Abstract
Purpose
The magnitude of heat acclimation (HA) adaptations varies largely among individuals, but it remains unclear what factors influence this variability. This study compared individual characteristics related to fitness status and body dimensions of low-, medium-, and high responders to HA.
Methods
Twenty-four participants (9 female, 15 male; maximum oxygen uptake [\(\dot{{V}}\)O2peak,kg] 52 ± 9 mL kg−1 min−1) completed 10 daily controlled-hyperthermia HA sessions. Adaptations were evaluated by heat stress tests (HST; 35 min cycling 1.5 W kg−1; 33 °C, 65% relative humidity) pre- and post-HA. Low-, medium-, and high responder groups were determined based on tertiles (n = 8) of individual adaptations for resting rectal temperature (Tre), exercise-induced Tre rise (ΔTre), whole-body sweat rate (WBSR), and heart rate (HR).
Results
Body dimensions (p > 0.3) and \(\dot{{V}}\)O2peak,kg (p > 0.052) did not differentiate low-, medium-, and high responders for resting Tre or ΔTre. High WBSR responders had a larger body mass and lower body surface area-to-mass ratio than low responders (83.0 ± 9.3 vs 67.5 ± 7.3 kg; 249 ± 12 vs 274 ± 15 cm2 kg−1, respectively; p < 0.005). Conversely, high HR responders had a smaller body mass than low responders (69.2 ± 6.8 vs 83.4 ± 9.4 kg; p = 0.02). \(\dot{{V}}\)O2peak,kg did not differ among levels of responsiveness for WBSR and HR (p > 0.3).
Conclusion
Individual body dimensions influenced the magnitude of sudomotor and cardiovascular adaptive responses, but did not differentiate Tre adaptations to HA. The influence of \(\dot{{V}}\)O2peak,kg on the magnitude of adaptations was limited.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heat acclimation (HA), i.e., repeated exposures to heat stress within a certain time frame, can be adopted to artificially induce improvements in sweat and skin blood flow responses, fluid balance, cardiovascular stability, and thermal tolerance. These adaptive responses result in a lower thermal strain during exercise at a given workload, which is usually reflected by a lower core temperature, heart rate (HR) and skin temperature, higher whole-body sweat rate (WBSR), and improved thermal comfort (Periard et al. 2015; Daanen et al. 2018).
The magnitude of HA adaptations depends on the intensity, duration, frequency, and number of heat exposures (Taylor and Cotter 2006; Periard et al. 2015). Furthermore, characteristics specific to the individual undertaking HA likely influence the development of adaptations; some authors have suggested the existence of low- and high responders to HA (Taylor and Cotter 2006; Racinais et al. 2012). Identification of low- and high responders to HA is of significant practical importance in occupational, athletic, or military settings, when practitioners or institutions desire to individualize strategies that reduce thermal strain. However, there is limited evidence-based knowledge on what factors may predispose individuals to either group. There is potentially a genetic component that mediates HA responsiveness (Bouchard et al. 2011; Taylor 2014), but phenotypic characteristics such as fitness status and body dimensions may also relate to inter-individual differences in the adaptive response to HA (Pandolf et al. 1977; Taylor 2014; Casadio et al. 2017; Corbett et al. 2018).
Fitness status, typically characterized as maximum oxygen uptake adjusted for body mass (\(\dot{{V}}\)O2peak,kg), has often been considered to affect HA responsiveness, ever since the findings of Pandolf et al. (1977). They observed that the most fit soldiers required only four HA days to achieve complete adaptation, as defined by a rectal temperature (Tre) adaptation plateau, while the least fit participants required 8 days. These findings suggest that well-trained individuals adapt more rapidly during HA, but the authors did not specify the magnitude of adaptation that was achieved by the individual participants. Thus, whether the absolute adaptive gains varied with baseline fitness status remains unknown.
Taylor and Cotter (2006) proposed that endurance-trained individuals have a relatively low adaptive response to HA. That is, the repeated exposure to high core temperatures and sweating inherent to prolonged physical exercise training confers partial heat adaptation (Avellini et al. 1982; Periard et al. 2015; Lamarche et al. 2018a). This exercise-induced partial heat adaptation may reduce the potential for further adaptation. To date, a few studies have examined the influence of baseline fitness status on the magnitude of the HA response. Early observations from Shvartz et al. (1977) supported the hypothesis that well-trained individuals have a reduced scope for HA adaptation. However, this outcome may be specific to their experimental design. The fixed absolute workload during their HA sessions induced greater Tre rises in untrained participants than in trained participants, which may have resulted in a higher adaptive stimulus for the untrained group. Inter-individual variation in Tre during HA can be reduced by implementing a controlled-hyperthermia HA protocol, in which core temperature is elevated to and maintained at a pre-determined value during each session. When this technique was adopted, no association was observed between \(\dot{{V}}\)O2peak,kg and magnitude of adaptations after 10 days of HA (Corbett et al. 2018). This suggests that baseline fitness status does not differentiate the magnitude of the adaptive response to HA, which is in contrast to the theoretical concept proposed by Taylor and Cotter (2006). It should be noted that \(\dot{{V}}\)O2peak,kg does not directly relate to the exercise-induced partial heat adaptation status. Researchers have proposed that habitual training activity might be more reflective of this partial adaptation status (Lamarche et al. 2018a; Ravanelli et al. 2020). Altogether, there is no consensus on the degree to which fit and unfit individuals benefit from HA.
Previous studies investigating inter-individual variation in the magnitude of HA adaptations have focused primarily on fitness status (Shvartz et al. 1977; Corbett et al. 2018), whilst the role of body characteristics such as body mass, body surface area (BSA), or its ratio (i.e., BSA-to-mass ratio) has not been assessed. In particular, BSA-to-mass ratio is considered to be an important covariant in individual thermoregulation, with heat exchange between body and environment being dependent upon BSA and body heat storage upon body mass (Havenith et al. 1998; Notley et al. 2016). Whether a high or low BSA-to-mass ratio provides an advantage during exercise heat stress depends on the mode of exercise, prescribed exercise intensity, and the environmental conditions (Havenith 2001; Cramer and Jay 2016; Notley et al. 2016). Notley et al. (2016) observed that during light and moderate exercise with matched heat loss requirements, individuals with a high BSA-to-mass ratio are predisposed to dissipate heat via cutaneous vasodilation, while those with a low BSA-to-mass ratio are more dependent on sweat evaporation for heat loss. The authors suggested that individuals have naturally adapted towards the heat loss mechanism that best suits their body size. In line with this, Taylor (2014) hypothesized that, during HA, individuals may predominantly develop their anthropometric-dependent “preferred” heat loss pathway. We speculate that the “preferred” thermoeffector, determined by one’s body dimensions, is more active during HA, resulting in a greater adaptation for that specific thermoeffector. For example, sweat gland activity during HA may be particularly high for individuals with a low BSA-to-mass ratio, resulting in a pronounced sweat gland adaptation. Although it is not known whether a dose–response relationship exists, Buono et al. (2009) did show that sweat gland activity during HA is essential to improve sweating capacity. Alternatively, one could argue that the less-developed thermoeffector has more potential for adaptation and therefore shows high responsiveness. Thus, it remains unknown how, if at all, one’s body dimensions influence the adaptive response to HA.
