Abstract
Purpose
Colorectal liver metastases (CRLM) are the predominant factor limiting survival in patients with colorectal cancer. Multimodal treatment strategies are frequently necessary to achieve total tumor elimination. This study examines the efficacy of liver resection combined with local ablative therapy in comparison to liver resection only, in the treatment of patients with ≥ 4 CRLM.
Methods
This retrospective cohort study was conducted at the University Hospital RWTH Aachen, Germany. Patients with ≥ 4 CRLM in preoperative imaging, who underwent curative resection between 2010–2021, were included. Recurrent resections and deaths in the early postoperative phase were excluded. Ablation modalities included radiofrequency or microwave ablation, and irreversible electroporation. Differences in overall- (OS) and recurrence-free-survival (RFS) between patients undergoing combined resection-ablation vs. resection only, were examined.
Results
Of 178 included patients, 46 (27%) underwent combined resection-ablation and 132 (73%) resection only. Apart from increased rates of adjuvant chemotherapy in the first group (44% vs. 25%, p = 0.014), there were no differences in perioperative systemic therapy. Kaplan–Meier and log-rank test analyses showed no statistically significant differences in median OS (36 months for both, p = 0.638) or RFS (9 months for combined resection-ablation vs. 8 months, p = 0.921). Cox regression analysis showed a hazard ratio of 0.891 (p = 0.642) for OS and 0.981 (p = 0.924) for RFS, for patients undergoing resection only.
Conclusion
For patients with ≥ 4 CRLM, combined resection-ablation is a viable option in terms of OS and RFS. Therefore, combined resection-ablation should be considered for complete tumor clearance, in patients with multifocal disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) remains among the commonest and deadliest diseases worldwide, with up to 80% of CRC patients developing colorectal liver metastases (CRLM) [1, 2]. Curative liver resection ensures the best survival outcomes, but patients frequently present with complex disease, requiring multimodal treatment strategies [3]. These include multi-stage hepatectomies (MSH), portal vein embolization (PVE) or Associating Liver Partition and Portal vein ligation for Staged hepatectomy (ALPPS), local ablative or chemoembolization therapies and perioperative chemotherapy [4].
Local ablative therapy (LAT) plays an increasingly important role in the management of CRLM, as an adjunct to resection or standalone treatment [5, 6]. Common modalities include radiofrequency ablation (RFA), microwave ablation (MWA) and irreversible electroporation (IRE) [5,6,7], and can be conducted intraoperatively or percutaneously [3, 6,7,8]. Technological advancements and accumulated experience have resulted in ever-improving results [7, 8], so that LAT is now included in official guidelines for multimodal therapy of CRLM [3, 9]. However, evidence on the efficacy of LAT compared to resection remains limited and heterogeneous [6, 9], partly because studies have focused on LAT of irresectable lesions, with inherent bias regarding long-term outcomes [6, 10].
Data regarding combined resection and LAT for multiple CRLM is also sparse [11], despite the advantages of LAT being particularly pertinent in these patients, which were historically deemed unresectable [12,13,14,15,16,17]. Even now, multiple CRLM are considered a predictor of poor survival or increased recurrence risk [12,13,14,15,16,17]. Although attitudes shifted to consider these patients as surgically treatable, especially in combination with LAT [18], it has been shown that patients with ≥ 4 CRLM remain at a particular disadvantage [15, 19, 20].
The aim of this study was to compare overall- (OS) and recurrence-free (RFS) survival between patients undergoing combined resection-ablation or resection only, for ≥ 4 CRLM.
Materials and methods
This study was conducted under ethical approval of the Institutional Review Board of the RWTH Aachen University (EK-001/21) and in accordance with the current version of the Declaration of Helsinki, the Declaration of Istanbul, and good clinical practice guidelines (ICHGCP). Informed consent was waived due to the retrospective study design and collection of readily available clinical data. Furthermore, the study was performed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [21].
Patient cohort and inclusion criteria
Consecutive adult patients with ≥ 4 CRLM undergoing elective liver resections in curative intent at the University Hospital RWTH Aachen between 2010 and 2021 were eligible for inclusion in this retrospective study. Patients operated for recurrences, those dying within 90 days postoperatively and those with unresected primary tumor (PT) or liver metastases were excluded. Patients were divided into those who underwent resection only (RES group), or additional intraoperative or percutaneous LAT (RESABL group). The primary and secondary endpoints were OS and RFS, respectively.
