Abstract
Purpose
To examine the acute effects of concurrent muscle power and sport-specific endurance exercises order on immunological stress responses, muscular-fitness, and rating-of-perceived-exertion (RPE) in highly trained youth male judo athletes.
Methods
Twenty male participants randomly performed two concurrent training (CT) sessions; power-endurance and endurance-power. Measures of immune response (e.g., white blood cells), muscular-fitness (i.e., counter-movement-jump [CMJ]), RPE, blood-lactate, and -glucose were taken at different time-point (i.e., pre, mid, post, and post6h).
Results
There were significant time*order interactions for white blood cells, lymphocytes, granulocytes, granulocyte-lymphocyte-ratio, and systemic-inflammation-index. Power-endurance resulted in significantly larger pre-to-post increases in white blood cells and lymphocytes while endurance-power resulted in significantly larger pre-to-post increases in the granulocyte-lymphocyte-ratio and systemic-inflammation-index. Likewise, significantly larger pre-to-post6h white blood cells and granulocytes increases were observed following power-endurance compared to endurance-power. Moreover, there was a significant time*order interaction for blood-glucose and -lactate. Following endurance-power, blood-lactate and -glucose increased from pre-to-mid but not from pre-to-post. Meanwhile, in power-endurance blood-lactate and -glucose increased from pre-to-post but not from pre-to-mid. A significant time*order interaction was observed for CMJ-force with larger pre-to-post decreases in endurance-power compared to power-endurance. Further, CMJ-power showed larger pre-to-mid performance decreases following power-endurance, compared to endurance-power. Regarding RPE, significant time*order interactions were noted with larger pre-to-mid values following endurance-power and larger pre-to-post values following power-endurance.
Conclusion
CT induced acute and delayed order-dependent immune cell count alterations in highly trained youth male judo athletes. In general, power-endurance induced higher acute and delayed immunological stress responses compared to endurance-power. CMJ-force and RPE fluctuated during both CT sessions but went back to baseline 6 h post-exercise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute immunological responses to exercise are multifactorial and marked by systemic alterations of hormone- and immune cell concentration (Gleeson 2007; Kraemer et al. 2020). Compelling evidence suggests that chronic exercise has anti-inflammatory effects (Gleeson et al. 2011). Meanwhile, acute immunological responses to exercise are yet to be discussed (Campbell and Turner 2018a). Nonetheless, it is well-established that exercise is associated with acute leucocytosis in healthy individuals (Walsh et al. 2011). In fact, leucocytosis is one of the most clinically accepted markers of inflammatory response and is often associated with infection (Opdenakker et al. 1998) and exercise-induced stresses (Walsh et al. 2011). The magnitude of leucocytosis is dependent on several training variables such as type of exercise, intensity, and duration (Walsh et al. 2011; Ghanbari-Niaki et al. 2011; Schlagheck et al. 2020; Bessa et al. 2016). In terms of youth athletes, only a few studies investigated acute immunological responses to exercise (Freitas et al. 2016; Moraes et al. 2017; Puta et al. 2018), making our understanding of the matter relatively deficient. Generally, growth and maturation are associated with changes within tissues, organs, body systems, body composition, and physical fitness (DiFiori et al. 2014). Thus, youth athletes are a vulnerable group since high training volumes at early ages may increase not only the risk for infection (Nieman and Wentz 2019; Moreira et al. 2009) but also the likelihood for drop out due to acute or chronic injury (Fabricant et al. 2016; Roberts 2014).
To succeed in competition, elite team and individual athletes seek the development of both, high levels of muscle strength and power as well as cardiorespiratory endurance. In this context, concurrent training (CT) is a commonly applied and effective training approach. It stands for the combination of strength/power and endurance exercises to improve both measures of muscular fitness (e.g., muscle strength and power) and cardiorespiratory endurance (e.g., maximal oxygen consumption). Immunological events in the context of CT received little attention in the literature with the majority of the available studies focused on hormonal responses (Schumann et al. 2013; Schumann et al. 2014; Sparkes 2020; Enright et al. 2018; Inoue et al. 2016). For instance, Schumann and colleagues (Schumann et al. 2013; Schumann et al. 2014) reported that strength followed by endurance exercises induced similar acute peripheral alterations of cortisol and testosterone compared to endurance followed by strength in physically active men. Similar results were reported for professional male soccer players when strength exercises were combined with soccer-specific endurance training or vice versa (Sparkes 2020; Enright et al. 2018). Meanwhile, Inoue et al. (Inoue et al. 2016) investigated the effects of concurrent endurance and strength exercises order on acute inflammatory responses in recreational male weightlifters and found similar acute alterations for interleukin-6, interleukin-10 and tumour-necrosis-factor-alpha immediately post-exercise, regardless of the order. In general, the number of studies in this area is limited and the available ones indicate that the acute immune response to CT may differ from those known after single-mode strength- or endurance training (Donges et al. 2014), yet supportive evidence for youth athletes is missing.
