Abstract
Purpose
This study evaluated the concurrent training (CT) effect in combination with either progressive energy restriction (PER) or severe energy restriction (SER) on body composition and strength-related variables in resistance-trained women.
Methods
Fourteen women (29.5 ± 3.8 years; 23.8 ± 2.8 kg·m−2) were randomly assigned to a PER (n = 7) or SER (n = 7) group. Participants performed an 8-week CT program. Pre- and post-intervention measures of fat mass (FM) and fat-free mass (FFM) were assessed by dual-energy X-ray absorptiometry and strength-related variables were assessed through 1-repetition maximum (in the squat and bench press) and countermovement jump.
Results
Significant reductions in FM were observed in PER and SER (Δ = − 1.7 ± 0.4 kg; P = < 0.001; ES = − 0.39 and Δ = − 1.2 ± 0.6 kg; P = 0.002; ES = − 0.20, respectively). After correcting FFM for fat-free adipose tissue (FFAT), no significant differences for this variable were found either in PER (Δ = − 0.3 ± 0.1; P = 0.071; ES = − 0.06) or in SER (Δ = − 0.2 ± 0.1; P = 0.578; ES = − 0.04). There were no significant changes in the strength-related variables. No between-group differences were found in any of the variables.
Conclusion
A PER has similar effects to a SER on body composition and strength in resistance-trained women performing a CT program. Given that PER is more flexible and thus may enhance dietary adherence, it might be a better alternative for FM reduction compared to SER.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe energy restriction (SER) strategies have been used for years for the purpose of reducing fat mass (FM) levels. SER is often combined with regimented fitness programs to enhance fat loss while preserving fat-free mass (FFM). Alternatively, competitive fitness and bodybuilding competitors often employ progressive energy restriction (PER) strategies of durations between two and four months, in conjunction with resistance training and often cardiovascular exercise (a.k.a., concurrent training) (Maestu et al. 2010). The duration of these restrictions depends on the athlete’s physical fitness, the time remaining for the competition, and the individual genetic characteristics of the athlete. Regardless, a reduction in FM requires an energy deficit (McGuire 2011) and/ or an increase in total daily energy expenditure through physical exercise (Deighton et al. 2014); both of which can compromise fat free mass (FFM) levels. Higher energy deficits are associated with more rapid weight loss; the more pronounced the deficit, the greater the possibility to lose lean mass (Hall 2008; Mettler et al. 2010; Garthe et al. 2011). Moreover, evidence shows that a prolonged energy deficit in natural bodybuilders could also lead to hormonal imbalances, fatigue and psychological issues (Fagerberg 2018).
To counteract the detrimental effects of a negative energy balance, fat loss interventions should seek to employ strategies that maintain as much FFM as possible (Helms et al. 2014). For example, research indicates that higher intakes of dietary protein aid in the preservation of FFM during periods of energy restriction (Wycherley et al. 2012; Walilko et al. 2021). For trained athletes under hypocaloric conditions, recommendations range from 1.8 to 2.7 g/protein/kg/day (Helms et al. 2014), and 1.6–2.4 g/protein/kg/day (Hector and Phillips 2018). Moreover, resistance training helps to enhance the maintenance of FFM during an energy deficit (Josse et al. 2011; Longland et al. 2016). Despite the efficacy of these strategies, the loss of FFM is not always attenuated during SER (Gornall and Villani 1996; Donnelly et al. 1991). For example, the only study to date that evaluated the effects of SER with a high protein diet on body composition and physical performance (including muscular strength) in well-trained female athletes of different disciplines (Pearson et al. 2021) found no differences in FFM between groups consuming a high (35% of energy intake) or modest (15% of energy intake) protein intake.
Due to a paucity of research in female athletes as to the effects of energy restriction combined with regimented exercise, the aim of this study was to compare the effects of an 8-week high-protein SER or PER dietary intervention accompanied by a high-volume concurrent training (CT) program on body composition and strength-related variables in resistance-trained women. We hypothesized that eight weeks of a PER intervention would elicit a greater reduction in FM while maintaining FFM and strength levels compared to a SER due to a better adherence to the nutritional strategy in resistance-trained women.