To summarize, there is little empirical evidence to support theoretical perspectives on the individual adaptive response to HA. Therefore, the objective of our explorative study was to compare individual characteristics related to fitness status and body dimensions of low-, medium-, and high responders to controlled-hyperthermia HA. Considering the limited research available on the factors that contribute to inter-individual variance during HA, we aimed to explore the potential contributors by including participants with a range of characteristics, rather than isolating one prospective influential characteristic. It was recently shown that most phenotypic adaptations within the individual were not interrelated (Corbett et al. 2018). That is, one phenotypic adaptation (e.g., WBSR) can develop independently of others (e.g., HR or resting Tre). In the present explorative study, participants were therefore classified into the respective responder groups for separate adaptation phenotypes; resting Tre, exercise-induced rise in Tre (ΔTre), WBSR and HR.
Methods
Participants
We recruited 24 healthy volunteers (15 male, 9 female; Table 1). Participants did not reside in a warm environment (> 25 °C air temperature) for longer than 7 days within the 3 months prior to the study. They did not smoke, had no history of heat-related illnesses or cardiovascular complications, and did not have any known issues with thermoregulation. Three participants were taking medication: 70 mg alendronic acid weekly, 500 mg calci-chew, and 7.5 mg mirtazapine daily (control of benign bone tumor); Ritalin (ADHD); methotrexate and folic acid (rheumatoid arthritis). Six females used the combined pill, one used a hormonal intrauterine device, and two reported regular natural menstrual cycles (25–35 days). Participants were asked to consistently maintain habitual medication and supplement intake over the course of the study. As a potential indicator of partial HA status, weekly exercise training time (min) was determined from the self-reported habitual training program (considering the 2 months preceding the study). With the aim of removing sessions that do not evoke thermal strain, swimming sessions were excluded (Avellini et al. 1982). Procedures were approved by the Ethics Committee of the Faculty of Behavioural and Movement Sciences of the Vrije Universiteit Amsterdam (VCWE-2018-160R1), conform the standards set out by the Declaration of Helsinki. Prior to the study, participants were informed about the procedures and provided verbal and written consent.
Study design
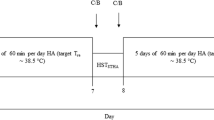
During the first visit to the laboratory, participants completed a graded exercise test in temperate conditions to determine baseline \(\dot{{V}}\)O2peak,kg and were, after a short break, familiarized with the heat stress test (HST). On a separate occasion, body dimensions and composition were assessed. Approximately 7 days after the participants first reported to the laboratory, they completed the first HST (HST1). The next day, participants commenced a 10-consecutive-day controlled-hyperthermia HA program. To evaluate adaptive responses, participants performed a second HST (HST2), which was scheduled 48 h after the last HA session. All HST and HA sessions were administered in an environmental chamber (b-Cat B.V., Tiel, The Netherlands), with air temperature 33 °C, relative humidity 65% and minimal air flow. During HA, participants did not engage in any additional exercise training (few exceptions for occasional short light-intensity exercise bouts in temperate conditions). The study was conducted during winter time (Netherlands; Jan–Apr) to minimize acclimatization status.
Experimental sessions
Body dimensions and composition
BSA was calculated from height (stadiometer; Seca 217, Seca, Hamburg, Germany) and body mass (Platform scale, SATEX 34 SA-1 250, Weegtechniek Holland B.V., Zeewolde, The Netherlands), according to the formula proposed by DuBois and DuBois (1916). BSA-to-mass ratio was calculated as BSA divided by body mass. Body fat percentage was assessed using a whole-body dual-energy X-ray absorptiometry scan (Discovery A, Hologic, Inc., Marlborough, MA, USA).
Graded exercise test (\(\dot{{V}}\)O2peak,kg)
To determine maximum oxygen uptake, participants completed a graded exercise test on an electrically braked cycle ergometer (Excalibur Sport, Lode B.V., Groningen, The Netherlands) in temperate conditions (22 °C, 32% relative humidity). Cycling started at a power output of 25 W, after which intensity increased with 25 W min−1 until volitional exhaustion. During exercise, strong verbal encouragement was given. The rate of oxygen consumption was monitored breath-by-breath using a metabolic cart (Quark CPET, COSMED, Rome, Italy). Values were discarded if they were higher than two standard deviations from the mean within a local 12-s window. The maximum oxygen uptake was defined as the highest 15-s moving average. Maximum oxygen uptake was divided by body mass (i.e., \(\dot{{V}}\)O2peak,kg) and used as an indicator of individual fitness level.
Heat stress test
Testing took place throughout the day, but each participant completed their own two HSTs at the same time of day. They were instructed to refrain from caffeine and alcohol consumption, to avoid strenuous exercise, and to report and replicate food and beverage intake during the 24 h preceding the HSTs. To encourage euhydration, participants were asked to drink 500 mL water the evening before and 10 mL kg body mass−1 of water during the 3 h prior to the HST. Upon arrival at the laboratory, participants provided a urine sample, from which urine-specific gravity was measured using a handheld refractometer (PAL-10S, Atago Co. Ltd, Tokyo, Japan). A urine-specific gravity value ≤ 1.025 was considered as an indication of sufficient hydration for adults engaging in daily exercise (Kenefick and Cheuvront 2012). Two participants had, each on one occasion, a urine-specific gravity value above 1.025; after consuming 5 mL kg−1 of water, they were allowed to resume the experiments. Upon entering the environmental chamber, participants first rested in a chair for 10 min, while stable baseline measures were obtained. They then mounted an electrically braked cycle ergometer (Excalibur Sport, Lode B.V., Groningen, The Netherlands) and commenced cycling at a power output of 1.5 W kg body mass−1 for 35 min. This was followed by a 5-min resting period, during which participants consumed a standardized volume of water (3 mL kg body mass−1). Next, participants performed a graded exercise test (GXTHST), starting at a power output of 1.5 W kg body mass−1 with subsequent increments of 25 W min−1 until volitional exhaustion. No feedback or encouragement was given during the GXTHST. WBSR was calculated as the difference between pre- and post-session nude body mass, corrected for exposure time (g h−1; Platform scale, SATEX 34 SA-1 250, Weegtechniek Holland B.V., Zeewolde, The Netherlands). With every weighing procedure, two measurements were performed from which body mass was determined as the average value over a stable 5-s assessment. If the difference between the two measurements was > 0.05 kg, a third measurement was performed.