Several factors (e.g., type of exercise, intensity, and duration) are likely to alter the effects of CT (Fyfe et al. 2014; Fyfe and Loenneke 2018; Ihalainen et al. 2017). Particularly, there are indications that the applied exercise order during CT determines the magnitude of the underlying physiological events (Schumann et al. 2013; Coffey and Hawley 2017; Taipale et al. 2014). However, there is hardly any study that examined the effects of CT exercise order on markers of acute immunological stress responses (e.g., While blood cells [WBC], lymphocytes [LYM], granulocytes [GRAN], granulocyte-lymphocyte-ratio [GLR] and the systemic inflammation index [SII]). Of note, the GLR and SII are newly emerging inflammatory markers, mostly used in clinical settings (Buonacera et al. 2022). However, while their use in exercise science is yet limited, they are attracting more attention. This is because the available evidence indicated moderate-to-high correlations of GLR and SII with other well-established inflammatory markers such as the C-reactive-protein and Interleukin-6 (Huang et al. 2018; Islas-Vazquez et al. 2020; Walzik et al. 2021).
Besides, youth elite athletes are yet underrepresented in the literature, although they are commonly exposed to high training loads, which could weaken their immune system, making them prone to injury and infection (DiFiori et al. 2014; Fabricant et al. 2016; Roberts 2014). Therefore, the primary aim of this study was to investigate the effect of CT order (i.e., power-endurance versus endurance-power) on acute (< 15 min) and delayed (≤ 6 h) immunological stress responses (i.e., WBC, LYM, GRAN, GLR, and SII) in highly trained youth male judo athletes. We additionally examined the effects of CT order on measures of muscular fitness (i.e., jump height, force, power) and rating of perceived exertion (RPE). We hypothesized that different exercise orders during CT would cause different acute and delayed alterations in peripheral immune cell counts, measures of muscular fitness, and RPE in highly trained youth male judo athletes (Schumann et al. 2013; Coffey and Hawley 2017; Taipale et al. 2014).
Methods
Participants
A total of 24 judo athletes were recruited for the study. To be included, participant had to be free from injuries or any signs of infectious diseases before and throughout the entire experimental period. All participants were enrolled in a national training centre and were members of the federal and/or national judo squat and can therefore be classified as highly trained (McKay et al. 2022). Additionally, they were all actively engaged in national and/or international judo competitions. Their general training routine consisted of two sessions per day, lasting one to two hours each on ≥ 5 days a week. The maturity status of participants was determined using the maturity offset method, which was estimated using the predictive equation of Mirwald et al. (2002) for males. During the wash-out period, four individuals dropped out of the study. Thus, 20 males completed the entire protocol. Details of the included participants at baseline are provided in Table 1. All participants as well as their legal guardians gave written informed consent to participate in the study. The Human Ethics Committee at Potsdam University approved the experimental procedure.
Procedure
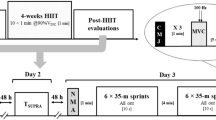
A schematic overview of the study is shown in Fig. 1. The experiments took place during May and June 2021. All participants visited the training/testing area on four different occasions. During the first visit, all participants were familiarised with all exercises (see “Muscle power and sport-specific endurance exercises” section) and the experimental protocol while during the second visit, the 1-repetition maximum protocol using the leg-press machine was carried out. Thirty-six hours before the third and fourth visits, participants were instructed to avoid any kind of physical activity. Further, all participants were advised to have breakfast on the day of the experiment but to follow their normal filling routine. Due to COVID-19 restrictions, participants were assigned to three groups. The first arrived at 7:00 am, the second at 9:30 am, and the third at 11:30 am. All participants performed a standardised warm-up routine, based on selected exercises of the FIFA 11 + program (FIFA 2022). Then, they were randomly assigned to either the power-endurance or endurance-power order. After the end of the protocols, all participants were informed to follow their daily routine but were instructed not to perform any additional sports activities for the rest of the day. Following a wash-out period of 2 weeks in which all participants followed their normal training routine, the procedure was repeated. More specifically, all participants from the power-endurance order ran endurance-power and vice versa.
Muscle power and sport-specific endurance exercises
With reference to the recommendations of Faigenbaum et al. (2009) for the progression of resistance training in youth athletes, the power exercise consisted of 4 sets of 8 repetitions using a leg-press machine (SCHNELL, Peutenhausen, Germany) at 30–40% of 1-repetition maximum with 4 min break between sets (total work time including breaks and post measures ⁓ 25 min). This setting is commonly used in practice and aims to improve muscular power. Participants were instructed to perform each repetition as fast as possible. For the sport-specific endurance exercise, the previously validated Special-Judo-Fitness-Test (Sterkowicz et al. 1999) was used. Specifically, four rounds of the Special-Judo-Fitness-Test with three sets (A = 15 s; B and C = 30 s) per round were carried out. The rest in between sets and rounds was 10 s and 4 min, respectively. Briefly, during each round, the evaluated participant throws two partners who are positioned 12 m apart as many times as possible using the ippon-seoi-nage technique (Franchini et al. 2011). The total work time including breaks and post measures was ⁓ 25 min. Due to their daily training routine and the previously applied familiarisation sessions, all participants were acquainted with the applied exercises.
Data collection
All time points of measurement (i.e. pre, mid, post, post6h) are shown in Fig. 1. Capillary blood markers of immune response were obtained from the earlobe (20 µl) at pre, post, and post6h. In this context, we did not measure immune responses between the power and sport-specific endurance protocol (i.e. mid). This was due to the fact, that an additional measure would have extended the timeframe between the strength and sport-specific endurance exercise. WBC, LYM, GRAN, and (blood) platelet were analysed immediately after taking the blood sample by using a haematology analyser system (Medonic M32, Boule Medical AB, Sweden). Medonic M32 uses a WBC-discriminator and operates due to the principle of impedance. Thereby, GRAN includes neutrophils, basophils, and eosinophils. Based on previous literature (Buonacera et al. 2022; Steidten et al. 2021), GLR and the SII were calculated as follows:
Additionally, 10 µl blood were taken from the earlobe at pre, mid, and post, to quantify blood lactate and blood glucose (Biosen S-Line, EKF-Diagnostics, Germany). The RPE using the BORG scale (Williams 2017) and CMJ-height, -force, and -power using a force plate (Leonardo Jumping Platform, Novotec, Germany) were assessed at pre, mid, post, and post6h.