Materials and methods
Experimental design
This was a repeated-measures study carried out in resistance-trained women who were recruited from a previous research project (Romance et al. 2019; Vargas-Molina et al. 2020) to participate immediately following completion of that protocol. After previously taking part in an 8-week training and nutritional protocol with a consumption of 45 kcal·kg−1 FFM, the participants were randomly assigned in a 1:1 fashion to either a SER or PER dietary intervention accompanied by a high-volume CT program. This design allowed the athletes to become adapted to the program protocols and thus help to ensure better internal validity.
Sample
Fourteen young women (29.5 ± 3.8 years; 164.9 ± 7.0 cm; 63.1 ± 9.0 kg; 23.8 ± 2.8 kg·m−2) with > 2 years of continuous experience in resistance training volunteered to participate in this study. Participants who self-reported the use of doping agents (e.g., anabolic–androgenic steroids) during the last two years were excluded from participation. Additionally, participants pledged not to consume ergogenic supplements during the study. Female athletes with oligomenorrhea or polycystic ovarian syndrome, as well as those not within the required age range of 18–35 years, were excluded as well.
All participants committed to following the CT and dietary protocols, and to be monitored during the 8-week study period. Participants were instructed to avoid performing any structured exercise during the study period other than that prescribed for the intervention. The participants were informed of the possible risks of the experiment and signed an informed consent form. The study was developed in accordance with the ethical guidelines of the World Medical Association Declaration of Helsinki (WMA 2013) and approved by the ethics committee at University of Málaga (code: 38-2019-H).
Exercise protocol
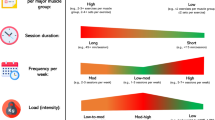
Both groups performed a CT program with a frequency of 6 sessions a week. The resistance training program consisted of four sessions a week, divided into upper-limb/lower-limb, with a 72-h recovery period between sessions that involved training the same muscle region. The program incorporated super-sets (two exercises are performed in succession), whereby the first exercise, a multi-joint movement, was performed with relatively heavy loads (~ 5–6 repetition maximum [RM]) and the second exercise, a single-joint movement, was performed with lighter loads (~ 20RM). A 3-min rest interval was implemented between each super-set. Participants trained to volitional failure during the first 3 weeks of the study, and in the fourth week the loads were decreased; this sequence was replicated over the ensuing 4 weeks of the study. At the end of the resistance training sessions, participants performed 20 min of cardiovascular exercise on a cycle ergometer at an intensity of 65% heart rate reserve. Additionally, the participants carried out two 45-min cardio sessions on non-resistance training days. Thus, the total weekly volume of cardiovascular exercise was 170 min, with the last day of the week allocated for recovery. The intensity of the cardiovascular exercise was controlled using the estimation of the heart rate reserve via the Karvonen method (Karvonen et al. 1957). The following formula was used. To calculate maximum heart rate: 206 − (0.88 × age) (Gulati et al. 2010) and apply it to the Karvonen formula. Participants were asked to record resting heart rate for three consecutive mornings (the mean of the records was used) immediately upon waking and before arising. Participants used heart rate sensor to assess heart rate (Sensors Polar H10, Tampere, Finland), wetting the band as indicated by the manufacturer. The programming variables, intensity, rest and cadences were adjusted to each type of training (Fig. 1). All participants performed the same exercise protocol for the duration of the program. During the intervention, all participants were monitored by a physical conditioning specialist, who supervised, controlled and adjusted the loads in each training session.
