Abstract
Purpose
Geographic atrophy (GA) is a late-stage form of age-related macular degeneration (AMD) characterized by the expansion of atrophic lesions in the outer retina. There are currently no approved pharmacological treatments to prevent or slow the progression of GA. This review describes the progression and assessment of GA, predictive imaging features, and complement-targeting investigational drugs for GA.
Methods
A literature search on GA was conducted.
Results
Expansion of atrophic lesions in patients with GA is associated with a decline in several measures of visual function. GA lesion size has been moderately associated with measures obtained through microperimetry, whereas GA lesion size in the 1-mm diameter area centered on the fovea has been associated with visual acuity. Optical coherence tomography (OCT) can provide 3-dimensional quantitative assessment of atrophy and is useful for identifying early atrophy in GA. Features that have been found to predict the development of GA include certain drusen characteristics and pigmentary abnormalities. Specific OCT features, including hyper-reflective foci and OCT-reflective drusen substructures, have been associated with AMD disease progression. Lesion characteristics, including focality, regularity of shape, location, and perilesional fundus autofluorescence patterns, have been identified as predictors of faster GA lesion growth. Certain investigational complement-targeting drugs have shown efficacy in slowing the progression of GA.
Conclusion
GA is a progressive disease associated with irreversible vision loss. Therefore, the lack of treatment options presents a significant unmet need. OCT and drugs under investigation for GA are promising future tools for disease management.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Geographic atrophy (GA) is a late-stage form of age-related macular degeneration (AMD) characterized by progressive degenerative lesions affecting photoreceptors, retinal pigment epithelium (RPE), and choriocapillaris primarily in the macula [1,2,3,4,5]. Although patients with AMD may benefit from supplements consisting of high-dose antioxidant vitamins (C, E, and beta-carotene) and zinc, which have been found to decrease the risk of progression to advanced AMD [6], there are currently no approved pharmacological treatments to prevent or slow the progression of GA [7]. Thus, management largely consists of monitoring disease progression [1]. Nevertheless, early identification of patients with AMD is important because patients may ultimately benefit from drugs that are currently under investigation for GA. This report provides an overview of the progression and assessment of GA, predictive imaging features, and treatments currently on the horizon.
Disease progression and visual function
Early and intermediate age-related macular degeneration
Features of AMD observed prior to the development of GA include the presence of medium or large drusen, which are deposits between Bruch membrane and the RPE, as well as AMD pigmentary abnormalities, which have been defined as hyper- or hypopigmentation (within 2-disc diameters of the center of the macula) associated with medium (63 to 125 µm) or large (> 125 µm) drusen and without other reasons for the abnormalities [8]. AMD has been classified into early, intermediate, and late stages [8]. Early AMD is defined by the presence of medium-size drusen without pigmentary abnormalities, whereas intermediate AMD is defined by the presence of large drusen and/or AMD pigmentary abnormalities [8]. Early stages of AMD have been reported to usually be asymptomatic [8]. Individuals with early or intermediate AMD may progress to late AMD, which includes neovascular AMD or GA [8].
Geographic atrophy
GA is characterized by well-demarcated atrophic lesions in the outer retina that expand over time [1, 4, 9, 10]. Lesions often begin in parafoveal regions and expand to the fovea later in the disease [10,11,12]. However, there is variability across eyes. In a study of patients with bilateral drusen who were examined annually, the location of incident GA was parafoveal (250–1500 µm) in 61.4%, ≤ 250 µm from the foveal center in 17.5%, and subfoveal in 20.2% [12]. Lesion growth rate varies across studies and individuals and is affected by baseline lesion size (in mm2) [13, 14]. In a recent meta-analysis, the pooled mean GA growth rate was shown to be 1.66 mm2/year or 0.33 mm/year, and regardless of how GA growth rate was calculated (non-square root transformed, mm2; or square root transformed, mm), a significant association was observed with baseline GA area (mm2) [13]. Since baseline GA area is 1 of the factors that affect GA growth, square root transformation methods can be used in clinical trials to minimize the impact of baseline GA size [13]. For eyes with GA with foveal sparing, GA progression toward the periphery has been found to be faster than progression toward the fovea [15].