Controlled-hyperthermia HA sessions
Every participant performed 10 controlled-hyperthermia HA sessions at approximately the same time of day (at least within ± 3 h of HST time). Prior to each session, a urine sample was collected to monitor hydration status over the course of HA. The controlled-hyperthermia protocol served to increase Tre to 38.5 °C in approximately 35 min (referred to as “thermal drive”) and subsequently maintain Tre slightly above 38.5 °C for 60 min (referred to as “thermal maintenance”). During thermal drive, participants cycled at a power output that was expected to cause an increase in Tre to 38.5 °C within the set time window (Excalibur Sport, Lode B.V., Groningen, The Netherlands; or Wattbike Pro, Wattbike B.V., Duivendrecht, The Netherlands). For the first HA session, power output during this phase was determined based on observations during familiarization and HST1. For the subsequent HA sessions, power output was based on observations during the previous HA sessions. Thermal maintenance was achieved by adjusting power output and introducing resting periods when necessary. Participants were allowed to drink ad libitum during all HA sessions. WBSR was calculated as the difference between pre- and post-session nude body mass plus drinking volume, corrected for exposure time.
General measurements and calculations
During all HST and HA sessions, HR, Tre, and mean skin temperature (Tsk) were monitored continuously. HR was measured at the chest (Polar Vantage-M, Kempele, Finland). Tre was used as an indicator for body core temperature and assessed using a rectal thermometer (MSR, Seuzach, Switzerland; or Yellow Springs Instruments, Yellow Springs, OH, USA), which was self-inserted 10 cm past the anal sphincter. Local skin temperature was measured at the chest, forearm, thigh, and calf using iButtons (DS1922, Maxim Integrated Products, Inc., San Jose, CA, USA), which were attached to the skin with tape (Fixomull Stretch ADH, BSN Medical GmbH, Hamburg, Germany). Tsk was calculated as a weighted average of the four local skin temperatures (Ramanathan 1964). To quantify the individual adaptive stimulus during HA, we calculated the approximate integral (trapezoidal numerical integration) of, respectively, the Tre and Tsk curves (1-min averages) for each session, and summated the approximated session integrals:
where X = physiological variable (i.e., Tre or Tsk [°C·min]); iHA = the ith HA session (starting from 1); NHA = the total number of HA sessions (i.e., 10); N = the total number of time intervals (i.e., session duration in minutes); Xi = X at the end of the ith interval; Δt = time interval in min (i.e., 1).
This approach was adapted from Taylor and Cotter (2006; Taylor 2014), who introduced the “cumulative adaption impulse” as the summated session integrals for mean body temperature. Total work done (J) during HA was calculated using the same equation, where X = power output (W) and Δt is expressed in s.
Data analysis
All data were synchronized and formatted using MATLAB (R2019a, The MathWorks Inc., Natick, MA, USA). Statistical analysis was performed using R software (version 3.6.3, R Foundation for Statistical Computing, Vienna, Austria) in the Rstudio environment (version 1.2.5033, Rstudio, Inc., Boston, MA, USA). Data were reported as mean ± standard deviation. The level of statistical significance was set at p ≤ 0.050. When non-parametric tests were performed (as outlined below), data were reported as median (first quartile, third quartile) and p values were reported with superscript “np”.
The overall group responses to HA (all participants) were assessed by comparing physiological measures for HST1 and HST2 using paired t tests. These HST values were determined as follows: resting Tre = average Tre during min 5–10 of baseline rest; end-exercise Tre, HR and Tsk = average value over the last 5 min of the fixed workload exercise; ΔTre = end-exercise Tre minus resting Tre; WBSR = WBSR over full HST (fixed workload exercise + GXTHST); and GXTHST time = time to exhaustion on graded exercise test in the heat. Normality of the HST2–HST1 differences was analyzed using the Shapiro–Wilk test. If the normality assumption was violated (Shapiro–Wilk test yielded p ≤ 0.05), the Wilcoxon Signed-Rank test was used to evaluate the overall group responses to HA. To investigate the inter-dependency of physiological adaptations, associations between adaptations were assessed using Pearson’s product–moment correlation coefficient rp or Spearman’s rank-order correlation coefficient rs (when non-normal or non-linear). The magnitude of adaptations was quantified by subtracting the value at HST1 from the value at HST2 (HST2–HST1). In addition, associations between individual characteristics were assessed to confirm independency. Linearity was confirmed visually and normality of the variables was evaluated using the Shapiro–Wilk test. The strength of the association was classified as trivial (r < 0.1), small (r = 0.1–0.3), moderate (r = 0.3–0.5), or large (r > 0.5) (Cohen 1988).
For each adaptation phenotype, participants were divided into one of three equally sized groups (i.e., tertiles); (1) low-, (2) medium- or (3) high responders. We performed multiple one-way analyses of variance (i.e., ANOVAs) to evaluate the null hypothesis that the low-, medium-, and high responders have similar individual characteristics and HST1 responses and received comparable adaptive stimulus during HA. Normality of the residuals was analyzed using the Shapiro–Wilk test. Homogeneity of variance was visually inspected by plotting the residuals against the fitted values. If the residuals were not normally distributed or when residuals showed considerable variance across the range of fitted values, the non-parametric Kruskal–Wallis test was performed to compare the three groups. When a significant effect was observed, post hoc pairwise comparisons were done using independent t tests (when assumptions were met) or Wilcoxon Rank Sum tests (when assumptions were not met) with Bonferroni correction. In addition, an alternative analysis using simple least-squares regression is reported in the electronic supplementary material (text and tables in supplementary file1, figures in supplementary file2).
Results
HA
Twenty-four participants completed all experimental trials. On average, the target Tre of 38.5 °C during HA was achieved in 37.5 ± 6.7 min. On 175 occasions, the target Tre was reached within 40 min, while on 65 occasions, this took between 41 and 61 min. The full HA session duration was 96.5 ± 6.9 min. The average Tre during the thermal drive and thermal maintenance phase was 37.9 ± 0.2 °C and 38.6 ± 0.09 °C, respectively. HA was performed with a power output of 91 ± 18 W, HR of 132 ± 14 bpm, and WBSR of 1244 ± 444 g h−1.