Dietary intervention
The intervention began immediately after participants completed an 8-week program designed to increase FFM with a daily intake of 45 kcal·kg−1 FFM (Romance et al. 2019; Vargas-Molina et al. 2020). The SER group was instructed to consume 25 kcal·kg−1 FFM from throughout the 8-week duration of the present investigation. The prescribed macronutrient distribution in this group was 2 g·kg−1·d−1 of protein, 1 g·kg−1·d−1 of fat, and the balance of total calories from carbohydrates. Alternatively, the PER group progressively restricted caloric intake, accomplished by a reduction in carbohydrates, as follows: Energy intake for the first two weeks (weeks 1 and 2) amounted to 40 kcal·kg−1 with a similar macronutrient prescription to the SER group. During the next two weeks (weeks 3 and 4), total calorie intake was reduced to 35 kcal·kg−1 FFM. The following month continued in the same fashion, with calories reduced to 30 kcal·kg−1 FFM in the fifth and sixth weeks and then further reduced to 25 kcal·kg−1 FFM in the final two weeks (weeks 7 and 8). To help ensure compliance, participants recorded their daily macronutrient intake via a smartphone app (MyFitnessPal, LLC, CA, USA), which has been validated as viable tool for energy and macronutrient assessment (Teixeira et al. 2018). A sports nutritionist with experience in RT instructed participants on the proper use of the app and managed dietary consumption over the course of the study.
Body composition
Body composition was measured seven days after menstruation in both the pre- and post-intervention periods to avoid potential confounding issues due to hormonal-induced fluctuations in extracellular water (Rosenfeld et al. 2008; Stachenfeld 2008). Total body and regional body composition were estimated via dual-energy X-ray absorptiometry (DXA). To eliminate the influence of fat-free adipose tissue (FFAT) and thus provide more accurate measures of changes in body composition, FFAT was eliminated on DXA-derived FFM (Heymsfield et al. 2002). The FFAT was estimated assuming that 85% of adipose tissue is fat using the equation (FM/0.85) × 0.15; the FFM-FFAT was then calculated and reported as we have done recently (Bonilla et al. 2021). This is recommended especially when large changes in body fat occur following an intervention (Abe et al. 2019).
Each subject was scanned by a certified technician, and computer algorithms distinguished bone and soft tissue, edge detection, and regional demarcations (software version APEX 5.6.0.7, Hologic Horizon A, Bedford, MA). For each scan, subjects wore sport clothes and were asked to remove all materials that could attenuate the X-ray beam (e.g., jewelry items, underwear containing wire, etc.). Calibration of the densitometer was checked daily against a standard calibration block supplied by the manufacturer. The abdominal region was delineated by an upper horizontal border located at half of the distance between acromion and external border of the iliac crests, a lower border determined by the external end of iliac crests and the lateral borders extending to the edge of the abdominal soft tissue. All trunk tissue within this standardized region was selected for analysis. To determine intertester reliability, two different observers manually selected the area for each subject with a coefficient of variation = 0.263%.
Strength- and power-related assessments
For measurement of variables related to muscular strength and power, subjects were instructed to avoid vigorous exercise for 48 h before the tests in both the pre- and post-study periods. Participants performed a general warm-up consisting of light stretching and stationary cycling for 7–10 min prior to testing.
The countermovement jump (CMJ) test was performed on a jump mat (Smart Jump; Fusion Sport, Coopers Plains, Australia). Subjects initiated movement by reaching 90º of knee flexion while keeping their hands at the waist and their trunk erect, and then jumped vertically as high as possible. Instructions emphasized that the movement should be performed without interruption from the beginning to the end of the jump. Subjects performed 3–5 practice attempts for familiarization. Thereafter, two jumps were provided with a rest interval of 1 min between each trial; the highest value was used for analysis.
Pre- and post-study RM was assessed in the squat (SQ) and bench press (BP) performed on a Smith machine (Gervasport, Madrid, Spain). A specific warm-up set of the given exercise was performed for 12–15 repetitions at ~ 40% of subjects self-estimated 1-RM followed by two to three sets of two to three repetitions at a load corresponding to approximately 60–80% 1-RM. Participants then performed sets of one repetition of increasing weight for 1-RM determination. A 3- to 5-min rest interval was provided between each successive attempt. Participants were required to reach parallel in the 1-RM SQ; confirmation of squat depth was obtained by a research assistant positioned laterally to the subject to ensure accuracy. Successful 1-RM BP was achieved if the subject displayed a five-point body contact position (head, upper back, and buttocks firmly on the bench with both feet flat on the floor) and executed full-elbow extension. 1-RM SQ testing was conducted before 1-RM BP with a 7-min rest period separating tests. Participants then performed as many attempts as necessary until repetition failure, using the protocol described by McGuigan (2016). Bench placement was set by marking the floor with adhesive tape, to maintain the same placement for both measurements. All testing sessions were supervised by the research team to achieve a consensus for success on each trial.