As GA progresses, visual function declines [3, 9, 16, 17]. In a retrospective analysis of the Age-Related Eye Disease Study, the median time to loss of 15 letters was 5.6 years for eyes with non-central GA at baseline (baseline visual acuity: 74.5 letters) and 6.3 years for eyes with central GA at baseline (baseline visual acuity: 52.8 letters) [3]. In addition, some reports have suggested that patients with GA may have deficits in visual-function measures that rely on the fovea even when visual acuity is still relatively preserved (20/50 or better), including low luminance visual acuity, foveal dark-adapted sensitivity, and contrast sensitivity [11]. Furthermore, due to absolute scotomas associated with the GA lesion, patients with GA may have deficits in tasks that require a wide visual field [11, 18].
In the large Chroma and Spectri studies, various measures of visual function were found to worsen over 48 weeks in patients with GA, including best corrected visual acuity, low luminance visual acuity, monocular and binocular maximum reading speed, macular sensitivity, and absolute number of scotomatous points (as measured by mesopic microperimetry), and patient-reported outcomes of visual function (25-item National Eye Institute Visual Function Questionnaire and the Functional Reading Independence Index) [16]; however, correlations between GA lesion area and measures of visual function were found to be weak, especially for best corrected visual acuity [16]. The only visual-function measures that showed moderate correlations (0.4 ≤ Spearman correlation coefficient ≤ 0.59) with GA lesion area at baseline or week 48 were the number of absolute scotomatous points and mean macular sensitivity as measured by microperimetry [16]. Other studies have demonstrated that the relationship between GA lesion area and visual acuity is stronger when the area of analysis is limited to the 1-mm diameter area centered around the fovea than when the total GA lesion area is considered [3, 17]. Although the association between GA lesion size and visual function remains unclear, low-luminance deficit has been shown to be a predictor of future vision loss in patients with GA and good vision (≥ 20/50), which may allow for the identification of patients with a high risk of losing vision [19].
Although previously considered as distinct entities, GA and choroidal neovascularization (CNV) are not mutually exclusive. Eyes with bilateral GA or an eye with GA and fellow eye CNV have a significant risk of developing CNV in the eye with GA [20, 21]. Sunness et al noted that the 2-year and 4-year rate of patients developing CNV in an eye with GA was 6% and 17%, respectively [21]. When broken down by fellow eye, the 2-year rate was 18% and the 4-year rate was 34% when fellow eye had CNV, and 2% and 11% in eyes with bilateral GA [21]. Furthermore, progression to advanced AMD (GA or CNV) in eyes with early or intermediate AMD is increased when there is GA or CNV in the fellow eye [20].
Assessment of geographic atrophy progression
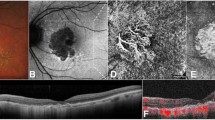
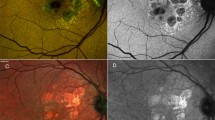
Several imaging modalities can be used to monitor the progression of GA, in particular color fundus photography (CFP), fundus autofluorescence (FAF), and optical coherence tomography (OCT) [22, 23]. CFP is the historical standard for imaging GA; however, it is limited in the contrast that it provides [22, 24]. GA on CFP appears as sharply demarcated areas of hypopigmentation [22]. FAF provides higher contrast than CFP [22]. Due to the loss of RPE cells and lipofuscin with GA, atrophy on FAF presents as regions of hypoautofluorescence [1, 22]. However, assessment of the foveal region is difficult as the macular pigment blocks blue light, making the signal intensity reduced, especially when the atrophic region is close to the fovea and therefore additional imaging modalities are needed [22]. Spectral domain OCT (SD-OCT) has notable advantages for visualizing GA in that it allows for cross-sectional and en-face images and for 3-dimensional quantitative assessment of atrophy of specific retinal layers [22, 25]. Swept-source OCT has further advantages in that it allows for wider imaging areas and greater detail of the choroid [22].