Physiological responses to HA and their interactions
During HST2, both resting Tre and end-exercise Tre were lower than during HST1 (HST1 37.5 ± 0.3 vs. HST2 37.3 ± 0.4 °C, p = 0.02; HST1 38.2 ± 0.3 vs. HST2 38.1 ± 0.4 °C, p = 0.005; respectively). ΔTre remained unchanged (HST1 0.7 ± 0.3 vs. HST2 0.8 ± 0.3 °C, p = 0.6). Post-HA, we observed lower values for end-exercise Tsk (HST1 36.5 ± 0.3 vs. HST2 36.2 ± 0.4 °C, p = 0.001) and end-exercise HR (HST1 149 ± 17 vs. HST2 139 ± 15 bpm, p < 0.001). WBSR increased following HA (HST1 1017 [787, 1326] vs. HST2 1237 [888, 1756] g h−1, p < 0.001np). Performance at the GXTHST was improved, with a longer time to exhaustion in HST2 (578 ± 132 s) than in HST1 (525 ± 138 s, p < 0.001). The resting Tre adaptation was related to the ΔTre adaptation (rp = − 0.72, p < 0.001) and end-exercise Tre adaptation (rp = 0.51, p = 0.01). There was an association between the WBSR adaptation and end-exercise Tsk adaptation (rs = − 0.53, p = 0.02). No significant associations were observed among any other adaptation indices.
Individual characteristics
Weekly exercise training time was related to both body mass (r = 0.52, p = 0.01) and BSA–mass ratio (r = − 0.63, p = 0.001). Body fat percentage and \(\dot{{V}}\)O2peak,kg were significantly correlated (r = − 0.79, p = < 0.001). Inherent to their calculation, body mass, BSA, and BSA–mass ratio were interrelated (r = − 0.91, r = − 0.76, r = 0.95, p < 0.001).
Resting T re responders
As per our grouping criteria, resting Tre increased or remained unchanged in low resting Tre responders, while resting Tre decreased in all high responders following HA (Fig. 1). Body mass and BSA-to-mass ratio were not significantly different between low-, medium-, and high resting Tre responders (Fig. 2). \(\dot{{V}}\)O2peak,kg did not vary significantly among resting Tre responders, but approached statistical significance (p = 0.052; Fig. 2). Body fat percentage varied among responder groups, with the highest values for low responders, but no pairwise differences were identified post hoc (p > 0.07; Table 2). Considering the inter-dependency of the resting Tre adaptation and ΔTre adaptation, we have displayed the Tre responses of low-, medium-, and high resting Tre responders to HST1 and HST2 in Fig. 3.
Physiological responses to HSTs in low-, medium-, and high responders for resting Tre (first row), ΔTre (second row), WBSR (third row), and HR (fourth row). Tre, rectal temperature; ΔTre, exercise-induced rise in rectal temperature; WBSR, whole-body sweat rate; End-ex, end-exercise (average over last 5 min of exercise); HR, heart rate; HST, heat stress test. Gray lines represent individual responses, with filled points showing males and open points showing females. Black lines and points represent the average group response
Individual characteristics of low-, medium-, and high responders for resting Tre (first column), ΔTre (second column), WBSR (third column), and HR (fourth column). Tre, rectal temperature; ΔTre, exercise-induced rise in rectal temperature; WBSR, whole-body sweat rate; HR, heart rate; BSA-to-mass ratio, body surface area-to-mass ratio; \(\dot{{V}}\)O2peak,kg, maximum oxygen uptake relative to body mass; Filled triangles represent males, open triangles represent females. Group means and standard deviations are presented in black. Significance denotations: *p ≤ 0.050, **p ≤ 0.010
ΔT re responders
As per our grouping criteria, ΔTre increased in all low ΔTre responders, while ΔTre decreased in all high responders following HA (Fig. 1). Body mass, BSA-to-mass ratio, and \(\dot{{V}}\)O2peak,kg were not significantly different between low-, medium-, and high ΔTre responders (Fig. 2). There were significant effects of ΔTre responder group on the ΔTre and WBSRBSA during HST1 (Table 3). Post hoc pairwise comparisons revealed that individuals with a large ΔTre (i.e., within-HST rise in Tre) adaptation had a greater ΔTre during HST1 than individuals with a medium ΔTre adaptation (high 1.0 ± 0.3 °C vs. medium 0.6 ± 0.2 °C, p = 0.047). High ΔTre responders had a lower WBSRBSA during HST1 than low ΔTre responders (high 411 ± 126 g h−1 m−2 vs. low 598 ± 117 g h−1 m−2, p = 0.03.
WBSR responders
As per our grouping criteria, WBSR increased in all responder groups, with the largest elevation in the high WBSR responders following HA (Fig. 1). High WBSR responders had a significantly larger body mass (p = 0.005), BSA (p = 0.02), and smaller BSA-to-mass ratio (p = 0.004) than low WBSR responders (Fig. 2; Table 2). \(\dot{{V}}\)O2peak,kg did not vary significantly among WBSR responder groups (p = 0.3, Fig. 2). There were significant effects of WBSR responder group on WBSR and WBSRBSA during HST1, the cumulative adaptation impulse for Tre, and the WBSR during HA and the end-exercise Tsk adaptation (Table 3). Post hoc pairwise comparisons revealed that high WBSR responders had a greater WBSR and WBSRBSA during HST1 than low responders (high 1287 ± 305 g h−1 vs. low 782 ± 198 g h−1, p = 0.005; high 621 ± 126 g h−1 m−2 vs. low 425 ± 108 g h−1 m−2, p = 0.02). The cumulative adaptation impulse for Tre was higher in high WBSR responders (37,983 [37211, 38925] °C·min) than low WBSR responders (34,976 [34919, 35926] °C·min, p = 0.04np). High WBSR responders sweat more during HA than low WBSR responders, as shown by a larger average WBSR (high 1524 ± 297 g h−1 vs. low 916 ± 239 g h−1, p = 0.005) and WBSRBSA (high 738 ± 129 g h−1 m−2 vs. low 499 ± 137 g h−1 m−2, p = 0.03) during HA.