Statistical analysis
The results are expressed as mean ± standard deviation. The normality of the data was assessed with the Shapiro–Wilk test and the equality of variance with the Levene test. The comparison of the means of the variables (pretest vs. posttest) was performed with the paired t-test, and the effect size (ES) was calculated with Hedges' g, considering a ≤ 0.2 as small effect, 0.5 moderate effect, > 0.8 as large effect, and ≥ 1.30 as a very large effect (Rosenthal 1996). Likewise, to evaluate the effects and the comparison between the intervention groups, a general linear model (GLM) of repeated measures was performed, considering the Time (pre-test vs. post-test) and Group (PER vs SER) factors, and the Time × Group interaction. Additionally, between-group comparisons were made with estimation statistics (Ho et al. 2019). A P-value less than 0.05 (P < 0.05) was considered statistically significant for all tests. The analyses were performed with SPSS version 26 (IBM Corp., Armonk, NY, USA) and the effect size was computed with the R package Data Analysis using Bootstrap-Coupled Estimation (DABEST) v0.3.0 (Ho et al. 2019) within the R statistical computing environment version 4.0.0 (R Core Team 2020).
Results
Of the 44 women who participated in the immediately preceding study, only 14 met inclusion criteria and, thereafter, were assigned to either the PER (n = 7) or SER (n = 7) group. Figure 2 presents a flow diagram of subject enrollment as recommended by the Consolidated Standards of Reporting Trials (CONSORT).
Baseline analysis of the participants showed no between-group differences in any of the assessed variables (Table 1).
As planned, energy intake of the PER group was higher compared to SER in weeks 1–2 (P = 0.010). No differences were registered in weeks 3–4 and 5–6 (P = 0.167 and P = 0.120, respectively), while PER energy intake was lower compared to SER in weeks 7–8 (P = 0.003). Table 2 shows the estimated caloric intake from the nutrient intake record in both groups.
In regard to body composition, body mass showed a significant decrease with a small effect in PER (Δ = − 2.3 ± 0.7 kg; P < 0.001; ES = − 0.27) and SER (Δ = − 1.6 ± 1.2 kg; P = 0.013; ES = − 0.18). Similarly, FM was significantly reduced with a small effect in PER (Δ = − 1.7 ± 0.4 kg; P = < 0.001; ES = − 0.39) and SER (Δ = − 1.2 ± 0.6 kg; P = 0.002; ES = − 0.20). FFM displayed a significant reduction with a small effect in PER (Δ = − 0.6 ± 0.4 kg; P = 0.008; ES = − 0.11); the reduction of FFM was not statistically significant in SER (Δ = − 0.4 ± 0.8 kg; P = 0.258; ES = − 0.08). However, when adjusting the FFM with the FFAT values (FFM–FFAT), no significant decrease was observed for the PER and SER groups (Δ = − 0.3 ± 0.1; P = 0.071; ES = − 0.06 and Δ = − 0.2 ± 0.1; P = 0.578, ES = − 0.04, respectively). No between-group differences were found on the change comparison (Fig. 3), nor in the GLM analysis for body composition variables (Table 2).
The difference between PER and SER in body composition. A Fat mass; B Fat free mass; C Fat-free adipose mass; D Fat free mas minus fat-free adipose tissue. The values presented are the post-intervention changes (post-test—pretest). In each figure, both groups are plotted on the left axes; the mean difference is plotted on a floating axes on the right as a bootstrap sampling distribution. The mean difference is depicted as a dot; the 95% confidence interval is indicated by the ends of the vertical error bar (Ho et al. 2019). PER, progressive energy restriction; SER, severe energy restriction
In regard to the strength-related variables, no significant differences were found for PER or SER in the BP (Δ = − 0.9 ± 1.1 kg; P = 0.095; ES = − 0.11 and Δ = − 0.9 ± 4.6 kg; P = 0.610; ES = − 0.10, respectively), SQ (Δ = 0.6 ± 4.3 kg; P = 0.708; ES = 0.06 and Δ = 2.8 ± 4.6 kg; P = 0.159; ES = 0.17, respectively) or CMJ (Δ = 0.5 ± 2.1 cm; P = 0.568; ES = 0.10 y Δ = − 0.1 ± 2.1 cm; P = 0.944; ES = − 0.01, respectively). No between-group differences were found on the change comparison (Fig. 4), nor in the GLM analysis for the strength-related variables (Tables 2 and 3).
