Abstract
Purpose
Real-time intraoperative perfusion assessment may reduce anastomotic leaks. Laser speckle contrast imaging (LSCI) provides dye-free visualization of perfusion by capturing coherent laser light scatter from red blood cells and displays perfusion as a colormap. Herein, we report a novel method to precisely quantify intestinal perfusion using LSCI.
Methods
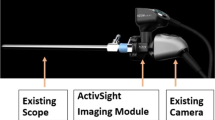
ActivSight™ is an FDA-cleared multi-modal visualization system that can detect and display perfusion via both indocyanine green imaging (ICG) and LSCI in minimally invasive surgery. An experimental prototype LSCI perfusion quantification algorithm was evaluated in porcine models. Porcine small bowel was selectively devascularized to create regions of perfused/watershed/ischemic bowel, and progressive aortic inflow/portal vein outflow clamping was performed to study arterial vs. venous ischemia. Continuous arterial pressure was monitored via femoral line.
Results
LSCI perfusion colormaps and quantification distinguished between perfused, watershed, and ischemic bowel in all vascular control settings: no vascular occlusion (p < 0.001), aortic occlusion (p < 0.001), and portal venous occlusion (p < 0.001). LSCI quantification demonstrated similar levels of ischemia induced both by states of arterial inflow and venous outflow occlusion. LSCI-quantified perfusion values correlated positively with higher mean arterial pressure and with increasing distance from ischemic bowel.
Conclusion
LSCI relative perfusion quantification may provide more objective real-time assessment of intestinal perfusion compared to conventional naked eye assessment by quantifying currently subjective gradients of bowel ischemia and identifying both arterial/venous etiologies of ischemia.




Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author, Peter CW Kim, upon reasonable request.
References
Turrentine FE et al (2015) Morbidity, mortality, cost, and survival estimates of gastrointestinal anastomotic leaks. J Am Coll Surg 220(2):195–206
Agzarian J et al (2019) The cost burden of clinically significant esophageal anastomotic leaks-a steep price to pay. J Thorac Cardiovasc Surg 157(5):2086–2092
Lee SW, Gregory D, Cool CL (2020) Clinical and economic burden of colorectal and bariatric anastomotic leaks. Surg Endosc 34(10):4374–4381
Lin J et al (2021) The efficacy of intraoperative ICG fluorescence angiography on anastomotic leak after resection for colorectal cancer: a meta-analysis. Int J Colorectal Dis 36(1):27–39
Cruz RJ Jr et al (2010) Regional blood flow distribution and oxygen metabolism during mesenteric ischemia and congestion. J Surg Res 161(1):54–61
Guzmán-de la Garza FJ et al (2009) Different patterns of intestinal response to injury after arterial, venous or arteriovenous occlusion in rats. World J Gastroenterol 15(31):3901
Kimura M et al (2003) Real-time energy metabolism of intestine during arterial versus venous occlusion in the rat. J Gastroenterol 38:849–853
Quero G et al (2019) Discrimination between arterial and venous bowel ischemia by computer-assisted analysis of the fluorescent signal. Surg Endosc 33(6):1988–1997
Chandler P, Wiesel O, Sherwinter DA (2021) Fluorescence-guided surgery of the esophagus. Ann Transl Med 9(10):908–908
Takeuchi M et al (2020) Effect of venous superdrainage on colon interposition for esophageal reconstruction. J UOEH 42(4):331–334
Tsao C-K et al (2004) Adequate venous drainage: the most critical factor for a successful free jejunal transfer. Ann Plast Surg 53(3):229–234
Ladak F et al (2019) Indocyanine green for the prevention of anastomotic leaks following esophagectomy: a meta-analysis. Surg Endosc 33(2):384–394
Nwaiwu CA et al (2023) Feasibility and comparison of laparoscopic laser speckle contrast imaging to near-infrared display of indocyanine green in intraoperative tissue blood flow/tissue perfusion in preclinical porcine models. Surg Endosc 37(2):1086–1095
Ambrus R et al (2017) Evaluation of gastric microcirculation by laser speckle contrast imaging during esophagectomy. J Am Coll Surg 225(3):395–402
Ambrus R et al (2017) A reduced gastric corpus microvascular blood flow during Ivor-Lewis esophagectomy detected by laser speckle contrast imaging technique. Scand J Gastroenterol 52(4):455–461
Briers D et al (2013) Laser speckle contrast imaging: theoretical and practical limitations. J Biomed Opt 18(6):066018
Draijer M et al (2009) Review of laser speckle contrast techniques for visualizing tissue perfusion. Lasers Med Sci 24(4):639–651
Zheng C, Lau LW, Cha J (2018) Dual-display laparoscopic laser speckle contrast imaging for real-time surgical assistance. Biomed Opt Express 9(12):5962–5981
Heeman W et al (2019) Application of laser speckle contrast imaging in laparoscopic surgery. Biomed Opt Express 10(4):2010–2019