HR responders
As per our grouping criteria, end-exercise HR decreased in all responder groups, with the largest reduction in the high HR responders following HA (Fig. 1). High HR responders had a significantly smaller body mass (p = 0.02) and BSA (p = 0.02) than low responders (Fig. 2; Table 2). \(\dot{{V}}\)O2peak,kg did not vary significantly among HR responder groups (p = 0.4, Fig. 2). There were significant effects of HR responder group on HR during HST1 and WBSR during HA (Table 3). Post hoc pairwise comparisons revealed that high HR responders had a higher HR during HST1 than low responders (high 158 ± 20 bpm vs. low 138 ± 10 bpm, p = 0.047). Low HR responders sweat more during HA than medium HR responders, as shown by a larger average WBSR (low 1515 ± 400 g h−1 m−2 vs. medium 969 ± 342 g h−1 m−2, p = 0.02) and WBSRBSA (low 724 ± 185 g h−1 m−2 vs. medium 494 ± 154 g h−1 m−2, p = 0.04) during HA.
Discussion
The objective of the current study was to compare individual characteristics related to fitness status and body dimensions of low-, medium-, and high responders to a 10-day controlled-hyperthermia HA protocol. The respective responder groups were determined for separate adaptation phenotypes; resting Tre, ΔTre, WBSR, and HR. Our findings suggest that high WBSR responders generally had a large body mass and BSA and low BSA-to-mass ratio, whereas high HR responders typically had a small body mass and BSA. Individuals with a medium or high resting Tre adaptation tended to be more fit than individuals with a low resting Tre adaptation, with a higher \(\dot{{V}}\)O2peak,kg and lower body fat percentage, but no statistically significant differences were observed. To our knowledge, this is the first experimental study that explores the role of body dimensions as an influential factor in HA responsiveness.
Physiological responses to HA and their interactions
Overall, our HA protocol successfully induced hallmark adaptations associated with HA; a reduced resting Tre, end-exercise Tre, HR, and Tsk and an elevated WBSR for exercise at a given workload (1.5 W kg body mass−1). The average reductions in resting Tre (− 0.17 °C) and end-exercise HR (− 10 bpm) were similar to previous studies that have adopted a comparable controlled-hyperthermia HA regimen (Patterson et al. 2004; Gibson et al. 2015). The WBSR adaptation following controlled-hyperthermia HA protocols varies, potentially caused by its dependency on ambient temperature during HA (Tyler et al. 2016). We observed no reduction in the exercise-induced Tre rise (i.e., ΔTre) following HA; the reduction in end-exercise Tre (− 0.14 °C) was simply the result of the resting Tre adaptation. The explanation for this unaltered exercise-induced Tre rise may be twofold. First, a humid environment, as used in the current study, may provide limited potential for enhanced evaporative cooling (Buono et al. 1998; Patterson et al. 2004). Indeed, we did not observe a statistically significant association between the ΔTre adaptation and WBSR adaptation, while Corbett et al. (2018) reported a moderate positive relationship between these variables following a more hot and dry HA (40 °C, 50%RH). We did find a more pronounced end-exercise Tsk adaptation along with a larger WBSR adaptation, suggesting that sweating cooled the skin. However, the small reduction in end-exercise Tsk (on average ~ 0.3 °C) was apparently not sufficient to reduce exercising Tre. Second, we observed that the ΔTre adaptation and resting Tre adaptation were inversely related; when individuals had a considerable resting Tre adaptation, they showed no or even a “negative” ΔTre adaptation (Fig. 3). From Newton’s Law of Cooling, it follows that the rate of temperature change of an object is related to the temperature gradient between that object and the environment (here 33 °C; Taylor 2014). The reduced starting temperature of the “object” (i.e., human) after HA may have diminished the potential for dry heat loss in HST2. The latter suggestion rests on the assumption that, before and after HA, sweating was initiated following a fixed change in core temperature rather than at an absolute core temperature threshold (Patterson et al. 2004; Tyler et al. 2016). Altogether, the magnitude of adaptations in the present study are comparable to previous reports. In accordance with Corbett et al. (2018), we show limited inter-dependency between adaptation phenotypes; high or low responsiveness to HA is likely phenotype-specific.
WBSR responders, HR responders, and body dimensions
We observed that high WBSR responders had a larger body mass and BSA and a lower BSA-to-mass ratio than those with a low sudomotor adaptation (i.e., low responders). Fitness status and body fat percentage did not differentiate low-, medium-, and high WBSR responders. High WBSR responders had a greater WBSR during HST1 than low responders. The latter is in line with Notley et al. (2016), who revealed that, in compensable conditions, large individuals (low BSA-to-mass ratio) were more reliant upon heat loss via the sudomotor pathway (i.e., sweating) than the vasomotor pathway. Our observation that these heavy sweaters develop superior sweat adaptation supports the notion by Taylor (2014) that individuals may adapt towards their “preferred” pathway for heat loss. Taylor (2014) approached this from an evolutionary perspective, but it is also plausible that increased activation of a thermoeffector during HA, by virtue of one’s body dimensions, results in a more pronounced adaptation of that thermoeffector. Since Buono et al. (2009) observed that sweat gland activity during HA is essential to develop sudomotor adaptations, one might suggest that a dose–response relationship exists; the more sweat gland activity during HA, the larger the sweat gland adaptation. Indeed, we found that high WBSR responders lost more sweat in total and per unit of BSA during HA than low responders. This might imply that the high WBSR responders, characterized by a low BSA-to-mass ratio, had a relatively large sweat gland output capacity or active sweat gland density during HA, which enabled superior sweat gland adaptation.
In addition, high WBSR responders showed an elevated cumulative adaptation impulse for Tre with respect to the low responders. The most conceivable explanation for this is a longer duration of the thermal drive phase during HA sessions; the time to reach a Tre of 38.5 °C tended to be longer (7 min per HA session) for high WBSR responders. This observation is likely related to the large thermal inertia inherent to their body mass. Since a Tre of at least 38.5 °C has been recommended to induce complete heat adaptation (Fox et al. 1963; Gibson et al. 2015; Racinais et al. 2015), we believe that this extended thermal drive phase in high WBSR responders did not notably enlarge the adaptive sudomotor stimulus.
In contrast to WBSR, high HR responders were mostly small individuals, with smaller body mass and BSA than low responders. Fitness status and body fat percentage did not differentiate low-, medium-, and high HR responders. HR reductions following HA occur as a result of a lower thermal strain (lower Tre) and/or an expanded plasma volume (Taylor 2014; Tyler et al. 2016; Periard et al. 2016). Among our HR responder groups, Tre adaptations did not differ, and therefore, it could be speculated that high HR responders had a greater plasma volume expansion than low responders. The HA-induced plasma volume expansion improves cardiovascular stability and increases the specific heat of the blood, with the latter supporting heat transfer from the core to the skin (Periard et al. 2016). This improved heat transfer potentially lowered the cutaneous blood flow demands (Sawka et al. 2011; Periard et al. 2016), allowing cardiovascular strain to decrease substantially during exercise. Thus, the large HR adaptation for small individuals may relate to a superior HA-induced plasma volume expansion, though we cannot confirm this in the present study.