The difference between PER and SER in strength. A Bench press; B Squat; C CMJ. In each figure, both groups are plotted on the left axes; the mean difference is plotted on a floating axes on the right as a bootstrap sampling distribution. The mean difference is depicted as a dot; the 95% confidence interval is indicated by the ends of the vertical error bar (Ho et al. 2019). PER, progressive energy restriction; SER, severe energy restriction
Discussion
The aim of this study was to compare two energy restriction protocols (PER vs SER) in combination with performance of a CT program on body composition and strength-related variables in trained women. Although our initial hypothesis was that the PER protocol would be more effective than SER for reducing FM and maintaining FFM and strength levels, no significant differences were observed between conditions. Consistent with our findings, Mero et al. (2010) reported statistically significant changes in FM reduction with no changes in lean body mass and bone mass in recreationally active, normal weight women when subjected to a deficit of 1000 or 500 kcal for 4 weeks (protein intake = 1.4–1.5 g·protein−1 per kilogram of BM). Alternatively, Garthe et al. (2011) found superior improvements in body composition and strength in a cohort of in 24 athletes (13 women) who targeted a slower vs faster weekly weight loss (0.7% vs 1.4% body mass per week) accompanied by 4 days/week of resistance training. Notably, our study is the first to compare a PER versus a SER in resistance-trained women and thus helps to fill current gaps in the literature. PER demonstrated a modestly greater magnitude of effect for changes in fat mass, which is of questionable practical significance.
Scrutiny of participants’ nutritional records in our study revealed that participants in the SER group did not consume the number of calories proposed (25 kcal·kg−1 FFM). This may help to explain the somewhat lower FM reduction (− 1.2 kg; ES = − 0.20) compared to the PER group (− 1.7 kg; ES = − 0.39), although at the same time it indicates a greater adherence for PER. It should be noted that self-report nutritional records are prone to error (Schoeller 1995). Moreover, individual components of energy expenditure (i.e., resting metabolic rate and non-exercise activity thermogenesis) can influence energy requirements. Thus, explanations for relative differences in body composition measure between conditions remains speculative; it is possible that discrepancies simply are indicative of random noise of the measurement.
Regarding the possible differences between the sexes, Longland et al. (2016) demonstrated an increase in FFM following a 4-week RT program combined with a 40% caloric deficit (33 kcal·kg−1 FFM and 2.4 g·protein−1 per kg of BM) in untrained, overweight young men. Although our study did not show significant changes in FFM (corrected for FFAT), the biological differences between men and women must be taken into account in addition to the resistance training experience of our participants. It should also be noted that some of the participants in Longland et al. (2016) purportedly had previously engaged in resistance training, thus raising the possibility that increases in FFM were at least partially due to the “muscle memory” effect (Snijders et al. 2020).
Our study has several limitations that should be acknowledged. First, our sample size was relatively small, limiting statistical power; larger samples are needed to draw stronger practical inferences on the topic. Second, mood state was not assessed (Helms et al. 2015) to determine the lack of adherence in any of the groups. Third, the variation in energy intake among groups made it difficult to draw definitive conclusions. We did not measure resting energy expenditure which limited our discussion. Although no statistically significant differences between groups was detected on primary measures, we partially confirmed our initial hypothesis that PER might favor outcomes on body composition; nevertheless, additional more research is warranted to confirm this hypothesis. Finally, our study investigated one possible dietary comparison of energy restriction strategies; further research is needed to compare other nutritional strategies that have been shown to improve body composition in resistance-trained individuals (e.g., intermittent energy restriction such as intermittent fasting, diet refeeds or diet breaks) (Campbell et al. 2020; Escalante et al. 2020; Ashtary-Larky et al. 2021).