Data collection
Data was obtained from a prospectively-maintained retrospective database [4]. The Union for International Cancer Control (UICC) tumor / lymph node /metastasis (TNM) system was used for PT staging and the Brisbane classification [22] was used to describe liver resections, which were designated major, when involving ≥ 3 segments. Preoperative computerized tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET) scans were used to determine the number, size, and location of CRLM, as well as the presence of extrahepatic metastases.
Metastases diagnosed within 3 months of the PT were defined as synchronous. Chemotherapy regimens were defined as previously described [4]: neoadjuvant before resection of rectum carcinoma; adjuvant after resection of advanced PT; inductive for initially unresectable CRLM; additive after liver resection, in patients with remaining systemic tumor load. Postoperative complications were stratified according to the Clavien-Dindo (CD) classification [23] and the Comprehensive Complication Index (CCI) [24].
Operative technique
Operative technique followed common clinical standards [4]. Intraoperative ultrasonography confirmed preoperative imaging findings and excluded new manifestations. In open surgery, the Cavitron Ultrasonic Surgical Aspirator (CUSA®, Integra LifeSciences, Plainsboro NJ, USA) was used for parenchymal transection, with clipping or ligation of vascular and biliary structures. In laparoscopic cases, either the THUNDERBEAT (Olympus K.K., Tokyo, Japan), HARMONIC ACE® (Ethicon Inc. Somerville, NJ, USA) or laparoscopic CUSA® (Integra LifeSciences, Plainsboro NJ, USA) devices were employed, combined with ECHELON™ vascular staplers (Ethicon, Somerville, New Jersey, USA) or Weck® Hem-o-lok® polymer clips (Teleflex Inc., Pennsylvania, USA). Pringle maneuvers were applied as needed. Anatomical or parenchyma-sparing resections were chosen according to general patient condition, preoperative liver function tests, and limiting factors, such as macrovascular invasion. Resection margins were controlled intraoperatively with frozen section examination.
Ablative technique
Ablations were performed intraoperatively under ultrasonography guidance, or percutaneously under CT control, in general anesthesia. Manufacturer recommendations and standardized treatment protocols were strictly adhered to. For RFA, a monopolar system (RF 3000, Boston Scientific, Marlborough, MA, USA) was used, with a 480 kHz frequency and variable output up to 200W through an umbrella-shaped electrode (LeVeen, Boston Scientific, Marlborough, MA, USA). Depending on lesion size, an array diameter of 2 cm-5 cm was used to ensure complete lesion coverage, including a safety margin of ≥ 5 mm. Ablation procedures comprised two cycles, separated by a 120-s pause. Each cycle stopped when tissue impedance reached > 500Ω. For MWA, the Emprint Ablation System with Thermosphere Technology (Medtronic, Dublin, Ireland) was used. Ablation parameters and antenna shaft length (15 cm or 20 cm) were chosen according to size and location of the target lesion, aiming for a 5 mm safety margin. Finally, IRE was conducted using unipolar, 19-gauge probes (NanoKnife, AngioDynamics, Latham, New York, USA) with an active tip length of 15 mm-25 mm. The number of probes depended on target lesion size and intended margin width. Under electrocardiographic gating, 70 pulses of 90 µs duration were applied per probe pair, with 3000 V maximum voltage. To confirm successful ablation and exclude complications, a triphasic liver CT was performed after each LAT.
Indication for ablation
Treatment strategies were set in multidisciplinary team meetings. For technically irresectable lesions, LAT was generally combined with systemic therapy and resection of further hepatic metastases, if present. Frequently, IRE was employed to protect vessels and bile ducts near target lesions. For resectable lesions, LAT was utilized to avoid staged resections in patients with comorbidities or to minimize loss of liver parenchyma, where disproportionately large resections were required. Concepts were often individualized, based on patient and tumor characteristics. The decision to ablate was sometimes made intraoperatively, for example, when new, functionally irresectable lesions were identified.
