of dystrophic skeletal muscles in the scid/mdx mouse. J Pathol 213(2):229–238
Glass DJ (2010) PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr Top Microbiol Immunol 346:267–278
Gundersen K (2011) Excitation-transcription coupling in skeletal muscle: the molecular pathways of exercise. Biol Rev Camb Philos Soc 86(3):564–600
Head SI (2010) Branched fibres in old dystrophic mdx muscle are associated with mechanical weakening of the sarcolemma, abnormal Ca2+ transients and a breakdown of Ca2+ homeostasis during fatigue. Exp Physiol 95(5):641–656
Ito N, Ruegg UT, Kudo A, Miyagoe-Suzuki Y, Takeda S (2013) Activation of calcium signaling through Trpv1 by nNOS and peroxynitrite as a key trigger of skeletal muscle hypertrophy. Nat Med 19(1):101–106
Jorgensen LH, Blain A, Greally E, Laval SH, Blamire AM, Davison BJ, Brinkmeier H, MacGowan GA, Schroder HD, Bushby K, Straub V, Lochmüller H (2011) Long-term blocking of calcium channels in mdx mice results in differential effects on heart and skeletal muscle. Am J Pathol 178(1):273–283
Kis K, Liu X, Hagood JS (2011) Myofibroblast differentiation and survival in fibrotic disease. Expert Rev Mol Med 13:e27
Kornegay JN, Childers MK, Bogan DJ, Bogan JR, Nghiem P, Wang J, Fan Z, Howard JF Jr, Schatzberg SJ, Dow JL, Grange RW, Styner MA, Hoffman EP, Wagner KR (2012) The paradox of muscle hypertrophy in muscular dystrophy. Phys Med Rehabil Clin N Am 23(1):149–172
Lang F, Böhmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V (2006) (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev 86(4):1151–1178
Lynch GS, Hinkle RT, Faulkner JA (2001) Force and power output of diaphragm muscle strips from mdx and control mice after clenbuterol treatment. Neuromuscul Disord 11(2):192–196
McPherron AC, Lawler AM, Lee SJ (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387(6628):83–90
Mezzano V, Cabrera D, Vial C, Brandan E (2007) Constitutively activated dystrophic muscle fibroblasts show a paradoxical response to TGF-beta and CTGF/CCN2. J Cell Commun Signal 1(3–4):205–217
Morrison J, Lu QL, Pastoret C, Partridge T, Bou-Gharios G (2000) T-cell-dependent fibrosis in the mdx dystrophic mouse. Lab Invest 80(6):881–891
Mosqueira M, Zeiger U, Förderer M, Brinkmeier H, Fink RH (2013) Cardiac and respiratory dysfunction in Duchenne muscular dystrophy and the role of second messengers. Med Res Rev 33(5):1174–1213
Onofre-Oliveira PC, Santos AL, Martins PM, Ayub-Guerrieri D, Vainzof M (2012) Differential expression of genes involved in the degeneration and regeneration pathways in mouse models for muscular dystrophies. Neuromolecular Med 14(1):74–83
Pritschow BW, Lange T, Kasch J, Kunert-Keil C, Liedtke W, Brinkmeier H (2011) Functional TRPV4 channels are expressed in mouse skeletal muscle and can modulate resting Ca2+ influx and muscle fatigue. Pflügers Arch 461(1):115–122
Shkryl VM, Martins AS, Ullrich ND, Nowycky MC, Niggli E, Shirokova N (2009) Reciprocal amplification of ROS and Ca2+ signals in stressed mdx dystrophic skeletal muscle fibers. Pflügers Arch 458(5):915–928
Spencer MJ, Tidball JG (2001) Do immune cells promote the pathology of dystrophin-deficient myopathies? Neuromuscul Disord 11(6–7):556–564
Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, Narusawa M, Leferovich JM, Sladky JT, Kelly AM (1991) The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature 352(6335):536–539
Stinckens A, Van den Maagdenberg K, Luyten T, Georges M, De Smet S, Buys N (2007) The RYR1 g.1843C>T mutation is associated with the effect of the IGF2 intron3-g.3072G>A mutation on muscle hypertrophy. Anim Genet 38(1):67–71
Sun G, Haginoya K, Wu Y, Chiba Y, Nakanishi T, Onuma A, Sato Y, Takigawa M, Iinuma K, Tsuchiya S (2008) Connective tissue growth factor is overexpressed in muscles of human muscular dystrophy. J Neurol Sci 267(1–2):48–56
Vallon V, Wyatt AW, Klingel K, Huang DY, Hussain A, Berchtold S, Friedrich B, Grahammer F, Belaiba RS, Gorlach A, Wulff P, Daut J, Dalton ND, Ross J Jr, Flogel U, Schrader J, Osswald H, Kandolf R, Kuhl D, Lang F (2006) SGK1-dependent cardiac CTGF formation and fibrosis following DOCA treatment. J Mol Med (Berl) 84(5):396–404
Wallace GQ, McNally EM (2009) Mechanisms of muscle degeneration, regeneration, and repair in the muscular dystrophies. Annu Rev Physiol 71:37–57
Webster C, Blau HM (1990) Accelerated age-related decline in replicative life-span of Duchenne muscular dystrophy myoblasts: implications for cell and gene therapy. Somat Cell Mol Genet 16(6):557–565
Wertz K, Füchtbauer EM (1998) Dmd(mdx-beta geo): a new allele for the mouse dystrophin gene. Dev Dyn 212(2):229–241
Wynn TA (2008) Cellular and molecular mechanisms of fibrosis. J Pathol 214(2):199–210
Yamahara H, Kishimoto N, Nakata M, Okazaki A, Kimura T, Sonomura K, Matsuoka E, Shiotsu Y, Adachi T, Matsubara H, Iwasaka T, Mori Y (2009) Direct aldosterone action as a profibrotic factor via ROS-mediated SGK1 in peritoneal fibroblasts. Kidney Blood Press Res 32(3):185–193
Acknowledgments
We are grateful to Ms. H. Kenk for the expert technical assistance and we thank U. Lendeckel for kindly providing the anti-SGK1 antibody. This work was supported by project grants from the European Commission (FP7-REGPOT-2010, grant no. 264143) and Deutsche Duchenne Stiftung der aktion benni & co. e.V. to H. B. and a Gerhardt Domagk scholarship of the University Medicine Greifswald to Martin Steinberger, F. L. obtained support from the Deutsche Forschungsgemeinschaft (DFG).
Conflict of interest
The authors declare that no conflict of interest exists.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 261 kb)
Rights and permissions
About this article
Cite this article
Steinberger, M., Föller, M., Vogelgesang, S. et al. Lack of the serum- and glucocorticoid-inducible kinase SGK1 improves muscle force characteristics and attenuates fibrosis in dystrophic mdx mouse muscle. Pflugers Arch - Eur J Physiol 467, 1965–1974 (2015). https://doi.org/10.1007/s00424-014-1645-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-014-1645-5