Heeman W et al (2019) Clinical applications of laser speckle contrast imaging: a review. J Biomed Opt 24(08):1
Davis MA, Kazmi SM, Dunn AK (2014) Imaging depth and multiple scattering in laser speckle contrast imaging. J Biomed Opt 19(8):086001
Oberlin J, Dimaio E (2021) U.S. Patent No. 11,206,991: systems and methods for processing laser speckle signals. Activ Surgical: United States
Ronn JH et al (2019) Laser speckle contrast imaging and quantitative fluorescence angiography for perfusion assessment. Langenbecks Arch Surg 404(4):505–515
Barberio M et al (2020) Quantitative fluorescence angiography versus hyperspectral imaging to assess bowel ischemia: a comparative study in enhanced reality. Surgery 168(1):178–184
Barberio M et al (2020) HYPerspectral Enhanced Reality (HYPER): a physiology-based surgical guidance tool. Surg Endosc 34(4):1736–1744
D’Urso A et al (2021) Computer-assisted quantification and visualization of bowel perfusion using fluorescence-based enhanced reality in left-sided colonic resections. Surg Endosc 35(8):4321–4331
Nadort A et al (2016) Quantitative blood flow velocity imaging using laser speckle flowmetry. Sci Rep 6:25258
Lütken CD et al (2021) Quantification of fluorescence angiography: toward a reliable intraoperative assessment of tissue perfusion - a narrative review. Langenbeck’s Arch Surg 406(2):251–259
Takeda FR et al (2021) Supercharged cervical anastomosis for esophagectomy and gastric pull-up. J Thorac Cardiovasc Surg 162(3):688-697.e3
Tsui C, Klein R, Garabrant M (2013) Minimally invasive surgery: national trends in adoption and future directions for hospital strategy. Surg Endosc 27(7):2253–2257
Percie Du Sert N et al (2020) The ARRIVE guidelines 20: updated guidelines for reporting animal research. PLOS Biol 18(7):e3000410
Zaiontz C (2021) Real Statistics Resource Pack software
Cruz RJ Jr et al (2004) Effects of intra-aortic balloon occlusion on intestinal perfusion, oxygen metabolism and gastric mucosal PCO2 during experimental hemorrhagic shock. Eur Surg Res 36(3):172–178
Jacquet-Lagrèze M et al (2014) A new device for continuous assessment of gut perfusion: proof of concept on a porcine model of septic shock. Crit Care 18(4):R153
Diana M et al (2017) Precision real-time evaluation of bowel perfusion: accuracy of confocal endomicroscopy assessment of stoma in a controlled hemorrhagic shock model. Surg Endosc 31(2):680–691
Diana M et al (2015) Intraoperative fluorescence-based enhanced reality laparoscopic real-time imaging to assess bowel perfusion at the anastomotic site in an experimental model. Br J Surg 102(2):e169–e176
Wada T et al (2017) ICG fluorescence imaging for quantitative evaluation of colonic perfusion in laparoscopic colorectal surgery. Surg Endosc 31(10):4184–4193
Yukaya T et al (2015) Indocyanine green fluorescence angiography for quantitative evaluation of gastric tube perfusion in patients undergoing esophagectomy. J Am Coll Surg 221(2):e37-42
Baiocchi GL, Diana M, Boni L (2018) Indocyanine green-based fluorescence imaging in visceral and hepatobiliary and pancreatic surgery: state of the art and future directions. World J Gastroenterol 24(27):2921–2930
Holmer A et al (2018) Hyperspectral imaging in perfusion and wound diagnostics - methods and algorithms for the determination of tissue parameters. Biomed Tech (Berl) 63(5):547–556
Acknowledgements
We acknowledge Robin Grandl, PhD, Activ Surgical for review and editing.
Author information
Authors and Affiliations
Contributions
• Study conception and design: Yao Z Liu, Saloni Mehrotra, Chibueze A Nwaiwu, Vasiliy E Buharin, Steven D Schwaitzberg, and Peter CW Kim. • Acquisition of data: Yao Z Liu, Saloni Mehrotra, Vasiliy E Buharin, John Oberlin, Roman Stolyarov, and Peter CW Kim. • Analysis and interpretation of data: Yao Z Liu, Saloni Mehrotra, Vasiliy E Buharin, Roman Stolyarov, and Peter CW Kim. • Drafting of the manuscript: Yao Z Liu, Saloni Mehrotra, Chibueze A Nwaiwu, and Peter CW Kim. • Critical revision of the manuscript: All authors.
Corresponding author
Ethics declarations
This study was funded by Activ Surgical, Inc. Authors Yao Z Liu, Saloni Mehrotra, Chibueze A Nwaiwu, Vasiliy E Buharin, John Oberlin, Roman Stolaryov, and Peter CW Kim are employees of Activ Surgical. Author Steven D Schwaitzberg is on the advisory board of Activ Surgical, Human Extensions, New View Surgical, HAI Technology, Levitra Magnetics, and Arch Therapeutics.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y.Z., Mehrotra, S., Nwaiwu, C.A. et al. Real-time quantification of intestinal perfusion and arterial versus venous occlusion using laser speckle contrast imaging in porcine model. Langenbecks Arch Surg 408, 114 (2023). https://doi.org/10.1007/s00423-023-02845-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-023-02845-0