Abstract
Purpose
To clarify the efficacy of perioperative chemotherapy for the patients with resectable colorectal liver metastases (CLM), we conducted a multicenter randomized phase III trial to compare surgery followed by postoperative FOLFOX regimen with perioperative FOLFOX regimen plus cetuximab in patients with KRAS wild-type resectable CLM.
Methods
Patients who had KRAS wild-type resectable CLM having one to eight liver nodules without extrahepatic disease were randomly assigned to the postoperative chemotherapy group, wherein up-front hepatectomy was performed followed by 12 cycles of postoperative modified FOLFOX6, and the perioperative chemotherapy group (experimental), wherein six cycles of preoperative modified FOLFOX6 plus cetuximab were performed followed by hepatectomy and six cycles of postoperative modified FOLFOX6 plus cetuximab. The primary endpoint was progression-free survival (PFS).
Results
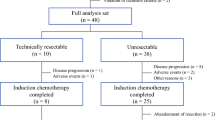
There were 37 patients in postoperative chemotherapy group and 40 patients in the perioperative chemotherapy group who were analyzed. Baseline characteristics were well-balanced between groups. The PFS and overall survival (OS) showed no significant difference (PFS, hazard ratio 1.18 [95% confidence interval 0.69–2.01], P = 0.539: OS, 1.03 [0.46–2.29], P = 0.950). In the postoperative chemotherapy group, 35.1% had a 3-year PFS, and 86.5% had a 3-year OS. Meanwhile, in the perioperative chemotherapy group, 30.0% had a 3-year PFS, and 74.4% had a 3-year OS.
Conclusion
There was no difference in survival found between the group of the perioperative chemotherapy plus cetuximab and that of the postoperative chemotherapy in the cohort of our study. The study was registered in the University Hospital Medical Information Network (UMIN000007787).



Similar content being viewed by others
Data availability
Not applicable.
Code Availability
Not applicable.
References
Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kawano H, Kinugasa Y, Kokudo N, Murofushi K, Nakajima T, Oka S, Sakai Y, Tsuji A, Uehara K, Ueno H, Yamazaki K, Yoshida M, Yoshino T, Boku N, Fujimori T, Itabashi M, Koinuma N, Morita T, Nishimura G, Sakata Y, Shimada Y, Takahashi K, Tanaka S, Tsuruta O, Yamaguchi T, Yamaguchi N, Tanaka T, Kotake K, Sugihara K (2018) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol 23:1–34. https://doi.org/10.1007/s10147-017-1101-6
Hasegawa K, Saiura A, Takayama T, Miyagawa S, Yamamoto J, Ijichi M, Teruya M, Yoshimi F, Kawasaki S, Koyama H, Oba M, Takahashi M, Mizunuma N, Matsuyama Y, Watanabe T, Makuuchi M, Kokudo N (2016) Adjuvant oral uracil-tegafur with leucovorin for colorectal cancer liver metastases: a randomized controlled trial. PLoS ONE 11:e0162400. https://doi.org/10.1371/journal.pone.0162400
Kawaguchi Y., Kopetz S., Newhook T. E., De Bellis M., Chun Y. S., Tzeng C. D., Aloia T. A., and Vauthey J. N. (2019) Mutation Status of RAS, TP53, and SMAD4 is superior to mutation status of RAS alone for predicting prognosis after resection of colorectal liver metastases clin cancer Res 25:5843–5851. https://doi.org/10.1158/1078-0432.Ccr-19-0863
Kawaguchi Y, Lillemoe HA, Panettieri E, Chun YS, Tzeng CD, Aloia TA, Kopetz S, Vauthey JN (2019) Conditional recurrence-free survival after resection of colorectal liver metastases: persistent deleterious association with RAS and TP53 co-mutation. J Am Coll Surg 229:286-294.e281. https://doi.org/10.1016/j.jamcollsurg.2019.04.027
Saiura A, Yamamoto J, Hasegawa K, Koga R, Sakamoto Y, Hata S, Makuuchi M, Kokudo N (2012) Liver resection for multiple colorectal liver metastases with surgery up-front approach: bi-institutional analysis of 736 consecutive cases. World J Surg 36:2171–2178. https://doi.org/10.1007/s00268-012-1616-y