Conclusions
PER and SER are effective strategies to reduce FM while maintaining FFM and strength levels in resistance-trained women undergoing an 8-week CT program. However, a progressive energy deficit appears to have a relatively higher magnitude of effect on FM reduction and might promote greater adherence in comparison to SER. Thus, our preliminary findings suggest that a PER may be considered a viable weight loss option in resistance-trained women.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BM:
-
Body mass
- BMI:
-
Body mass index
- BP:
-
Bench press
- CMJ:
-
Countermovement jump
- CONSORT:
-
Consolidated standards of reporting trials
- CT:
-
Concurrent training
- DXA:
-
Dual-energy X-ray absorptiometry
- ES:
-
Effect size
- FM:
-
Fat mass
- FFM:
-
Fat-free mass
- FFAT:
-
Fat-free adipose tissue
- GLM:
-
General linear model
- RM:
-
Repetition maximum
- PER:
-
Progressive energy restriction
- SER:
-
Severe energy restriction
- SQ:
-
Squat jump
- VO2max:
-
Maximal oxygen consumption
References
Abe T, Loenneke JP, Thiebaud RS (2019) Fat-free adipose tissue mass: impact on peak oxygen uptake (VO(2peak)) in adolescents with and without obesity. Sports Med (auckland, NZ) 49(1):9–15. https://doi.org/10.1007/s40279-018-1020-3
Ashtary-Larky D, Bagheri R, Tinsley GM, Asbaghi O, Paoli A, Moro T (2021) Effects of intermittent fasting combined with resistance training on body composition: a systematic review and meta-analysis. Physiol Behav 237:113453. https://doi.org/10.1016/j.physbeh.2021.113453
Association GAotWM (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent 81(3):14. https://pubmed.ncbi.nlm.nih.gov/24141714/
Bonilla DA, Kreider RB, Petro JL, Romance R, Garcia-Sillero M, Benitez-Porres J, Vargas-Molina S (2021) Creatine enhances the effects of cluster-set resistance training on lower-limb body composition and strength in resistance-trained men: a pilot study. Nutrients. https://doi.org/10.3390/nu13072303
Campbell BI, Aguilar D, Colenso-Semple LM, Hartke K, Fleming AR, Fox CD, Longstrom JM, Rogers GE, Mathas DB, Wong V, Ford S, Gorman J (2020) Intermittent energy restriction attenuates the loss of fat free mass in resistance trained individuals. A randomized controlled trial. J Funct Morphol Kinesiol. https://doi.org/10.3390/jfmk5010019
Deighton K, Batterham RL, Stensel DJ (2014) Appetite and gut peptide responses to exercise and calorie restriction. The effect of modest energy deficits. Appetite 81:52–59. https://doi.org/10.1016/j.appet.2014.06.003
Donnelly JE, Pronk NP, Jacobsen DJ, Pronk SJ, Jakicic JM (1991) Effects of a very-low-calorie diet and physical-training regimens on body composition and resting metabolic rate in obese females. Am J Clin Nutr 54(1):56–61. https://doi.org/10.1093/ajcn/54.1.56
Escalante G, Campbell BI, Norton L (2020) Effectiveness of diet refeeds and diet breaks as a precontest strategy. Strength Cond J 42(5):102–107. https://doi.org/10.1519/ssc.0000000000000546
Fagerberg P (2018) Negative consequences of low energy availability in natural male bodybuilding: a review. Int J Sport Nutr Exerc Metab 28(4):385–402. https://doi.org/10.1123/ijsnem.2016-0332
Garthe I, Raastad T, Refsnes PE, Koivisto A, Sundgot-Borgen J (2011) Effect of two different weight-loss rates on body composition and strength and power-related performance in elite athletes. Int J Sport Nutr Exerc Metab 21(2):97–104. https://doi.org/10.1123/ijsnem.21.2.97
Gornall J, Villani RG (1996) Short-term changes in body composition and metabolism with severe dieting and resistance exercise. Int J Sport Nutr 6(3):285–294. https://doi.org/10.1123/ijsn.6.3.285