Furthermore, high HR responders had a higher exercising HR during HST1 than low HR responders. In line with this, Corbett et al. (2018) observed that a higher end-exercise HR prior to HA was associated with a larger subsequent HR adaptation. The higher pre-HA cardiovascular strain for high HR responders, most of whom were small individuals (i.e., small body mass) in our study, might be a result of their elevated reliance on the vasomotor pathway to dissipate heat (Notley et al. 2016). That is, during exercise heat stress, HR rises in response to the concurrent blood flow demands of the cutaneous circulation and working skeletal muscle (Sawka et al. 2011; Periard et al. 2016). Increased reliance on dry heat loss might therefore impose higher cardiovascular strain upon small individuals.
The contrasting body dimensions of high WBSR and high HR responders suggest a morphological dependency of the adaptive response to HA. One’s “preferred” heat loss avenue, which is related to one’s body dimensions (Notley et al. 2016), may dictate the adaptive pathway during HA (Taylor 2014). This notion is supported by our observation that heavy sweaters developed superior sweat adaptation. This notion also implies that small individuals, who mainly rely upon dry heat exchange, would develop a more pronounced vasomotor adaptation. The pronounced HR adaptation in small individuals may relate to this hypothesis, but in the present study, we did not implement the appropriate measures to directly confirm this. These inferences may only apply to HA in a warm humid environment, which allows both dry and wet heat exchange (present study; 33 °C, 65% relative humidity). Distinct outcomes may be observed when one of these heat loss pathways is restricted by high ambient temperatures (i.e., exceeding Tsk) or a higher humidity. Data from Notley (2016; dissertation) indicated that HA adaptations were similar for small (273 cm2 kg−1) and large (244 cm2 kg−1) individuals in a more hot-dry environment. Future studies should investigate the isolated effect of body dimensions on sudomotor and vasomotor adaptations following HA in various environmental conditions.
Resting T re and fitness status
Medium and high resting Tre responders tended to be more fit than low responders, with a higher \(\dot{{V}}\)O2peak,kg and lower body fat percentage, but the evidence was not sufficiently strong to reject the null hypothesis. Body dimensions did not differentiate low-, medium-, and high resting Tre responders. Our findings contradict the notion that well-trained individuals have a reduced scope for adaptation (Taylor and Cotter 2006). Support for this notion comes from earlier work by Shvartz et al. (1977), who showed greater adaptation in untrained individuals (~ 36 mL kg−1 min−1) for exercising HR, resting Tre, and exercising Tre than in trained individuals (~ 60 mL kg−1 min−1). In that study, however, the untrained individuals had a higher Tre during HA sessions, resulting in a higher adaptive stimulus during HA, which complicates interpretation of their findings. Our controlled-hyperthermia HA reduced potential bias resulting from inter-individual variation in cumulative adaptation impulse. Indeed, in accordance with our results, Corbett et al. (2018) recently reported that \(\dot{{V}}\)O2peak,kg (range 45–75 mL kg−1 min−1) was not associated with the increase in WBSR or the reduction in end-exercise HR, end-exercise Tre and ΔTre after 10 controlled-hyperthermia HA days. Thus, in recreationally active and well-trained participants, fitness status may not affect the magnitude of adaptation following 10 days of controlled-hyperthermia HA.
The notion that \(\dot{{V}}\)O2peak,kg would affect HA responsiveness rests on the assumption that \(\dot{{V}}\)O2peak,kg represents partial adaptation status (Taylor and Cotter 2006; Ravanelli et al. 2020). However, this assumption may lack validity, given the variability in \(\dot{{V}}\)O2peak,kg trainability (Bouchard et al. 2011) and exercise environment (e.g., water vs. land; Avellini et al. 1982) or modality (e.g., sprinters vs. endurance athletes; Amano et al. 2013). We therefore included the self-reported weekly exercise training time (swimming excluded) into our analysis. However, our results must be interpreted with caution considering the response bias in self-reported data. Also, we did not implement a validated physical activity questionnaire (e.g., as in Lamarche et al. 2018b), which might be a more sensitive measure of training-induced thermoregulatory status. To circumvent these issues, future studies may want to standardize physical activity prior to HA when investigating the influence of exercise-induced partial heat adaptation on HA induction (e.g., as in Ravanelli et al. 2018).
General considerations
It should be noted that the explorative nature of the current study made it impossible to disentangle body dimensions and fitness status characteristics from sex. Although overlap existed, the females in the current study generally had a smaller body mass, higher BSA-to-mass ratio, higher body fat percentage, and a lower \(\dot{{V}}\)O2peak,kg than the males. However, the independent effect of sex on thermoregulation may be limited. Recent research suggests that sex differences in vasomotor and sudomotor activity during compensable heat stress can mainly be explained by divergence in BSA-to-mass ratio (Notley et al. 2017). Moreover, we did not control for menstrual cycle phase, which may have increased the variability in our data (Lei et al. 2019). Menstrual cycle phases during the HSTs were randomly distributed over our female participants, so it is not likely that the thermoregulatory fluctuations associated with the menstrual cycle introduced a systematic bias.
Seeking understanding of individual responses to an intervention is rather complex. Ideally, one should implement a control group as well as repeated interventions, to exclude sources of variability that are not related to the “true” individual’s response, such as random variation and within-subject variability (Hecksteden et al. 2015). Following, the variability observed in the present study cannot be attributed only to “true” inter-individual variation in HA responsiveness. In addition, various statistical analysis techniques can be employed to support data interpretation. We aimed to determine a set of characteristics that differentiated participants with a high adaptive response from those with a low response. This analysis occasionally resulted in separation of individuals with a similar adaptive response into two distinctive groups. However, the overall adaptation response differed considerably among groups. Also, by introducing a medium responder group, we created a substantial distinction between low and high responders. The interested reader is referred to the electronic supplementary material (file1 and file2) for alternative analysis using simple least-squares linear regressions; similar conclusions can be drawn from this.