Statistical analyses
To examine the effects of CT exercise order on the dependent variables (i.e., WBC; LYM; GRAN; GLR; SII; blood lactate and glucose; CMJ-performance [i.e., power; force; height] as well as RPE) a 2 (order: endurance-power and power-endurance) * 3 or 4 (time: Pre, Mid, Post, Post6h) repeated measure analysis of variance was computed. Data were tested and confirmed for normal distribution using the Shapiro–Wilk test. To correct for violations of sphericity, the degrees of freedom were corrected in the normal way, using the Huynh–Feldt (ε > 0.75) or Greenhouse–Geisser (ε < 0.75) values for ε, as appropriate (Field and Discovering statistics using SPSS 2009). In the case of significant order*time interactions, Bonferroni pairwise comparisons were conducted (Field and Discovering statistics using SPSS 2009; Cohen 1988). Effect size (ES) was interpreted as trivial (ES < 0.20), small (0.2 ≤ ES < 0.50), moderate (0.50 ≤ ES < 0.80) or large (ES ≥ 0.80) (Cohen 1988). The results are presented as mean ± standard deviation (SD). Statistical significance was set at p < 0.05. The data were analysed using the Statistical Package for Social Science (SPSS, Chicago, IL, USA, version 29.0).
Results
Mean values and standard deviations for all measures are displayed in Table 2. There were no baseline differences between the two exercise orders for all measurements.
Blood markers of immune response
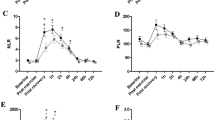
Results showed significant time*order interactions for WBC (p = 0.022), LYM (p < 0.001), GRAN (p = 0.004), GLR and SII (p < 0.001, respectively). More specifically, power-endurance resulted in significantly larger pre-to-post (∆ + 64%; ES = 2.20) and pre-to-post6h (∆ + 98%; ES = 3.40) increases for WBC compared to endurance-power (∆ + 33%; ES = 0.90 and ∆ + 55%; ES = 1.50, respectively). For LYM, significantly larger pre-to-post increases (∆ + 68%; ES = 1.87) in power-endurance compared to endurance-power (∆ − 13%, ES = 0.40) were noted. In terms of GRAN, results indicated significantly larger pre-to-post6h elevations (∆ + 160%; ES = 4.82) for power-endurance compared to endurance-power (∆ + 94%; ES = 1.68). Regarding GLR and SII, findings indicated significantly larger pre-to-post increases for endurance-power (∆ + 106%; ES = 2.07 and ∆ + 109%; ES = 2.05, respectively), compared to power-endurance (∆ + 1%; ES = 0.02 and ∆ + 3%; ES = 0.08, respectively). A graphical representation of these results can be found in Fig. 2.
Means and standard deviation for all immunological blood markers measured at pre, post, and 6 h post for the power-endurance (blue coloured line) and endurance-power order (orange coloured line). The graph highlights that concurrent training induced order-dependent immune cell count alterations in healthy youth male judo athletes. From an acute (≤ 15 min) perspective, significant differences between the two exercise orders in white blood cells, lymphocytes, granulocyte-lymphocyte-ratio, and the systemic inflammation index were observed. From a delayed (≤ 6 h) perspective, there were significant differences in white blood cells and granulocytes. *Stands for significant time*order interaction effect
Metabolic response
Our findings indicated significant time*order interactions for blood glucose and lactate (p < 0.001, respectively). Results showed significantly larger pre-to-mid increases in blood glucose and lactate following the endurance exercise (as part of the endurance-power order, ∆ + 18%; ES = 0.78 and ∆ + 947%; ES = 40.50, respectively) compared to the power exercise (as part of the power-endurance order, ∆ − 4%; ES = 0.23 and ∆ + 52%; ES = 1.72, respectively). From pre-to-post, changes in blood glucose and lactate were significantly larger for the power-endurance order (∆ + 17%; ES = 0.91 and ∆ + 814%; ES = 24.38, respectively), compared to the endurance-power order (∆ − 7%; ES = 0.30 and ∆ + 280%; ES = 8.75, respectively). A graphical representation of these results can be found in Fig. 3.
Means and standard deviation for all metabolic values collected at pre, mid, post, and 6 h post for the power-endurance (i.e., blue coloured line) and endurance-power order (i.e., orange coloured line). The graph highlights significant time*order interaction effects with significantly larger pre-to-mid increases in blood glucose and lactate following the endurance exercise (as part of the endurance-power order) compared to significantly larger pre-to-post increases in blood glucose and lactate following the endurance exercise (as part of the power-endurance order). *Stands for significant time*order interaction effect
Physical performance and subjective perception of effort
For physical performance, a significant time*order interaction was observed for CMJ-force (p = 0.045) with significantly larger pre-to-post performance decreases for the endurance-power order (∆ − 5%; ES = 0.19), compared to the power-endurance order (∆ + 1%; ES = 0.02). Further, there was a significant time*order interaction for CMJ-power (p = 0.019) with larger pre-to-mid performance decreases for the power-endurance order (∆ − 1%; ES = 0.03), compared to the endurance-power order (∆ + 3%; ES = 0.09). Regarding RPE, there was a significant time*order interaction (p < 0.001) showing larger pre-to-mid values following the endurance exercise (as part of the endurance-power order, ∆ + 157%; ES = 11.00), compared to the power exercise (as part of the power-endurance order, ∆ + 29%; ES = 2.00). Additionally, significantly larger pre-to-post RPE values were observed for power-endurance order (∆ + 157%; ES = 11.00), compared to endurance-power (∆ + 57%; ES = 4.00). A graphical representation of these results can be found in Fig. 4.