Ayez N, van der Stok EP, Grunhagen DJ, Rothbarth J, van Meerten E, Eggermont AM, Verhoef C (2015) The use of neo-adjuvant chemotherapy in patients with resectable colorectal liver metastases: clinical risk score as possible discriminator. Eur J Surg Oncol 41:859–867. https://doi.org/10.1016/j.ejso.2015.04.012
Adam R., Pascal G., Castaing D., Azoulay D., Delvart V., Paule B., Levi F., and Bismuth H. (2004) Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg 240:1052–1061; discussion 1061–1054. https://doi.org/10.1097/01.sla.0000145964.08365.01
Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, Xiong HQ, Eng C, Lauwers GY, Mino-Kenudson M, Risio M, Muratore A, Capussotti L, Curley SA, Abdalla EK (2006) Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol 24:2065–2072. https://doi.org/10.1200/jco.2005.05.3074
Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Collette L, Praet M, Bethe U, Van Cutsem E, Scheithauer W, Gruenberger T (2008) Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 371:1007–1016. https://doi.org/10.1016/s0140-6736(08)60455-9
Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Mauer M, Tanis E, Van Cutsem E, Scheithauer W, Gruenberger T (2013) Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 14:1208–1215. https://doi.org/10.1016/s1470-2045(13)70447-9
Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher J, Weitz J, Konopke R, Stroszczynski C, Liersch T, Ockert D, Herrmann T, Goekkurt E, Parisi F, Kohne CH (2010) Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol 11:38–47. https://doi.org/10.1016/s1470-2045(09)70330-4
Beppu T, Sakamoto Y, Hasegawa K, Honda G, Tanaka K, Kotera Y, Nitta H, Yoshidome H, Hatano E, Ueno M, Takamura H, Baba H, Kosuge T, Kokudo N, Takahashi K, Endo I, Wakabayashi G, Miyazaki M, Uemoto S, Ohta T, Kikuchi K, Yamaue H, Yamamoto M, Takada T (2012) A nomogram predicting disease-free survival in patients with colorectal liver metastases treated with hepatic resection: multicenter data collection as a Project Study for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 19:72–84. https://doi.org/10.1007/s00534-011-0460-z
Sakamoto K, Honda G, Beppu T, Kotake K, Yamamoto M, Takahashi K, Endo I, Hasegawa K, Itabashi M, Hashiguchi Y, Kotera Y, Kobayashi S, Yamaguchi T, Tabuchi K, Kobayashi H, Yamaguchi K, Morita S, Miyazaki M, Sugihara K (2020) Comprehensive data of 3525 patients newly diagnosed with colorectal liver metastasis between 2013 and 2014: 2nd report of a nationwide survey in Japan. J Hepatobiliary Pancreat Sci 27:555–562. https://doi.org/10.1002/jhbp.738
Yamamoto M, Yoshida M, Furuse J, Sano K, Ohtsuka M, Yamashita S, Beppu T, Iwashita Y, Wada K, Nakajima TE, Sakamoto K, Hayano K, Mori Y, Asai K, Matsuyama R, Hirashita T, Hibi T, Sakai N, Tabata T, Kawakami H, Takeda H, Mizukami T, Ozaka M, Ueno M, Naito Y, Okano N, Ueno T, Hijioka S, Shikata S, Ukai T, Strasberg S, Sarr MG, Jagannath P, Hwang TL, Han HS, Yoon YS, Wang HJ, Luo SC, Adam R, Gimenez M, Scatton O, Oh DY, Takada T (2021) (2021) Clinical practice guidelines for the management of liver metastases from extrahepatic primary cancers. J Hepatobiliary Pancreat Sci 28:1–25. https://doi.org/10.1002/jhbp.868
Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kinugasa Y, Murofushi K, Nakajima TE, Oka S, Tanaka T, Taniguchi H, Tsuji A, Uehara K, Ueno H, Yamanaka T, Yamazaki K, Yoshida M, Yoshino T, Itabashi M, Sakamaki K, Sano K, Shimada Y, Tanaka S, Uetake H, Yamaguchi S, Yamaguchi N, Kobayashi H, Matsuda K, Kotake K, Sugihara K (2020) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 25:1–42. https://doi.org/10.1007/s10147-019-01485-z