Propensity-score-matching analysis
To address our study’s inherent selection bias, a propensity score matching (PSM) analysis was carried out. Variables chosen for matching were age, sex, ASA score, synchronicity of metastases, chemotherapy regimens, major and multi-stage resections, number of metastases and MDLL.
Statistical analysis
Groups were compared using Mann–Whitney U, Chi-square, or Fisher’s exact tests. Independent risk factors for OS and RFS were identified through uni- and multivariable Cox regression analyses and hazard ratios (HR) were given with 95% confidence intervals (CI). Factors with p < 0.10 in the univariable analysis were considered for inclusion in the multivariable model. Median follow-up time was calculated using the reverse Kaplan–Meier technique. Differences in survival were compared with Kaplan–Meier analysis and the log-rank test. Results were reported as medians and interquartile range (IQR, given as 1st-3rd quartiles) for continuous variables, or absolute and relative frequencies for categorical and ordinal variables. All p-values < 0.05 were considered statistically significant. Statistical analysis and graph generation was performed using SPSS Statistics v29 (IBM Corp., Armonk, NY, USA) and Prism v9.0 (GraphPad Software, La Jolla, CA, USA), respectively.
Results
Patient characteristics
One hundred seventy-eight patients were included (Fig. 1), with a median age of 62 (53–67) years. The commonest PT sites were rectum (46%), sigmoid (24%) and ascending colon (14%). Most PT were staged T3 (68%) and/or N1 (40%). Metastases were overwhelmingly bilateral (87%) and mostly synchronous (79%). The RESABL group comprised 46 (26%) patients. The RES group had more synchronous metastases (83% vs. 67%, p = 0.030), fewer lesions (67% vs 48% with 4–6 lesions, 17% vs 39% with 7–9 lesions, p = 0.007), larger median diameter of the largest lesion (MDLL, 3.0 cm vs. 2.2 cm, p = 0.01), higher preoperative serum carcinoembryonic antigen (CEA, 7.0 µg/l vs. 4.0 µg/l, p = 0.03) and lower rates of adjuvant chemotherapy (25% vs. 44%, p = 0.01), Detailed information is provided in Table 1. Fifty-three ablative procedures were carried out, of which 21(40%) were RFA, 20 (37%) MWA and 12(23%) IRE. Six (13%) patients underwent > 1 LAT, including a simultaneous left-sided IRE and right-sided RFA for one patient (Table 2).
Operative data and postoperative morbidity
Intra- and perioperative data is summarized in Table 3. Sixty-eight per cent of patients underwent major resections and 26% required MSH, including ALPPS. Patients in the RES group underwent significantly more major resections (75% vs. 48%, p < 0.001), especially extended hepatectomies or trisectionectomies (24% vs. 0%, p < 0.001). On the other hand, patients in the RESABL group underwent up to 3 atypical resections significantly more often (26% vs 7%, p < 0.001). The RES group underwent significantly more anatomical resections (38% vs. 15%, p = 0.005), whereas the opposite was true for atypical resections (37% vs. 15%, p = 0.002). Finally, there was a significantly higher proportion of patients without complications (CD = 0) in the RESABL group (24% vs. 11%, p = 0.038), as well as a significantly lower median CCI (15.0 vs. 24.2, p = 0.019).
Survival analysis
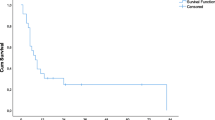
Median follow-up was 50 months, with no differences between the two groups (p = 0.28). Median OS and RFS for the study cohort were 36 months (95%CI 31–41) and 8 months (95%CI 6–10), respectively. There were no differences in median OS (RESABL: 36 months, 95%CI 19–53, vs. RES: 36 months, 95%CI 28–44, p = 0.64) or RFS (RESABL: 9 months, 95%CI 4–14, vs. RES: 8 months, 95%CI 6–10, p = 0.92) between the two groups (Figs. 2 and 3). Furthermore, there were no differences in median OS (RFA 52 months vs. MWA 39 months vs. IRE 32 months, p = 0.248) or RFS (RFA 14 months vs. MWA 6 months vs. IRE 4 months, p = 0.111) according to ablation type (Supplementary Fig. 1). Moreover, there were local recurrences after 25 (47%) ablative procedures, with the median local-recurrence-free survival (LRFS) being 19 months for all 53 procedures and no significant differences in local recurrence rates between ablation modalities (Table 2). As shown in Supplementary Fig. 2, there were no differences in median LRFS according to ablation type (RFA 20 months vs. MWA 18 months vs. IRE 20 months, p = 0.664).