Means and standard deviation for all physical performance and perceived exertion values collected at pre, mid, post, and 6 h post for the power-endurance (i.e., blue coloured line) and endurance-power order (i.e., orange coloured line). The graph highlights significant time*order interactions for CMJ-performance and rate of perceived exertion. CMJ-force showed significantly larger pre-to-post performance decreases within the endurance-power order while CMJ-power showed larger pre-to-mid performance decreases following the power-endurance order. Rate of perceived exertion was significantly higher following the endurance exercise (as part of the endurance-power order. *Stands for significant time*order interaction effect
Discussion
The main findings of this study indicated that CT induced acute (≤ 15 min) and delayed (≤ 6 h), order-dependent immune cell count alterations in healthy youth male judo athletes. In general, the power-endurance order induced higher increases in WBC compared to the endurance-power order. Further, we found order-dependent fluctuations in CMJ-force with larger performance decreases after endurance-power compared to power-endurance while CMJ-power significantly increased from pre-to-mid following the endurance-power order. For RPE, findings indicated larger values from pre-to-post following power-endurance while endurance-power induced larger values from pre-to-mid.
This is the first study investigating the acute effects of CT across two different exercise orders on immunological stress responses in youth male judo athletes. Consequently, comparative literature is sparse. In general, physical exercise that involves repetitive muscle contraction acutely stimulates the activity of the central nervous system and causes systemic substrate metabolism (Valencia-Sánchez et al. 2019). This would alter immune cell activity/function and further, stimulate the release of glucocorticoid hormones (e.g., cortisol), catecholamines (e.g., adrenaline), cytokines (e.g., Interleukin-6), and WBC into peripheral circulation (Valencia-Sánchez et al. 2019; Rosa-Neto et al. 2022). Thereby, the increase in WBC seems to be due to demargination from the vessel walls and to cell releases from organic storage (e.g., liver, lung), as well as the thymus gland, bone marrow lymph nodes, and skeletal muscle (Simpson et al. 2015). The magnitude and the time course of exercise-induced leucocytosis and thus, any alteration within circulating immune cell counts is dependent on several factors, such as age, duration and intensity and/or the type of the applied exercise (Walsh et al. 2011; Schlagheck et al. 2020; Natale et al. 2003). To the best of our knowledge, acute leucocytosis in responses to power-based exercises is yet to be investigated. Previous studies reported acute leucocytosis after strength (Ihalainen et al. 2014; Nieman et al. 1995), endurance (Nielsen et al. 1996; Shek et al. 1995; Vider et al. 2001; Wahl et al. 2020), and concurrent strength and endurance exercises (Bessa et al. 2016), in trained (Bessa et al. 2016; Nielsen et al. 1996; Vider et al. 2001; Wahl et al. 2020) and recreationally active (Ihalainen et al. 2014; Nieman et al. 1995; Shek et al. 1995) adults. Although a direct comparison to most of the forenamed studies is difficult, our findings generally corroborate with the literature since both exercise orders induced acute (≤ 15 min) and delayed (≤ 6 h) immune cell count alterations (i.e. WBC, LYM, GRAN) in youth highly trained athletes. However, based on our results it seems that the magnitude of acute and delayed immune cell count alterations to CT is exercise order-dependent with particularly higher increases in WBC following the power-endurance order compared to the endurance-power order (Fig. 2).
Generally, LYM make up to 40% of the total WBC count in the bloodstream (Kverneland et al. 2016). LYM are made of lymphoid stem cells within the bone marrow and act as a crucial part of the adaptive immune system. It is accepted that lymphocytosis originates during and immediately after exercise, mainly due to an adrenergic stimulation forcing LYM to detach from vessel walls into circulation (Campbell and Turner 2018b). However, LYM fall back and could reach values even below baseline shortly after exercise (≤ 30 min). Several attempts have previously been made to explain the rapid fall-back of LYM (Vider et al. 2001; Nieman 2000; Kakanis et al. 2010; Shinkai et al. 1996). More specifically, recent literature indicates that LYM shift to peripheral tissue to obtain an increased state of immune surveillance (Campbell and Turner 2018b; Krüger et al. 2008; Kruger and Mooren 2007). It is widely accepted that the magnitude of lymphocytosis and lymphocytopenia is proportional to the applied duration and intensity of exercise (Walsh et al. 2011; Campbell and Turner 2018b). With respect to the current study, LYM solely increased in the power-endurance order, from pre-to-post but then decreased from post towards post6h. For the endurance-power order, LYM decreased from pre-to-post, before slightly increasing towards post6h. Therefore, we would report an acute but not delayed LYM difference between the two exercise orders. In fact, acute lymphocytosis was expected after both exercise orders. This is because the overall exercise load was equal between both exercise orders. In this context, we need to note that we did not measure cell kinetics between the power and sport-specific endurance protocol (i.e., mid). As such, it could be assumed that we have missed the time point in which LYM were elevated within the endurance-power order.