OCT can be used to identify early atrophy in AMD, as was noted by the Classification of Atrophy Meeting (CAM) group [25]. Identification of early atrophy is important for determining whether investigational therapeutics can reduce GA progression at early stages [25, 26]. The CAM group proposed 4 categories of GA based on OCT criteria, which describe whether the atrophy is incomplete or complete and whether it occurs in the presence of RPE atrophy: (1) complete RPE and outer retinal atrophy (cRORA); (2) incomplete RPE and outer retinal atrophy (iRORA); (3) complete outer retinal atrophy (cORA); and (4) incomplete outer retinal atrophy (iORA) [25]. According to this framework, GA and nascent GA are considered subsets of cRORA and iRORA, respectively, which occur in the absence of choroidal neovascularization [25].
Imaging predictors of disease progression
Several imaging features have been found to predict the development of GA, including certain drusen characteristics and pigmentary abnormalities. These features are valuable in identifying patients who may benefit from future treatments for GA. In a large study of individuals ≥ 49 years of age, characteristics of drusen that were strongly associated with the development of GA over a 15-year period included soft indistinct drusen, drusen within 500 µm from the foveal center, and a total drusen area > 375 µm in diameter [27]. The presence of reticular drusen, RPE depigmentation, and hyperpigmentation were also strongly associated with the development of GA [27].
Other imaging features, specifically on OCT, have been identified as predictors of disease progression for individuals with intermediate AMD. The presence of intraretinal hyper-reflective foci was found to correlate with progression to either late AMD (cRORA or choroidal neovascularization) or cRORA alone over 1 year [28]. The presence of OCT-reflective drusen substructures, which include low-reflective cores, high-reflective cores, conical debris, and split drusen, was associated with an increase in GA area over 2 years [29]. Furthermore, the presence of heterogeneous internal reflectivity within drusen (ie, calcified drusen) was associated with progression to advanced AMD (neovascular AMD or GA) over 1 year [30]. In addition, the appearance of persistent hyper-transmission defects with a minimum size of 250 µm on en-face swept-source OCT scans was found to increase the risk of progression to GA [31].
Recent studies have also examined imaging features on SD-OCT, specifically, a focus on photoreceptor integrity as photoreceptor loss is indeed 1 of the characteristics of GA [32, 33]. Reiter and colleagues demonstrated a significant association between the junctional zone (area surrounding the GA lesion) and GA growth after 12 months, suggesting that the junctional zone may be a predictor of GA growth [33]. It has also been demonstrated by Pfau et al that qualitatively, progressive photoreceptor degeneration outside GA correlated with GA progression rates [32]. Quantitatively, ellipsoid zone-loss-to-GA boundary distance and thickness of the outer nuclear layer, outer segment, and inner segment were all associated with future progression rates [32]. These studies suggest that by utilizing SD-OCT, photoreceptor loss and thinning could be an outcome measure beyond GA lesion size progression [32, 33].
For individuals with GA, certain features of the lesion have been found to predict faster rates of GA growth. Studies have found that multifocal lesions have higher growth rates than unifocal lesions and that irregularly shaped lesions have higher growth rates than more circular lesions [34, 35]. In addition, extrafoveal lesions have been found to have higher growth rates than foveal lesions [36]. When perilesional FAF patterns have been analyzed, lesions with banded or diffuse FAF patterns have been found to have higher growth rates than lesions with no abnormal FAF pattern or focal FAF patterns [37].