In the current study, adaptive responses were deduced from the pre- and post-HA HSTs, which employed an external workload of 1.5 W kg body mass−1. It can be argued that the higher absolute requirement for evaporation (W) in large individuals led to the greater WBSR observed during HST1 in high WBSR responders, and that this may have introduced a bias in our evaluation of the WBSR adaptation. However, high WBSR responders showed a greater WBSR and WBSRBSA during HA as well, where no difference in power output existed between groups. This suggests that the greater WBSR in high WBSR responders seems unrelated to the delivered power output. In addition, Ravanelli et al. (2017) recommended to normalize the exercise-induced heat production to body mass when comparing ΔTre and sweating responses in groups with distinct body dimensions during uncompensable heat stress. It should be noted, however, that we standardized external work rate, introducing variance in the normalized heat production that is related to the individual’s cycling efficiency. Although our approach did not eliminate variations in heat production (W kg−1), it presumably minimized systematic differences related to individual body dimensions. This supports the assumption that our study design facilitated unbiased comparisons among our groups with varying body dimensions.
As specified in Methods section, the WBSR was determined for the full HST (i.e., including GXTHST). Since we investigated pre- to post-HA changes (HST2-HST1), with similar procedures pre- and post-HA (average performance time improvement 53 s), we do not believe that this feature influenced the WBSR comparisons in the present study.
Conclusion
The findings of our study indicate that body dimensions influence the pathway of adaptation following a 10-day controlled-hyperthermia HA protocol in warm humid conditions. Participants with a high sudomotor adaptation generally had large body dimensions (large body mass and BSA, low BSA-to-mass ratio), while participants with a high end-exercise heart rate adaptation were typically small (small body mass and BSA). Body dimensions did not vary among different levels of resting Tre and ΔTre responsiveness. Medium and high resting Tre responders tended to have a higher baseline fitness level than low responders, but no statistically significant differences were observed. Our novel findings shed new light on the individual adaptive responses observed after HA, by identifying the individual body dimensions as an influential factor. We encourage future research into the isolated effect of body dimensions on HA induction to expand upon our observations.
Abbreviations
- BSA:
-
Body surface area
- HA:
-
Heat acclimation
- HR:
-
Heart rate
- HST:
-
Heat stress test
- T re :
-
Rectal temperature
- ΔT re :
-
Exercise-induced rise in Tre
- T sk :
-
Mean skin temperature
- \(\dot{{V}}\)O2peak,kg :
-
Maximum oxygen uptake in mL kg−1 min−1
- WBSR:
-
Whole-body sweat rate
- GXTHST :
-
Graded exercise test during heat stress test
References
Amano T, Koga S, Inoue Y, Nishiyasu T, Kondo N (2013) Characteristics of sweating responses and peripheral sweat gland function during passive heating in sprinters. Eur J Appl Physiol 113:2067–2075. https://doi.org/10.1007/s00421-013-2641-8
Avellini BA, Shapiro Y, Fortney SM, Wenger CB, Pandolf KB (1982) Effects on heat tolerance of physical training in water and on land. J Appl Physiol 53:1291–1298. https://doi.org/10.1152/jappl.1982.53.5.1291
Bouchard C, Rankinen T, Timmons JA (2011) Genomics and genetics in the biology of adaptation to exercise. Compr Physiol 1:1603–1648. https://doi.org/10.1002/cphy.c100059
Buono MJ, Heaney JH, Canine KM (1998) Acclimation to humid heat lowers resting core temperature. Am J Physiol Integr Comp Physiol 274:R1295–R1299. https://doi.org/10.1152/ajpregu.1998.274.5.R1295
Buono MJ, Numan TR, Claros RM, Brodine SK, Kolkhorst FW (2009) Is active sweating during heat acclimation required for improvements in peripheral sweat gland function? Am J Physiol Integr Comp Physiol 297:R1082–R1085. https://doi.org/10.1152/ajpregu.00253.2009
Casadio JR, Kilding AE, Cotter JD, Laursen PB (2017) From lab to real world: heat acclimation considerations for elite athletes. Sports Med 47:1467–1476. https://doi.org/10.1007/s40279-016-0668-9
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum Associates, New York
Corbett J, Rendell RA, Massey HC, Costello JT, Tipton MJ (2018) Inter-individual variation in the adaptive response to heat acclimation. J Therm Biol 74:29–36. https://doi.org/10.1016/j.jtherbio.2018.03.002
Cramer MN, Jay O (2016) Biophysical aspects of human thermoregulation during heat stress. Auton Neurosci Basic Clin 196:3–13. https://doi.org/10.1016/j.autneu.2016.03.001
Daanen HAM, Racinais S, Périard JD (2018) Heat acclimation decay and re-induction: a systematic review and meta-analysis. Sports Med 48:1–22. https://doi.org/10.1007/s40279-017-0808-x
DuBois D, DuBois EF (1916) A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 17:863–871. https://doi.org/10.1001/archinte.1916.00080130010002
Fox RH, Goldsmith R, Kidd DJ, Lewis HE (1963) Acclimatization to heat in man by controlled elevation of body temperature. J Physiol 166:530–547. https://doi.org/10.1113/jphysiol.1963.sp007121
Gibson OR, Mee JA, Tuttle JA, Taylor L, Watt PW, Maxwell NS (2015) Isothermic and fixed intensity heat acclimation methods induce similar heat adaptation following short and long-term timescales. J Therm Biol 49–50:55–65. https://doi.org/10.1016/j.jtherbio.2015.02.005
Havenith G (2001) Human surface to mass ratio and body core temperature in exercise heat stress—a concept revisited. J Therm Biol 26:387–393. https://doi.org/10.1016/S0306-4565(01)00049-3
Havenith G, Coenen JML, Kistemaker L, Kenney WL (1998) Relevance of individual characteristics for human heat stress response is dependent on exercise intensity and climate type. Eur J Appl Physiol Occup Physiol 77:231–241. https://doi.org/10.1007/s004210050327
Hecksteden A, Kraushaar J, Scharhag-Rosenberger F, Theisen D, Senn S, Meyer T (2015) Individual response to exercise training—a statistical perspective. J Appl Physiol 118:1450–1459. https://doi.org/10.1152/japplphysiol.00714.2014
Kenefick RW, Cheuvront SN (2012) Hydration for recreational sport and physical activity. Nutr Rev 70:S137–S142. https://doi.org/10.1111/j.1753-4887.2012.00523.x