GRAN represents the main component of total WBC (up to 60%) in healthy young adults (Kverneland et al. 2016). GRAN mature within bone marrow (Walsh et al. 2011) and rise during and after exercise several folds (Schlagheck et al. 2020; Natale et al. 2003; Nieman et al. 1995; Nieman et al. 1989). Unlike LYM, GRAN are made of myeloid stem cells and are responsible for the innate immune response. GRAN partly circulates throughout the peripheral system while adhering to the endothelial surface. Exercise-induced haemodynamics (e.g. increased blood flow, shear stress) trigger GRAN to demarginate from the blood vessel walls, which acutely increases the number of circulating cells (Simpson et al. 2015). The general trend of post-exercise GRAN kinetics was previously shown as a continuous increase, which starts during exercise but lasts up to six hours, depending on the applied exercise (Nieman et al. 1989; Ramel et al. 2003; Gabriel et al. 1992; Mayhew et al. 2005). Therefore, our results corroborate with the existing literature as GRAN gradually increased regardless of the applied exercise order. Although, results indicated a markedly higher delayed (i.e., pre-to-post6h) increase in GRAN following power-endurance (∆161%) compared to endurance-power (∆94%). However, the significant difference between the two exercise orders in the delayed cell count was unexpected. In this context, cortisol and catecholamines play a key role in activating the immune system and particularly, GRAN release from the bone marrow (Peake et al. 2017). Interestingly, it was previously suggested that the release of cortisol and catecholamines, is intensity-dependent (Hill et al. 2008; Kindermann et al. 1982). Although we did not measure hormone levels, it was interesting to find significant time*order interactions for RPE from pre-to-mid, indicating that the sport-specific endurance exercise imposed a higher physiological stress compared to the muscle power exercise. Consequently, it seems that the sport-specific endurance exercise was the main driver of the observed immune responses but that, in the endurance-power order, the applied power exercise may have partly attenuated the delayed GRAN increase. Based on our findings we would argue that the innate (i.e. GRAN) and adaptive (i.e., LYM) immune systems displayed a distinct time-dependent activity. However, this is a preliminary interpretation that should be confirmed by future studies.
The application of GLR and SII within the area of exercise science is yet limited. Meanwhile, from a clinical perspective, they are well-established markers of diseases (Buonacera et al. 2022) and death (Fois et al. 2020; Moisa et al. 2021). For example, Fois et al. (2020) reported that the SII on admission independently predicts in-hospital mortality in COVID-19 patients. It is worth noting though, that earlier studies demonstrated moderate-to-high correlations of GLR and SII with other well-established inflammatory markers such as the C-reactive-protein and Interleukin-6 (Huang et al. 2018; Islas-Vazquez et al. 2020; Zhu et al. 2020). A recent review by Walzik and colleagues (2021) suggested the application of GLR and SII as feasible tools to assess exercise-induced strain and indicators of recovery processes. Most of the available studies which measured GLR and SII in an exercise setting assessed the effects of endurance exercises on GLR and/or SII. For instance, Wahl et al. (2020) compared different recovery strategies following either high-intensity or sprint interval cycling and its effects on immune cell kinetics in male cyclists/triathletes aged 25 years. The authors reported that irrespective of the applied exercise, GLR and SII increased significantly up to 3 h post-exercise. In another study, Bessa et al. (2016) examined the effects of CT on biomarkers of injury and inflammation in elite cyclists aged 28 years. The authors reported a significant increase in GLR, lasting for 3 h while dropping significantly below baseline 12 h post-exercise. With respect to our results, there were significant time*order interaction effects for GLR and SII. Following endurance-power, GLR and SII increased one-fold immediately post-exercise and stayed elevated after 6 h. Meanwhile, in power-endurance, both parameters did not change from pre-to-post but rise towards post6h to a similar level compared to endurance-power. In general, GLR (SII)-values rise when GRAN (and platelets) are high while LYM are low (Walzik et al. 2021). Our results seem to be reasonable considering the elevated LYM following the power-endurance order but not endurance-power.
A secondary aim of this study was to examine the effect of CT order on CMJ performance and RPE. Our results showed a significantly larger pre-to-post decrease in CMJ-force following endurance-power and larger pre-to-mid performance decreases in CMJ-power for the power-endurance order, compared to the endurance-power order. Additionally, significantly larger pre-to-post RPE values were observed for the power-endurance order compared to endurance-power. However, from pre-to-post6h RPE and CMJ-force and power went back towards baseline. Based on the literature, it is highly recommended to report aspects of internal and external load to give the most accurate feedback about the experienced effort (Balsalobre-Fernández et al. 2014; Halson 2014; Cardinale and Stone 2006; García-Pinillos et al. 2021; Impellizzeri et al. 2019; McLaren et al. 2018). Regarding CT, the study of Bessa et al. (2016) showed an inverse relationship between GLR and upper-body muscle strength in elite male cyclists. More specifically, the authors revealed that GLR significantly increased 3 h post-exercise while muscle strength significantly decreased. However, both values returned to baseline levels after 48 h. With reference to Fig. 4, CMJ-force significantly decreased from pre-to-post and CMJ-power significantly increased from pre-to-mid following the endurance-power order. CMJ-height, on the other hand, did not exhibit a clear fluctuation throughout the two CT orders. Meanwhile, subjective RPE showed changes throughout the protocols. Specifically, compared to the immune cell kinetics which showed elevated cell counts at post and post6h, RPE went up throughout the protocols but went back to baseline 6 h post-exercise. This would indicate that both exercise orders were in fact, physiologically demanding. Of note, our findings pointed towards a disparity between immunological, perceived as well as physical responses following both CT orders. More specifically, while the immunological responses indicated the need for an extended rest to regain immunological homeostasis (Fig. 2), perceived and physical responses showed that athletes seemed to be ready for another session, 6 h post-CT (Fig. 4). Therefore, practitioners should be aware that measures of internal load may differ from those of external load. Nevertheless, these findings should be confirmed by future studies.