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Primrose J, Falk S, Finch-Jones M, Valle J, O’Reilly D, Siriwardena A, Hornbuckle J, Peterson M, Rees M, Iveson T, Hickish T, Butler R, Stanton L, Dixon E, Little L, Bowers M, Pugh S, Garden OJ, Cunningham D, Maughan T, Bridgewater J (2014) Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol 15:601–611. https://doi.org/10.1016/s1470-2045(14)70105-6
Hasegawa K, Oba M, Kokudo N (2014) Cetuximab for resectable colorectal liver metastasis: new EPOC trial. Lancet Oncol 15:e305-306. https://doi.org/10.1016/s1470-2045(14)70216-5
Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda AE, Bardelli A, Benson A, Bodoky G, Ciardiello F, D’Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Köhne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Österlund P, Oyen WJ, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taïeb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D (2016) ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 27:1386–1422. https://doi.org/10.1093/annonc/mdw235
Kawaguchi Y, De Bellis M, Panettieri E, Duwe G, Vauthey JN (2021) Debate: improvements in systemic therapies for liver metastases will increase the role of locoregional treatments. Surg Oncol Clin N Am 30:205–218. https://doi.org/10.1016/j.soc.2020.08.009
De Roock W, Piessevaux H, De Schutter J, Janssens M, De Hertogh G, Personeni N, Biesmans B, Van Laethem JL, Peeters M, Humblet Y, Van Cutsem E, Tejpar S (2008) KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol 19:508–515. https://doi.org/10.1093/annonc/mdm496
Di Fiore F, Blanchard F, Charbonnier F, Le Pessot F, Lamy A, Galais MP, Bastit L, Killian A, Sesboüé R, Tuech JJ, Queuniet AM, Paillot B, Sabourin JC, Michot F, Michel P, Frebourg T (2007) Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy. Br J Cancer 96:1166–1169. https://doi.org/10.1038/sj.bjc.6603685
Lièvre A, Bachet JB, Boige V, Cayre A, Le Corre D, Buc E, Ychou M, Bouché O, Landi B, Louvet C, André T, Bibeau F, Diebold MD, Rougier P, Ducreux M, Tomasic G, Emile JF, Penault-Llorca F, Laurent-Puig P (2008) KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol 26:374–379. https://doi.org/10.1200/jco.2007.12.5906
Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, Cote JF, Tomasic G, Penna C, Ducreux M, Rougier P, Penault-Llorca F, Laurent-Puig P (2006) KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 66:3992–3995. https://doi.org/10.1158/0008-5472.Can-06-0191
Van Cutsem E, Kohne CH, Lang I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, Schlichting M, Zubel A, Celik I, Rougier P, Ciardiello F (2011) Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 29:2011–2019. https://doi.org/10.1200/jco.2010.33.5091
Bridgewater JA, Pugh SA, Maishman T, Eminton Z, Mellor J, Whitehead A, Stanton L, Radford M, Corkhill A, Griffiths GO, Falk S, Valle JW, O’Reilly D, Siriwardena AK, Hornbuckle J, Rees M, Iveson TJ, Hickish T, Garden OJ, Cunningham D, Maughan TS, Primrose JN (2020) Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 21:398–411. https://doi.org/10.1016/s1470-2045(19)30798-3
Kishi Y, Zorzi D, Contreras CM, Maru DM, Kopetz S, Ribero D, Motta M, Ravarino N, Risio M, Curley SA, Abdalla EK, Capussotti L, Vauthey JN (2010) Extended preoperative chemotherapy does not improve pathologic response and increases postoperative liver insufficiency after hepatic resection for colorectal liver metastases. Ann Surg Oncol 17:2870–2876. https://doi.org/10.1245/s10434-010-1166-1
Stintzing S, Wirapati P, Lenz HJ, Neureiter D, Fischer von Weikersthal L, Decker T, Kiani A, Kaiser F, Al-Batran S, Heintges T, Lerchenmuller C, Kahl C, Seipelt G, Kullmann F, Moehler M, Scheithauer W, Held S, Modest DP, Jung A, Kirchner T, Aderka D, Tejpar S, Heinemann V (2019) Consensus molecular subgroups (CMS) of colorectal cancer (CRC) and first-line efficacy of FOLFIRI plus cetuximab or bevacizumab in the FIRE3 (AIO KRK-0306) trial. Ann Oncol 30:1796–1803. https://doi.org/10.1093/annonc/mdz387