Gulati M, Shaw LJ, Thisted RA, Black HR, Bairey Merz CN, Arnsdorf MF (2010) Heart rate response to exercise stress testing in asymptomatic women: the St. James Women Take Heart Project. Circulation 122(2):130–137. https://doi.org/10.1161/CIRCULATIONAHA.110.939249
Hall KD (2008) What is the required energy deficit per unit weight loss? Int J Obes (lond) 32(3):573–576. https://doi.org/10.1038/sj.ijo.0803720
Hector AJ, Phillips SM (2018) Protein recommendations for weight loss in elite athletes: a focus on body composition and performance. Int J Sport Nutr Exerc Metab 28(2):170–177. https://doi.org/10.1123/ijsnem.2017-0273
Helms ER, Zinn C, Rowlands DS, Brown SR (2014) A systematic review of dietary protein during caloric restriction in resistance trained lean athletes: a case for higher intakes. Int J Sport Nutr Exerc Metab 24(2):127–138. https://doi.org/10.1123/ijsnem.2013-0054
Helms ER, Zinn C, Rowlands DS, Naidoo R, Cronin J (2015) High-protein, low-fat, short-term diet results in less stress and fatigue than moderate-protein moderate-fat diet during weight loss in male weightlifters: a pilot study. Int J Sport Nutr Exerc Metab 25(2):163–170. https://doi.org/10.1123/ijsnem.2014-0056
Heymsfield SB, Gallagher D, Kotler DP, Wang Z, Allison DB, Heshka S (2002) Body-size dependence of resting energy expenditure can be attributed to nonenergetic homogeneity of fat-free mass. Am J Physiol Endocrinol Metab 282(1):E132-138. https://doi.org/10.1152/ajpendo.2002.282.1.E132
Ho J, Tumkaya T, Aryal S, Choi H, Claridge-Chang A (2019) Moving beyond P values: data analysis with estimation graphics. Nat Methods 16(7):565–566. https://doi.org/10.1038/s41592-019-0470-3
Josse AR, Atkinson SA, Tarnopolsky MA, Phillips SM (2011) Increased consumption of dairy foods and protein during diet- and exercise-induced weight loss promotes fat mass loss and lean mass gain in overweight and obese premenopausal women. J Nutr 141(9):1626–1634. https://doi.org/10.3945/jn.111.141028
Karvonen MJ, Kentala E, Mustala O (1957) The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn 35(3):307–315
Longland TM, Oikawa SY, Mitchell CJ, Devries MC, Phillips SM (2016) Higher compared with lower dietary protein during an energy deficit combined with intense exercise promotes greater lean mass gain and fat mass loss: a randomized trial. Am J Clin Nutr 103(3):738–746. https://doi.org/10.3945/ajcn.115.119339
Maestu J, Eliakim A, Jurimae J, Valter I, Jurimae T (2010) Anabolic and catabolic hormones and energy balance of the male bodybuilders during the preparation for the competition. J Strength Cond Res 24(4):1074–1081. https://doi.org/10.1519/JSC.0b013e3181cb6fd3
McGuigan M (2016) Administration, scoring, and interpretation of selected tests. In: Haff G, Triplett T (eds) Essentials of strength training and conditioning. Human Kinetics, Champaign, pp 265–266
McGuire S (2011) U.S. Department of Agriculture and U.S. Department of Health and Human Services, Dietary Guidelines for Americans, 2010. 7th Edition, Washington, DC: U.S. Government Printing Office, January 2011. Adv Nutr 2(3):293–294. https://doi.org/10.3945/an.111.000430
Mero AA, Huovinen H, Matintupa O, Hulmi JJ, Puurtinen R, Hohtari H, Karila TA (2010) Moderate energy restriction with high protein diet results in healthier outcome in women. J Int Soc Sports Nutr 7(1):4. https://doi.org/10.1186/1550-2783-7-4
Mettler S, Mitchell N, Tipton KD (2010) Increased protein intake reduces lean body mass loss during weight loss in athletes. Med Sci Sports Exerc 42(2):326–337. https://doi.org/10.1249/MSS.0b013e3181b2ef8e