Limitations and future research perspectives
This study has some limitations that warrant discussion. First, cell kinetics were measured at pre, post, and post6h but not in between the power and sport-specific endurance protocol (i.e., mid) or beyond the 6 h post-effort (e.g., 12 h or 24 h post). Additional measures at mid and/or post 12 h and 24 h would have helped provide a better overview of the delayed effects of CT order on the selected markers of the immunological stress response. However, because of the athletes’ congested training schedule besides the COVID-19 restrictions, it was not possible to collect further blood samples beyond 6 h post. Also, blood samples required immediate analyses. This would have led to experimental delays, due to the short time frame between the power- and endurance exercises. Second, contrasting endurance and/or power training alone with endurance-power and power-endurance would have led to a more comprehensive comparison. These particular shortcomings should be considered in future investigations. Third, we applied one power exercise, only. Indeed, to be in line with real-world scenarios, including several exercises may be more suitable when investigating combined power and endurance sessions. Nonetheless, the applied procedure is common practice and was agreed upon in consultation with the coaching staff. Of note, studies that investigated the effects of power exercises and CT on WBC alterations are yet missing. Accordingly, any comparison made to other studies should be interpreted with caution. Also, we considered male athletes only, making any conclusions regarding female athletes difficult. Lastly, it needs to be stressed that we have relied on indirect markers of inflammation (i.e., GLR and SII). There is evidence that both GLR and SII display moderate-to-high associations with other well-established inflammatory markers such as the C-reactive-protein and Interleukin-6 (Huang et al. 2018; Islas-Vazquez et al. 2020; Zhu et al. 2020). Nevertheless, future studies should favour more prominent markers (e.g., cytokines) to provide a more distinct inflammatory status.
Conclusion
The main findings of this study indicated that concurrent power and sport-specific endurance exercises induced acute (≤ 15 min) and delayed (≤ 6 h), order-dependent immune cell count alterations in healthy youth male judo athletes. Specifically, results showed that immunological stress responses were generally higher after power-endurance compared to endurance-power. However, the mechanisms behind this phenomenon remain speculative. The second finding of this study was that measures of muscular fitness (e.g., CMJ-force) and RPE fluctuated throughout the CT protocol but went back to baseline values 6 h post-exercise. Lastly, it is worth noting that our findings pointed towards a disparity between immunological, perceived as well as physical responses following both CT orders.
Availability of data and materials
All data are freely available on repositories of the Open Science Framework (https://osf.io/snqkb/).
Abbreviations
- CT:
-
Concurrent training
- CMJ:
-
Countermovement jump
- GRAN:
-
Granulocytes
- GLR:
-
Granulocyte-lymphocyte-ratio
- LYM:
-
Lymphocytes
- RPE:
-
Rate of perceived exertion
- SII:
-
Systemic inflammation index
- WBC:
-
White blood cells
References
Balsalobre-Fernández C, Tejero-González CM, del Campo-Vecino J (2014) Relationships between training load, salivary cortisol responses and performance during season training in middle and long distance runners. PLoS ONE 9(8):e106066
Bessa AL et al (2016) Exercise intensity and recovery: biomarkers of injury, inflammation, and oxidative stress. J Strength Cond Res 30(2):311–319
Buonacera A et al (2022) Neutrophil to lymphocyte ratio: an emerging marker of the relationships between the immune system and diseases. Int J Mol Sci 23(7):3636
Campbell JP, Turner JE (2018a) Debunking the myth of exercise-induced immune suppression: redefining the impact of exercise on immunological health across the lifespan. Front Immunol 9:648
Campbell JP, Turner JE (2018b) Debunking the myth of exercise-induced immune suppression: redefining the impact of exercise on immunological health across the lifespan. Front Immunol 9:648
Cardinale M, Stone MH (2006) Is testosterone influencing explosive performance? J Strength Cond Res 20(1):103–107
Coffey VG, Hawley JA (2017) Concurrent exercise training: do opposites distract? J Physiol 595(9):2883–2896
Cohen J (1988) Statistical power analysis for the behavioral sciences, no 1. Lwrence Earlbaum Associates Publishers
DiFiori JP et al (2014) Overuse injuries and burnout in youth sports: a position statement from the American Medical Society for Sports Medicine. Br J Sports Med 48(4):287–288
Donges CE et al (2014) Cytokine mRNA expression responses to resistance, aerobic, and concurrent exercise in sedentary middle-aged men. Appl Physiol Nutr Metab 39(2):130–137
Enright K et al (2018) Hormonal responses during two different concurrent-training trials in youth elite soccer players: does changing the organization of training impact the hormonal response to concurrent exercise? J Sports Med Phys Fitness 58(5):699–706
Fabricant PD et al (2016) Youth sports specialization and musculoskeletal injury: a systematic review of the literature. Phys Sportsmed 44(3):257–262
Faigenbaum AD et al (2009) Youth resistance training: updated position statement paper from the national strength and conditioning association. J Strength Cond Res 23:S60–S79