As shown in Tables 4 and 5, inductive chemotherapy was an independent risk factor for OS (HR 1.761, 95%CI 1.006–3.066, p = 0.047) and RFS (HR 1.488, 95%CI 1.042–2.125, p = 0.03), and bilateral metastases for RFS only (HR 1.886, 95%CI 1.069–3.328, p = 0.03). Treatment strategy (RESABL or RES) was neither a risk factor for OS (p = 0.64), nor for RFS (p = 0.92).
A subgroup analysis was carried out on patients with bilateral CRLM (n = 155, 87%), to further investigate the effect on survival. Bilateral metastasis was associated with significantly reduced RFS (7 months vs. 12 months, p = 0.008), but not OS (36 months vs. 39 months, p = 0.21), compared to the rest of the cohort (Supplementary Fig. 3). However, in patients with bilateral metastases, no differences between the RESABL and RES groups regarding OS (36 months vs. 35 months, p = 0.78) or RFS (9 months vs. 6 months, p = 0.63) were observed (Supplementary Fig. 4).
Propensity-score-matching analysis
The PSM analysis resulted in 44 matched pairs, leaving two patients in the RESABL group unmatched, with a total cohort size of 88. As can be seen in Supplementary Table 1, most previously significant differences between the groups have either no or reduced statistical significance after PSM. The only exception are major resections, which even after PSM are significantly increased in the RES group (p < 0.001). Survival analysis still showed no differences in median OS (RES 29 months vs. RESABL 39 months, p = 0.292) or RFS (RES 6 months vs. RESABL 9 months, p = 0.797), after PSM (Supplementary Fig. 5). Cox regression excluded the treatment group as a predictor of OS or RFS, with HR of 0.745 (95%CI 0.427–1.300, p = 0.294) and 0.941 (95%CI 0.582–1.524, p = 0.805), respectively.
Discussion
In this study, we compared combined resection-ablation with resection alone, in patients with ≥ 4 CRLM, undergoing curative-intent treatment. We found no difference in OS or RFS between the groups, suggesting that multimodal therapy is a viable alternative to resection alone.
A higher rate of synchronous metastases, higher median CEA, larger MDLL, and higher rate of major resections were seen in the RES group. The higher rate of synchronous disease is probably linked to the treatment of PT and CRLM, which may involve multiple operations. This provides an opportunity to resect bilateral metastases in multiple steps, obviating the need for LAT. On the contrary, combining resection with LAT may spare multiple operations in patients with metachronous disease. The higher rate of synchronous disease would explain the higher median CEA, stemming from the combined tumor burden of PT and metastases. Furthermore, the larger MDLL reflects the limits of LAT regarding target lesion size. Generally, ablation of CRLM larger than 3 cm is not recommended, regardless of LAT modality [25]. Finally, the higher rate of major resections is probably due to large metastases, which were non-amenable to LAT or other parenchyma-sparing techniques.
In the RESABL group, more patients with ≥ 7 lesions and a higher rate of adjuvant chemotherapy were observed. As the chances of deep-lying or irresectable lesions are increased in patients with multiple lesions, adjunct LAT is more often applied. Finally, the higher rate of adjuvant therapy is probably linked to the frequency of metachronous metastases. In the case of resectable synchronous disease, there is no proven benefit of adjuvant therapy after resection of PT and CRLM (unless remaining systemic tumor or other risk factors are present) [3]. On the contrary, patients with UICC Stage III CRC and/or risk factors receive adjuvant treatment as standard, after which metachronous metastases may be diagnosed. Therefore, the group with more metachronous metastases should also exhibit higher rates of adjuvant therapy after resection of the PT.