Kemeny M. M., Adak S., Gray B., Macdonald J. S., Smith T., Lipsitz S., Sigurdson E. R., O'Dwyer P. J., and Benson A. B., 3rd (2002) Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy--an intergroup study. J Clin Oncol 20:1499–1505. https://doi.org/10.1200/jco.2002.20.6.1499
Kemeny N, Huang Y, Cohen AM, Shi W, Conti JA, Brennan MF, Bertino JR, Turnbull AD, Sullivan D, Stockman J, Blumgart LH, Fong Y (1999) Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med 341:2039–2048. https://doi.org/10.1056/nejm199912303412702
Lorenz M, Müller HH, Schramm H, Gassel HJ, Rau HG, Ridwelski K, Hauss J, Stieger R, Jauch KW, Bechstein WO, Encke A (1998) Randomized trial of surgery versus surgery followed by adjuvant hepatic arterial infusion with 5-fluorouracil and folinic acid for liver metastases of colorectal cancer. German Cooperative on Liver Metastases (Arbeitsgruppe Lebermetastasen). Ann Surg 228:756–762. https://doi.org/10.1097/00000658-199812000-00006
Mitry E, Fields AL, Bleiberg H, Labianca R, Portier G, Tu D, Nitti D, Torri V, Elias D, O’Callaghan C, Langer B, Martignoni G, Bouché O, Lazorthes F, Van Cutsem E, Bedenne L, Moore MJ, Rougier P (2008) Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol 26:4906–4911. https://doi.org/10.1200/jco.2008.17.3781
Portier G, Elias D, Bouche O, Rougier P, Bosset JF, Saric J, Belghiti J, Piedbois P, Guimbaud R, Nordlinger B, Bugat R, Lazorthes F, Bedenne L (2006) Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol 24:4976–4982. https://doi.org/10.1200/jco.2006.06.8353
Innocenti F, Ou FS, Qu X, Zemla TJ, Niedzwiecki D, Tam R, Mahajan S, Goldberg RM, Bertagnolli MM, Blanke CD, Sanoff H, Atkins J, Polite B, Venook AP, Lenz HJ, Kabbarah O (2019) Mutational analysis of patients With colorectal cancer in CALGB/SWOG 80405 identifies new roles of microsatellite instability and tumor mutational burden for patient outcome. J Clin Oncol 37:1217–1227. https://doi.org/10.1200/jco.18.01798
Acknowledgements
This study was supported by Translational Research Center for Medical Innovation in Kobe, Japan as trial data center. The authors would like to thank Enago (www.enago.jp) for the English language review.
Funding
This study is funded by Translational Research Center for Medical Innovation in Kobe, Japan.
Author information
Authors and Affiliations
Contributions
Study conception and design: KH, MO, KY, HU, TY, S M, KT, MU, YS, KM, MM, HB, MS, AS, KS, and NK. Acquisition of data: MM, KH, MO, KY, HU, TY, KT, MU, YS, KM, NM, MM, HB, MS, YM, YK, TK, KI, AS, and KS. Analysis and interpretation of data: MM, KH, KY, HU, SM, KT, YK, TK, AS, KS, and NK. Drafting of manuscript: MM, YK, and KH. Critical revision of manuscript: KH, MO, KY, HU, TY, SM, KT, MU, YS, KM, MM, HB, MS, YK, AS, KS, and NK.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the institutional ethics committees of the participating institutions and was conducted in accordance with good clinical practice guidelines and the Helsinki Declaration.
Consent to participate
All the patients provided written informed consent.
Consent for publication
Consent for publication was obtained from all the patients.
Conflict of interest
Takayuki Yoshino received lecture fee from Merck biopharma, Japan. All remaining authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Matsumura, M., Hasegawa, K., Oba, M. et al. A randomized controlled trial of surgery and postoperative modified FOLFOX6 versus surgery and perioperative modified FOLFOX6 plus cetuximab in patients with KRAS wild-type resectable colorectal liver metastases: EXPERT study. Langenbecks Arch Surg 407, 1345–1356 (2022). https://doi.org/10.1007/s00423-022-02434-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-022-02434-7