Complement-targeting treatments for geographic atrophy
Several studies suggest that the complement system, a component of the innate immune system, plays an important role in the development of GA. Various complement genes have been linked to AMD in genome-wide association studies [38, 39]. The odds of GA were estimated to be 2.5 times higher per copy of a common risk allele (Y402H) of the CFH gene in individuals of European ancestry [40]. In addition to genetic studies, histologic studies have found complement proteins in drusen as well as elevated levels of complement proteins in the outer retinal tissue of postmortem eyes with AMD [41,42,43]. Furthermore, elevated levels of activated complement products in plasma have been found in individuals with GA [44]. Based on these findings, several drugs targeting the complement system are currently in clinical investigational phases for GA treatment.
Drugs targeting the complement system with phase 3 efficacy and safety data in GA to date include pegcetacoplan and avacincaptad pegol, which are complement C3 and complement C5 inhibitors, respectively [7, 45]. The efficacy and safety of pegcetacoplan administered via intravitreal injections every month or every other month in patients with GA were examined in randomized, double-masked, sham-controlled phase 2 (FILLY; NCT02503332) and phase 3 studies (OAKS and DERBY; NCT03525600 and NCT03525613) [45, 46]. In the phase 3 studies, OAKS found a significant reduction in GA lesion growth (mm2) with pegcetacoplan monthly (22% reduction) and pegcetacoplan every other month (16% reduction) compared with sham at month 12, whereas DERBY failed to find a significant reduction [46]. A combined analysis of OAKS and DERBY found a reduction in growth with pegcetacoplan monthly (17% reduction) and pegcetacoplan every other month (14% reduction) at month 12 [46]. Topline safety results showed rates of serious ocular treatment-emergent adverse events of 1.4% for pegcetacoplan monthly, 1.9% for pegcetacoplan every other month, and 0.0% for sham in OAKS; and 0.5% for pegcetacoplan monthly, 0.0% for pegcetacoplan every other month, and 1.0% for sham in DERBY [47]. In the OAKS and DERBY studies, exudative AMD rates determined by the investigators were 5.2%, 4.7%, and 1.4% for pegcetacoplan monthly, every other month, and sham in OAKS, and 6.8%, 3.4%, and 3.4% in DERBY [47, 48]. The efficacy and safety of avacincaptad pegol 2 mg and 4 mg administered through monthly intravitreal injections in patients with GA were examined in a randomized, double-masked, and sham-controlled phase 2/3 study (GATHER1; NCT02686658) [7]. GATHER1 found a significant reduction in GA growth rate (mm) with both avacincaptad pegol 2 mg (27% reduction) and avacincaptad pegol 4 mg (28% reduction) compared with sham at month 12 [7]. There were no reported serious ocular adverse events in avacincaptad pegol groups or sham groups at 12 months [7]. The most common ocular adverse events related to the injection procedure were conjunctival hemorrhage, conjunctival hyperemia, punctate keratitis, and increased intraocular pressure [7]. CNV rates reported by investigators were 9.0%, 9.6%, and 2.7% in the avacincaptad pegol 2 mg and 4 mg and sham groups, respectively [7, 48]. There is an ongoing phase 3, randomized, double-masked, sham-controlled, 24-month, trial that is evaluating monthly and every-other-month intravitreal injections of avacincaptad pegol 2 mg compared with sham in patients with GA (GATHER2; NCT04435366). Recently, the data for the first 12 months, for which patients received monthly avacincaptad pegol 2 mg or sham, became available. GATHER2 met its primary objectives with a significant reduction in observed GA growth rate (mm2) with avacincaptad pegol 2 mg (17.7% reduction) compared with sham at month 12 [49]. GATHER2 had a consistent safety profile with GATHER1 with the most frequently reported ocular adverse events were related to the injection procedure. Following a comprehensive surveillance of CNV, the reported incidence of CNV in the study eye at 12 months was 6.7% for avacincaptad pegol 2 mg compared with 4.1% for sham [50]. Post hoc analyses of the FILLY study have demonstrated that pegcetacoplan lowered the rate of iRORA progression to cRORA as well as reduced photoreceptor loss and thinning compared with sham [51, 52]. Similarly, a post hoc analysis of GATHER1 has demonstrated that avacincaptad pegol reduced the progression of drusen to iRORA/cRORA and the progression from iRORA to cRORA compared with sham, and a reduction in the growth of ellipsoid zone degradation [53]. Future analyses of the phase 3 studies for both pegcetacoplan and avacincaptad pegol, as well as other investigational drugs, will provide a further understanding of the complement system in GA and potential treatments for the management of GA.