So far, results from studies comparing resection and LAT for CRLM are inconsistent. Some reports show no difference in 3- or 5-year OS or RFS after propensity-score- [6, 26,27,28] or case-matching [29]. On the contrary, a systematic review by Kron et al. summarizing 18 non-randomized studies for resection vs. RFA, found a significantly better OS, RFS, and local recurrence rate in patients undergoing surgery [5]. However, these studies were heterogeneous regarding ablation modality (RFA, MWA or both) and route (laparoscopic, open, or interventional), were partly in the early 00’s (before modern chemotherapy and tumor biology concepts), and some investigated fairly small cohorts. Others compared MWA against combined resection-MWA, with contradicting results. For example, Stattner et al. demonstrated a significantly reduced OS and RFS with MWA alone [30], while Philips et al. found a longer median OS in the MWA group, and no difference in RFS [31]. None of these studies included resection alone [30, 31]. To date, few randomized controlled trials (RCT) have investigated this topic. The LAVA trial compared RFS after resection or ablation in high-risk surgical patients eligible for resection, but closed prematurely because of poor recruitment [32]. Final results are pending from the COLLISION RCT, which includes patients with at least one resectable and ablatable CRLM, ≤ 3 cm in diameter [33].
Contrary to our study, some previous reports have shown superior OS and RFS for patients with CRLM undergoing resection, compared to combined resection-ablation. Particularly, Abdalla et al. [34] and Gleisner et al. [35] investigated RFA alone, RFA combined with resection, and resection alone. Both studies showed significantly better OS and RFS for resection, compared to the other options [34, 35]. However, they did not focus on patients with multiple metastases and their cohorts stemmed mainly from the 90’s to early 00’s, before modern chemotherapy regimens were introduced. Similarly, Amerongen et al. found better 5-year OS and RFS in patients undergoing resection, compared to combined resection and intraoperative RFA [36]. However, they excluded patients undergoing MSH or synchronous resections of PT and CRLM, and those with extrahepatic disease. Additionally, significantly fewer R0 resections were reported in the combined group [36]. Other studies, including a meta-analysis by Meijerink et al., found no significant differences in OS or RFS between combined resection-ablation and resection alone [10, 37,38,39].
Regarding patients with ≥ 4 CRLM, De Jong et al. investigated a subgroup (n = 192) of an international cohort and demonstrated a higher risk of intrahepatic recurrence in those undergoing combined resection-ablation, compared to resection alone. However, no difference in OS or 5-year survival was observed [11]. More recently, Masuda et al. compared the two treatment strategies in a patient cohort divided into < 4 (n = 568) and ≥ 4 (n = 149) lesions [40]. Combined resection with RFA resulted in poorer OS in the < 4 lesions group, whereas no difference was seen in patients with ≥ 4 lesions. Additionally, KRAS mutation, positive PT N-status, and extrahepatic metastases were independent predictors of poor survival, contrary to our study. In agreement with our study, the combination of resection with RFA was not a predictor of poor prognosis [40]. A limitation of both studies is the inclusion of patients operated before modern chemotherapy regimens and concepts of tumor biology, especially in the first study, whose cohort originated between 1984–2009.
Our analysis precluded treatment strategy (RESABL or RES) as a risk factor for OS or RFS. In fact, only inductive chemotherapy was an independent risk factor fo r both measures of survival. One might speculate, that this was an indicator of advanced, initially irresectable disease, rather than a biological effect of the chemotherapy itself. The same association between inductive chemotherapy and OS was previously observed in our data [4]. Regarding RFS only, bilateral metastasis proved to be an additional independent risk factor. To further investigate this, we conducted a subgroup analysis in patients with bilateral disease, which found no differences in OS or RFS between the RESABL or RES groups. From this we conclude, that the increased recurrence risk in bilateral CRLM is an effect of tumor load and biology, rather than the choice of treatment strategy.
Some studies investigated the effect of risk factors, such as tumor biology, on survival after combined resection-ablation. For example, Sasaki et al. compared combined resection-ablation to resection alone and stratified patients according to risk factors, such as PT N-status, KRAS status and bilateral metastases [41]. Patients stratified as low-risk had similar 5-year OS to the resection-only group, whereas outcomes in the high-risk group were significantly worse [41]. In our study, KRAS status was only significant in the univariable analysis (trending towards significance in the multivariable analysis) for OS. Moreover, PT N-status was not a predictor of OS or RFS. As mentioned above, bilateral metastases were an independent risk factor for RFS. Furthermore, a study by Vles et al. demonstrated worse OS and local tumor progression rates in patients with non-desmoplastic histopathological growth patterns undergoing combined resection-ablation for CRLM [42]. We currently have no such information regarding our cohort.