Field A (2009) Discovering statistics using SPSS. Sage publications
FIFA, FIFA 11+. https://www.f-marc.com/files/downloads/posters_generic/english.pdf
Fois AG et al (2020) The systemic inflammation index on admission predicts in-hospital mortality in COVID-19 patients. Molecules 25(23):5725
Franchini E et al (2011) Energy system contributions to the special judo fitness test. Int J Sports Physiol Perform 6(3):334–343
Freitas CG et al (2016) Monitoring salivary immunoglobulin a responses to official and simulated matches in elite young soccer players. J Hum Kinet 53:107
Fyfe JJ, Loenneke JP (2018) Interpreting adaptation to concurrent compared with single-mode exercise training: some methodological considerations. Sports Med 48(2):289–297
Fyfe JJ, Bishop DJ, Stepto NK (2014) Interference between concurrent resistance and endurance exercise: molecular bases and the role of individual training variables. Sports Med 44(6):743–762
Gabriel H, Urhausen A, Kindermann W (1992) Mobilization of circulating leucocyte and lymphocyte subpopulations during and after short, anaerobic exercise. Eur J Appl Physiol 65(2):164–170
García-Pinillos F et al (2021) Vertical jumping as a monitoring tool in endurance runners: a brief review. J Hum Kinet 80(1):297–308
Ghanbari-Niaki A, Saghebjoo M, Hedayati M (2011) A single session of circuit-resistance exercise effects on human peripheral blood lymphocyte ABCA1 expression and plasma HDL-C level. Regul Pept 166(1–3):42–47
Gleeson M (2007) Immune function in sport and exercise. J Appl Physiol 103(2):693–699
Gleeson M et al (2011) The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 11(9):607–615
Halson SL (2014) Monitoring training load to understand fatigue in athletes. Sports Med 44(2):139–147
Hill E et al (2008) Exercise and circulating cortisol levels: the intensity threshold effect. J Endocrinol Invest 31(7):587–591
Huang Y et al (2018) Relationship between monocytes to lymphocytes ratio and axial spondyloarthritis. Int Immunopharmacol 57:43–46
Ihalainen J et al (2014) Acute leukocyte, cytokine and adipocytokine responses to maximal and hypertrophic resistance exercise bouts. Eur J Appl Physiol 114(12):2607–2616
Ihalainen JK et al (2017) Resistance training status modifies inflammatory response to explosive and hypertrophic resistance exercise bouts. J Physiol Biochem 73(4):595–604
Impellizzeri FM, Marcora SM, Coutts AJ (2019) Internal and external training load: 15 years on. Int J Sports Physiol Perform 14(2):270–273
Inoue DS et al (2016) Immunometabolic responses to concurrent training: the effects of exercise order in recreational weightlifters. J Strength Cond Res 30(7):1960–1967
Islas-Vazquez L et al (2020) IL-6, NLR, and SII markers and their relation with alterations in CD8+ T lymphocyte subpopulations in patients treated for lung adenocarcinoma. Biology 9(11):376
Kakanis M et al (2010) The open window of susceptibility to infection after acute exercise in healthy young male elite athletes. J Sci Med Sport 13:e85–e86
Kindermann W et al (1982) Catecholamines, growth hormone, cortisol, insulin, and sex hormones in anaerobic and aerobic exercise. Eur J Appl Physiol 49(3):389–399
Kraemer WJ et al (2020) Growth hormone (s), testosterone, insulin-like growth factors, and cortisol: roles and integration for cellular development and growth with exercise. Front Endocrinol 11:33
Krüger K et al (2008) Exercise-induced redistribution of T lymphocytes is regulated by adrenergic mechanisms. Brain Behav Immun 22(3):324–338
Kruger K, Mooren F (2007) T cell homing and exercise. Exerc Immunol Rev 13:37–54
Kverneland AH et al (2016) Age and gender leucocytes variances and references values generated using the standardized ONE-Study protocol. Cytometry A 89(6):543–564
Mayhew DL, Thyfault JP, Koch AJ (2005) Rest-interval length affects leukocyte levels during heavy resistance exercise. J Strength Cond Res 19(1):16–22
McKay AK et al (2022) Defining training and performance caliber: a participant classification framework. Int J Sports Physiol Perform 1(2):1–15
McLaren SJ et al (2018) The relationships between internal and external measures of training load and intensity in team sports: a meta-analysis. Sports Med 48(3):641–658
Mirwald RL et al (2002) An assessment of maturity from anthropometric measurements. Med Sci Sports Exerc 34(4):689–694
Moisa E et al (2021) Dynamic changes of the neutrophil-to-lymphocyte ratio, systemic inflammation index, and derived neutrophil-to-lymphocyte ratio independently predict invasive mechanical ventilation need and death in critically ill COVID-19 patients. Biomedicines 9(11):1656
Moraes H et al (2017) SIgA response and incidence of upper respiratory tract infections during intensified training in youth basketball players. Biol Sport 34(1):49
Moreira A et al (2009) Does exercise increase the risk of upper respiratory tract infections? Br Med Bull 90(1):111–131
Natale VM et al (2003) Effects of three different types of exercise on blood leukocyte count during and following exercise. Sao Paulo Med J 121:09–14
Nielsen H et al (1996) Lymphocyte, NK and LAK cell responses to maximal exercise. Int J Sports Med 17(01):60–65
Nieman DC (2000) Exercise effects on systemic immunity. Immunol Cell Biol 78(5):496–501
Nieman DC, Wentz LM (2019) The compelling link between physical activity and the body’s defense system. J Sport Health Sci 8(3):201–217
Nieman D et al (1989) Effects of long-endurance running on immune system parameters and lymphocyte function in experienced marathoners. Int J Sports Med 10(05):317–323