Lamarche DT, Notley SR, Louie JC, Poirier MP, Kenny GP (2018a) Fitness-related differences in the rate of whole-body evaporative heat loss in exercising men are heat-load dependent. Exp Physiol 103:101–110. https://doi.org/10.1113/EP086637
Lamarche DT, Notley SR, Poirier MP, Kenny GP (2018b) Self-reported physical activity level does not alter whole-body total heat loss independently of aerobic fitness in young adults during exercise in the heat. Appl Physiol Nutr Metab 44:99–102. https://doi.org/10.1139/apnm-2018-0211
Lei T-H, Cotter JD, Schlader ZJ, Stannard SR, Perry BG, Barnes MJ, Mündel T (2019) On exercise thermoregulation in females: interaction of endogenous and exogenous ovarian hormones. J Physiol 597:71–88. https://doi.org/10.1113/JP276233
Notley SR (2016) An examination of the inter-dependence of heat exchange pathways and morphology in humans. Dissertation, University of Wollongong
Notley SR, Park J, Tagami K, Ohnishi N, Taylor NAS (2016) Morphological dependency of cutaneous blood flow and sweating during compensable heat stress when heat-loss requirements are matched across participants. J Appl Physiol 121:25–35. https://doi.org/10.1152/japplphysiol.00151.2016
Notley SR, Park J, Tagami K, Ohnishi N, Taylor NAS (2017) Variations in body morphology explain sex differences in thermoeffector function during compensable heat stress. Exp Physiol 102:545–562. https://doi.org/10.1113/EP086112
Pandolf KB, Burse RL, Goldman RF (1977) Role of physical fitness in heat acclimatisation, decay and reinduction. Ergonomics 20:399–408. https://doi.org/10.1080/00140137708931642
Patterson MJ, Stocks JM, Taylor NAS (2004) Humid heat acclimation does not elicit a preferential sweat redistribution toward the limbs. Am J Physiol Integr Comp Physiol 286:R512–R518. https://doi.org/10.1152/ajpregu.00359.2003
Periard JD, Racinais S, Sawka MN (2015) Adaptations and mechanisms of human heat acclimation: applications for competitive athletes and sports. Scand J Med Sci Sports 25:20–38. https://doi.org/10.1111/sms.12408
Periard JD, Travers GJS, Racinais S, Sawka MN (2016) Cardiovascular adaptations supporting human exercise-heat acclimation. Auton Neurosci 196:52–62. https://doi.org/10.1016/j.autneu.2016.02.002
Racinais S, Mohr M, Buchheit M, Voss SC, Gaoua N, Grantham J, Nybo L (2012) Individual responses to short-term heat acclimatisation as predictors of football performance in a hot, dry environment. Br J Sport Med 46:810–815. https://doi.org/10.1136/bjsports-2012-091227
Racinais S, Alonso JM, Coutts AJ et al (2015) Consensus recommendations on training and competing in the heat. Br J Sports Med 49:1164–1173. https://doi.org/10.1136/bjsports-2015-094915
Ramanathan NL (1964) A new weighting system for mean surface temperature of the human body. J Appl Physiol 19:531–533. https://doi.org/10.1152/jappl.1964.19.3.531
Ravanelli N, Cramer M, Imbeault P, Jay O (2017) The optimal exercise intensity for the unbiased comparison of thermoregulatory responses between groups unmatched for body size during uncompensable heat stress. Physiol Rep 5:e13099. https://doi.org/10.14814/phy2.13099
Ravanelli N, Coombs GB, Imbeault P, Jay O (2018) Maximum skin wettedness after aerobic training with and without heat acclimation. Med Sci Sports Exerc 50:299–307. https://doi.org/10.1249/MSS.0000000000001439
Ravanelli N, Gagnon D, Imbeault P, Jay O (2020) A retrospective analysis to determine if exercise training-induced thermoregulatory adaptations are mediated by increased fitness or heat acclimation. Exp Physiol. https://doi.org/10.1113/EP088385
Sawka MN, Leon LR, Montain SJ, Sonna LA (2011) Integrated physiological mechanisms of exercise performance, adaptation, and maladaptation to heat stress. Compr Physiol 1:1883–1928. https://doi.org/10.1002/cphy.c100082
Shvartz E, Shapiro Y, Magazanik A, Meroz A, Birnfeld H, Mechtinger A, Shibolet S (1977) Heat acclimation, physical fitness, and responses to exercise in temperate and hot environments. J Appl Physiol Respir Environ Exerc Physiol 43:678–683. https://doi.org/10.1152/jappl.1977.43.4.678
Taylor NAS (2014) Human heat adaptation. Compr Physiol 4:325–365. https://doi.org/10.1002/cphy.c130022
Taylor NAS, Cotter JD (2006) Heat adaptation: guidelines for the optimisation of human performance. Int Sport J 7:33–57
Tyler CJ, Reeve T, Hodges GJ, Cheung SS (2016) The effects of heat adaptation on physiology, perception and exercise performance in the heat: a meta-analysis. Sports Med 46:1699–1724. https://doi.org/10.1007/s40279-016-0538-5
Acknowledgements
The authors thank the following people for their assistance during the data collection: Lisa Klous, Mireille Folkerts, Giulio Tan, Romin Gaalman, Mae El Hamoud, Iris Dijkstra, Amber Berlijn, Michelle van Delden, and Emma Wiedenmann. We would like to express our gratitude to the Amsterdam Nutritional Assessment Center, with in particular Robert Memelink and Yiming Ma, for execution and analysis of the dual-energy X-ray absorptiometry scans. Special thanks are given to the participants for their dedication to this study.
Funding
This research was funded by ZONMW and the Dutch Research Council (NWO) with grants held by Hein Daanen and Thijs Eijsvogels. Puck Alkemade was funded by the Citius Altius Sanius project (P16-28) from NWO and Nicola Gerrett through ZONMW (#546001003).
Author information
Authors and Affiliations
Contributions
PA, NG, TE, and HD collaborated in designing the study. PA and NG recruited participants and conducted the experiments. PA processed and analyzed the data and wrote the manuscript. PA, NG, TE, and HD collaborated in data interpretation, and reviewing and editing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
All procedures were approved by the Ethics Committee of the Faculty of Behavioural and Movement Sciences of the Vrije Universiteit Amsterdam (VCWE-2018-160R1), conform the standards set out by the Declaration of Helsinki.
Informed consent
Prior to the study, participants were informed about the procedures and provided verbal and written consent.
Additional information
Communicated by George Havenith.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alkemade, P., Gerrett, N., Eijsvogels, T.M.H. et al. Individual characteristics associated with the magnitude of heat acclimation adaptations. Eur J Appl Physiol 121, 1593–1606 (2021). https://doi.org/10.1007/s00421-021-04626-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-021-04626-3