Our study has certain strengths, such as its focus on patients with ≥ 4 metastases. This is particularly relevant in an era of increasingly aggressive multimodal strategies, for the treatment of patients with multiple CRLM. Compared to ours, many of the aforementioned studies are older, before modern chemotherapy regimens and surgical techniques became widespread, and some even excluded staged resections, such as ALPPS. We included all operative strategies and demonstrated homogeneity regarding the frequency of major resections, MSH, and PVE, between the two groups. Certain limitations should be considered when interpreting the results of this study, including its retrospective design, monocentric nature, and modest cohort size. Additionally, we analyzed different ablation modalities, conducted both intraoperatively and percutaneously, which may exhibit different outcomes when analyzed individually. However, this reflects modern clinical practice, where various LAT are used, according to tumor localization and general treatment concept. Additionally, there is an inherent bias in our study, as generally only irresectable tumors were ablated, or those that would significantly increase the extent of liver resection. Finally, as with any retrospective study, missing data is also a problem.
Despite these limitations, we have shown combined resection-ablation to be a viable solution for patients with multiple CRLM, which does not confer any disadvantages in terms of OS or RFS. In general, oncological results after RFA / MWA are continuously improving and the two modalities are similar in terms of local disease control [8]. More data is required concerning the role and efficacy of IRE in CRLM, which is promising, especially in cases where thermal ablation is contraindicated [7]. In any case, LAT presents a viable treatment option, particularly for metastases in unfavorable localizations, as its parenchyma-sparing properties allow for re-resection or re-ablation of recurrences [7, 43]. Further studies are warranted, especially prospective, multicentric RCT. Results from the aforementioned COLLISION trial are eagerly awaited. Meanwhile, well-designed retrospective or non-randomized prospective studies are necessary to further explore the role of LAT in patients with CRLM.
Conclusion
The combination of resection with LAT constitutes a viable treatment option in patients with multiple (≥ 4) CRLM. Overall- and recurrence-free survival in these patients is comparable to those undergoing resection alone. Further studies are necessary to better define the role of LAT in the multimodal treatment of patients with CRLM.
Data availability
Data can be made available upon reasonable request to the corresponding author.
Abbreviations
- ALPPS:
-
Associating liver partition and portal vein ligation for staged hepatectomy
- ASA:
-
American society of anesthesiology
- BMI:
-
Body mass index
- CCI:
-
Comprehensive complication index.
- CD:
-
Clavien-Dindo grade
- CEA:
-
Carcinoembryonic antigen
- CI:
-
Confidence interval
- CRC:
-
Colorectal cancer
- CRLM:
-
Colorectal liver metastases
- CT:
-
Computerized tomography
- CUSA:
-
Cavitron ultrasonic surgical aspirator
- DFS:
-
Disease-free survival
- ESMO:
-
European society for medical oncology
- HR:
-
Hazard ratio
- ICU:
-
Intensive care unit
- IMC:
-
Intermediate care unit
- IQR:
-
Interquartile range
- IRE:
-
Irreversible electroporation
- KRAS:
-
Kirsten rat sarcoma viral oncogene homolog
- LAT:
-
Local ablative therapy
- LRFS:
-
Local-recurrence-free survival
- MDLL:
-
Median diameter of the largest lesion
- MRI:
-
Magnetic resonance imaging
- MSH:
-
Multi-stage hepatectomy
- MWA:
-
Microwave ablation
- OS:
-
Overall survival
- PET:
-
Positron emission tomography
- PT:
-
Primary tumor
- PVE:
-
Portal vein embolization
- PSM:
-
Propensity score matching
- R0:
-
Resection margin negative
- R1:
-
Resection margin microscopically positive
- RAS:
-
Rat sarcoma viral oncogene homolog
- RCT:
-
Randomised controlled trial
- RES:
-
Resection-only group
- RESABL:
-
Combined resection-ablation group
- RFA:
-
Radiofrequency ablation
- RFS:
-
Recurrence-free survival
- TNM:
-
Tumor, lymph node, metastasis
- UICC:
-
Union for international cancer control
References
Sung H et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin 71(3):209–249