Pearson AG, Alexander L, Witard OC, Coughlin TE, Tipton KD, Walshe IH (2021) A hypoenergetic diet with decreased protein intake does not reduce lean body mass in trained females. Eur J Appl Physiol 121(3):771–781. https://doi.org/10.1007/s00421-020-04555-7
R Core Team (2020) R: a language and environment for statistical computing. https://www.R-project.org. Accessed Oct 2021 2020
Romance R, Vargas S, Espinar S, Petro JL, Bonilla DA, Schoenfeld BJ, Kreider RB, Benitez-Porres J (2019) Oral contraceptive use does not negatively affect body composition and strength adaptations in trained women. Int J Sports Med 40(13):842–849. https://doi.org/10.1055/a-0985-4373
Rosenfeld R, Livne D, Nevo O, Dayan L, Milloul V, Lavi S, Jacob G (2008) Hormonal and volume dysregulation in women with premenstrual syndrome. Hypertension 51(4):1225–1230. https://doi.org/10.1161/HYPERTENSIONAHA.107.107136
Rosenthal JA (1996) Qualitative descriptors of strength of association and effect size. J Soc Serv Res 21(4):37–59. https://doi.org/10.1300/J079v21n04_02
Schoeller DA (1995) Limitations in the assessment of dietary energy intake by self-report. Metabolism 44(2 Suppl 2):18–22. https://doi.org/10.1016/0026-0495(95)90204-x
Snijders T, Aussieker T, Holwerda A, Parise G, van Loon LJC, Verdijk LB (2020) The concept of skeletal muscle memory: Evidence from animal and human studies. Acta Physiol (oxf) 229(3):e13465. https://doi.org/10.1111/apha.13465
Stachenfeld NS (2008) Sex hormone effects on body fluid regulation. Exerc Sport Sci Rev 36(3):152–159. https://doi.org/10.1097/JES.0b013e31817be928
Teixeira V, Voci SM, Mendes-Netto RS, da Silva DG (2018) The relative validity of a food record using the smartphone application MyFitnessPal. Nutr Diet 75(2):219–225. https://doi.org/10.1111/1747-0080.12401
Vargas-Molina S, Petro JL, Romance R, Kreider RB, Schoenfeld BJ, Bonilla DA, Benitez-Porres J (2020) Effects of a ketogenic diet on body composition and strength in trained women. J Int Soc Sports Nutr 17(1):19. https://doi.org/10.1186/s12970-020-00348-7
Walilko E, Napierala M, Bryskiewicz M, Fronczyk A, Majkowska L (2021) High-protein or low glycemic index diet-which energy-restricted diet is better to start a weight loss program? Nutrients. https://doi.org/10.3390/nu13041086
Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD (2012) Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr 96(6):1281–1298. https://doi.org/10.3945/ajcn.112.044321
Acknowledgements
Supported by University of Málaga (Campus of International Excellence Andalucía Tech). Funding for open access charge: Universidad de Málaga/CBUA.
Funding
Funding for open access publishing: Universidad Málaga/CBUA.
Author information
Authors and Affiliations
Contributions
SVM and JBP conceived and designed the experiments. JBP served as lab coordinator and project manager. SVM, MGS and LC assisted in data collection. SVM designed and oversight the nutritional protocols and training. JLP and DAB analyzed the data. SVM, MGS, LC, DAB, BJS, JMJC and JBP, assisted in analysis and manuscript review. SVM, DAB and LC wrote the first draft. SVM, MGS, LC, DAB, BJS, JMJC, JLP and JBP, assisted in the statistics advice, discussion analysis, and manuscript preparation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest are declared by the authors.
Additional information
Communicated by William J. Kraemer.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vargas-Molina, S., Bonilla, D.A., Petro, J.L. et al. Efficacy of progressive versus severe energy restriction on body composition and strength in concurrent trained women. Eur J Appl Physiol 123, 1311–1321 (2023). https://doi.org/10.1007/s00421-023-05158-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-023-05158-8