Nieman D et al (1995) The acute immune response to exhaustive resistance exercise. Int J Sports Med 16(05):322–328
Opdenakker G, Fibbe WE, Van Damme J (1998) The molecular basis of leukocytosis. Immunol Today 19(4):182–189
Peake JM et al (2017) Recovery of the immune system after exercise. J Appl Physiol 122(5):1077–1087
Puta C et al (2018) Standardized assessment of resistance training-induced subjective symptoms and objective signs of immunological stress responses in young athletes. Front Physiol 9:698
Ramel A, Wagner K-H, Elmadfa I (2003) Acute impact of submaximal resistance exercise on immunological and hormonal parameters in young men. J Sports Sci 21(12):1001–1008
Roberts WO (2014) Overuse injuries and burnout in youth sports. LWW. 24:1–2
Rosa-Neto JC et al (2022) Immunometabolism-fit: how exercise and training can modify T cell and macrophage metabolism in health and disease. Exerc Immunol Rev 28:29–46
Schlagheck ML et al (2020) Cellular immune response to acute exercise: comparison of endurance and resistance exercise. Eur J Haematol 105(1):75–84
Schumann M et al (2013) Acute neuromuscular and endocrine responses and recovery to single-session combined endurance and strength loadings: “order effect” in untrained young men. J Strength Cond Res 27(2):421–433
Schumann M et al (2014) The order effect of combined endurance and strength loadings on force and hormone responses: effects of prolonged training. Eur J Appl Physiol 114(4):867–880
Shek PN et al (1995) Strenuous exercise and immunological changes. Int J Sports Med 16(07):466–474
Shinkai S et al (1996) Cortisol response to exercise and post-exercise suppression of blood lymphocyte subset counts. Int J Sports Med 17(08):597–603
Simpson RJ et al (2015) Exercise and the regulation of immune functions. Prog Mol Biol Transl Sci 135:355–380
Sparkes W et al (2020) The effect of training order on neuromuscular, endocrine and mood response to small-sided games and resistance training sessions over a 24-h period. J Sci Med Sport 23:866–871
Steidten T et al (2021) Overnight immune regulation and subjective measures of sleep: a three night observational study in adolescent track and field athletes. Front Sports Active Living 3:270
Sterkowicz S, Zuchowicz A, Kubica R (1999) Levels of anaerobic and aerobic capacity indices and results for the special fitness test in judo competitors. J Hum Kinet 2(1):115–135
Taipale RS et al (2014) Acute neuromuscular and metabolic responses to combined strength and endurance loadings: the “order effect” in recreationally endurance trained runners. J Sports Sci 32(12):1155–1164
Valencia-Sánchez S et al (2019) Effects of exercise upon immunoregulation: facts and a modern view of its molecular mechanisms. Adv Neuroimm Biol 7(3–4):187–198
Vider J et al (2001) Acute immune response in respect to exercise-induced oxidative stress. Pathophysiology 7(4):263–270
Wahl P et al (2020) Acute impact of recovery on the restoration of cellular immunological homeostasis. Int J Sports Med 41(01):12–20
Walsh NP et al (2011) Position statement part one: immune function and exercise
Walzik D et al (2021) Transferring clinically established immune inflammation markers into exercise physiology: focus on neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and systemic immune-inflammation index. Eur J Appl Physiol 121(7):1803–1814
Williams N (2017) The Borg rating of perceived exertion (RPE) scale. Occup Med 67(5):404–405
Zhu Z et al (2020) Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis 95:332–339
Acknowledgements
We would like to thank the Olympic Testing and Training Center Brandenburg as well as the coaching stuff and the participating athletes in Frankfurt (Oder)/Germany. Particularly, the authors give thanks to Kerstin Graf and Annett Bergmann for their support in capillary blood collection. Also, we want to thank the respective head coach Michael Rex, for ensuring the implementation of a controlled study design within a real-world environment.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study is part of the project “Applied training sciences in Brandenburg” funded by the federal government of Education, Youth, and Sport, Brandenburg/Germany. (RN: 249/89111/OSPB/2019/31) and originated in collaboration with the research project “Resistance Training in Youth Athletes” funded by the German Federal Institute of Sports Science (ZMVI4-081901/20-23).
Author information
Authors and Affiliations
Contributions
AM, JB, NH, HC: made substantial contributions to conception and design; AM, NH, LK, and JB: contributed to data collection; AM carried out data analysis; All authors interpreted the data; AM: wrote the original draft of the manuscript and all authors were involved in revising it critically for important intellectual content; all authors provided the final approval of the version to be published and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest relevant to the content of this original study.
Additional information
Communicated by Philip D. Chilibeck.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Markov, A., Bussweiler, J., Helm, N. et al. Acute effects of concurrent muscle power and sport-specific endurance exercises on markers of immunological stress response and measures of muscular fitness in highly trained youth male athletes. Eur J Appl Physiol 123, 1015–1026 (2023). https://doi.org/10.1007/s00421-022-05126-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-022-05126-8