Osterlund P et al (2021) Repeated centralized multidisciplinary team assessment of resectability, clinical behavior, and outcomes in 1086 Finnish metastatic colorectal cancer patients (RAXO): a nationwide prospective intervention study. Lancet Reg Health Eur 3:100049
Cervantes A et al (2023) Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 34(1):10–32. https://doi.org/10.1016/j.annonc.2022.10.003
Amygdalos I et al (2023) Novel machine learning algorithm can identify patients at risk of poor overall survival following curative resection for colorectal liver metastases. J Hepatobiliary Pancreat Sci 30(5):602-614. https://doi.org/10.1002/jhbp.1249
Kron P et al (2019) Ablation or resection for colorectal liver metastases? A systematic review of the literature. Front Oncol 9:1052
Tinguely P et al (2020) Microwave ablation versus resection for colorectal cancer liver metastases - a propensity score analysis from a population-based nationwide registry. Eur J Surg Oncol 46(3):476–485
Hitpass L et al (2022) Recurrent colorectal liver metastases in the liver remnant after major liver surgery-IRE as a salvage local treatment when resection and thermal ablation are unsuitable. Cardiovasc Intervent Radiol 45(2):182–189
Puijk RS et al (2022) Improved outcomes of thermal ablation for colorectal liver metastases: a 10-year Analysis from the Prospective Amsterdam CORE Registry (AmCORE). Cardiovasc Intervent Radiol 45(8):1074–1089
German Guideline Program in Oncology (German Cancer Society, German Cancer Aid, AWMF): S3-Guideline Colorectal Cancer, long version 2.1, 2019, AWMF registrationnumber: 021–007OL. Available from: http://www.leitlinienprogramm-onkologie.de/leitlinien/kolorektales-karzinom/
Meijerink MR et al (2018) Radiofrequency and microwave ablation compared to systemic chemotherapy and to partial hepatectomy in the treatment of colorectal liver metastases: a systematic review and meta-analysis. Cardiovasc Intervent Radiol 41(8):1189–1204
de Jong MC et al (2011) Therapeutic efficacy of combined intraoperative ablation and resection for colorectal liver metastases: an international, multi-institutional analysis. J Gastrointest Surg 15(2):336–344
Buisman FE et al (2022) Predicting 10-year survival after resection of colorectal liver metastases; an international study including biomarkers and perioperative treatment. Eur J Cancer 168:25–33
Torzilli G et al (2016) Surgery of colorectal liver metastases: pushing the limits. Liver Cancer 6(1):80–89
Fong Y et al (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230(3):309–18 (discussion 318-21)
Minagawa M et al (2007) Simplified staging system for predicting the prognosis of patients with resectable liver metastasis: development and validation. Arch Surg 142(3):269–276
Wong GYM et al (2022) Prognostic models incorporating RAS mutation to predict survival in patients with colorectal liver metastases: a narrative review. Cancers (Basel) 14(13):3223. https://doi.org/10.3390/cancers14133223
Kawaguchi Y et al (2021) Contour prognostic model for predicting survival after resection of colorectal liver metastases: development and multicentre validation study using largest diameter and number of metastases with RAS mutation status. Br J Surg 108(8):968–975. https://doi.org/10.1093/bjs/znab086
Pawlik TM et al (2006) Debunking dogma: surgery for four or more colorectal liver metastases is justified. J Gastrointest Surg 10(2):240–248
Schindl M et al (2005) Prognostic scoring in colorectal cancer liver metastases: development and validation. Arch Surg 140(2):183–189
Hokuto D et al (2016) The prognosis of liver resection for patients with four or more colorectal liver metastases has not improved in the era of modern chemotherapy. J Surg Oncol 114(8):959–965
von Elm E et al (2008) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 61(4):344–349
Strasberg SM et al (2000) The Brisbane 2000 terminology of liver anatomy and resections. HPB 2(3):333–339
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Slankamenac K et al (2013) The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 258(1):1–7
Crocetti L et al (2020) CIRSE standards of practice on thermal ablation of liver tumours. Cardiovasc Intervent Radiol 43(7):951–962
Tinguely P et al (2023) Ablation versus resection for resectable colorectal liver metastases - health care related cost and survival analyses from a quasi-randomised study. Eur J Surg Oncol 49(2):416–425. https://doi.org/10.1016/j.ejso.2022.09.006
Xu Y et al (2022) Thermal ablation versus hepatic resection for colorectal cancer with synchronous liver metastases: a propensity score matching study. Eur Radiol 32(10):6678-6690. https://doi.org/10.1007/s00330-022-09080-z
van de Geest TW et al (2022) Propensity score matching demonstrates similar results for radiofrequency ablation compared to surgical resection in colorectal liver metastases. Eur J Surg Oncol 48(6):1368–1374. https://doi.org/10.1016/j.ejso.2022.01.008
Guadagni S et al (2022) Surgery combined with intra-operative microwaves ablation for the management of colorectal cancer liver metastasis: a case-matched analysis and evaluation of recurrences. Front Oncol 12:1023301
Stattner S et al (2013) Microwave ablation with or without resection for colorectal liver metastases. Eur J Surg Oncol 39(8):844–849
Philips P et al (2016) Single-stage resection and microwave ablation for bilobar colorectal liver metastases. Br J Surg 103(8):1048–1054
Davidson B et al (2020) Liver resection surgery compared with thermal ablation in high surgical risk patients with colorectal liver metastases: the LAVA international RCT. Health Technol Assess 24(21):1–38
Puijk RS et al (2018) Colorectal liver metastases: surgery versus thermal ablation (COLLISION) - a phase III single-blind prospective randomized controlled trial. BMC Cancer 18(1):821
Abdalla EK et al (2004) Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 239(6):818–25 (discussion 825-7)
Gleisner AL et al (2008) Colorectal liver metastases: recurrence and survival following hepatic resection, radiofrequency ablation, and combined resection-radiofrequency ablation. Arch Surg 143(12):1204–1212
van Amerongen MJ et al (2016) Short term and long term results of patients with colorectal liver metastases undergoing surgery with or without radiofrequency ablation. Eur J Surg Oncol 42(4):523–530
Eltawil KM et al (2014) Patterns of recurrence following selective intraoperative radiofrequency ablation as an adjunct to hepatic resection for colorectal liver metastases. J Surg Oncol 110(6):734–738
Faitot F et al (2014) Two-stage hepatectomy versus 1-stage resection combined with radiofrequency for bilobar colorectal metastases: a case-matched analysis of surgical and oncological outcomes. Ann Surg 260(5):822–7 (discussion 827-8)
Liu M et al (2021) Short- and long-term outcomes of hepatectomy combined with intraoperative radiofrequency ablation for patients with multiple primarily unresectable colorectal liver metastases: a propensity matching analysis. HPB (Oxford) 23(10):1586–1594
Masuda T et al (2018) Combined hepatic resection and radio-frequency ablation for patients with colorectal cancer liver metastasis: a viable option for patients with a large number of tumors. Anticancer Res 38(11):6353–6360
Sasaki K et al (2016) Combined resection and RFA in colorectal liver metastases: stratification of long-term outcomes. J Surg Res 206(1):182–189
Vles MD et al (2022) Local tumour control after radiofrequency or microwave ablation for colorectal liver metastases in relation to histopathological growth patterns. HPB (Oxford) 24(9):1443–1452. https://doi.org/10.1016/j.hpb.2022.01.010
Hof J et al (2016) Outcomes after resection and/or radiofrequency ablation for recurrence after treatment of colorectal liver metastases. Br J Surg 103(8):1055–1062
Funding
Open Access funding enabled and organized by Projekt DEAL. This project was supported by internal departmental funding.
Author information
Authors and Affiliations
Contributions
Study conception and design: IA, LH, PB, SAL. Acquisition of data: IA, LH, AC, KJ, GJ, FS. Analysis and interpretation of data: IA, LH, AC, KJ, GJ, FS. Drafting of manuscript: IA, LH, PB, SAL. Critical revision of manuscript: JB, MLB, TL, SWMOD, TFU, UPN.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflicts of interest
The authors of this manuscript have no conflicts of interest to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amygdalos, I., Hitpass, L., Schmidt, F. et al. Survival after combined resection and ablation is not inferior to that after resection alone, in patients with four or more colorectal liver metastases. Langenbecks Arch Surg 408, 343 (2023). https://doi.org/10.1007/s00423-023-03082-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-023-03082-1