Conclusion
GA is a progressive disease associated with irreversible vision loss. Therefore, the lack of treatment options presents a significant unmet need. OCT has been identified as a useful tool for characterizing early atrophy. Furthermore, several imaging features have been identified as predictors of disease progression. Pharmacological agents currently under investigation for GA are being examined for their ability to slow the growth of GA and potentially preserve visual function. Given the irreversible nature of the disease, it is also important to understand whether these drugs can reduce the rates of onset of GA in patients at earlier stages. These investigational drugs are promising future tools for the management of patients with GA.
References
Fleckenstein M, Mitchell P, Freund KB, Sadda S, Holz FG, Brittain C, Henry EC, Ferrara D (2018) The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology 125:369–390. https://doi.org/10.1016/j.ophtha.2017.08.038
Fleckenstein M, Keenan TDL, Guymer RH, Chakravarthy U, Schmitz-Valckenberg S, Klaver CC, Wong WT, Chew EY (2021) Age-related macular degeneration. Nat Rev Dis Primers 7:31. https://doi.org/10.1038/s41572-021-00265-2
Shen LL, Sun M, Ahluwalia A, Young BK, Park MM, Toth CA, Lad EM, Del Priore LV (2021) Relationship of topographic distribution of geographic atrophy to visual acuity in nonexudative age-related macular degeneration. Ophthalmol Retina 5:761–774. https://doi.org/10.1016/j.oret.2020.11.003
Sadda SR, Chakravarthy U, Birch DG, Staurenghi G, Henry EC, Brittain C (2016) Clinical endpoints for the study of geographic atrophy secondary to age-related macular degeneration. Retina 36:1806–1822. https://doi.org/10.1097/IAE.0000000000001283
Flores R, Carneiro A, Vieira M, Tenreiro S, Seabra MC (2021) Age-related macular degeneration: pathophysiology, management, and future perspectives. Ophthalmologica 244:495–511. https://doi.org/10.1159/000517520
Age-Related Eye Disease Study Research Group (2001) A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 119:1417–1436. https://doi.org/10.1001/archopht.119.10.1417
Jaffe GJ, Westby K, Csaky KG, Monés J, Pearlman JA, Patel SS, Joondeph BC, Randolph J, Masonson H, Rezaei KA (2021) C5 inhibitor avacincaptad pegol for geographic atrophy due to age-related macular degeneration: a randomized pivotal phase 2/3 trial. Ophthalmology 128:576–586. https://doi.org/10.1016/j.ophtha.2020.08.027
Ferris FL 3rd, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, Sadda SR, Beckman Initiative for Macular Research Classification Committee (2013) Clinical classification of age-related macular degeneration. Ophthalmology 120:844–851. https://doi.org/10.1016/j.ophtha.2012.10.036
Schatz H, McDonald HR (1989) Atrophic macular degeneration. Rate of spread of geographic atrophy and visual loss. Ophthalmology 96:1541–1551. https://doi.org/10.1016/s0161-6420(89)32694-7
Lindblad AS, Lloyd PC, Clemons TE, Gensler GR, Ferris FL 3rd, Klein ML, Armstrong JR (2009) Change in area of geographic atrophy in the Age-Related Eye Disease Study: AREDS report number 26. Arch Ophthalmol 127:1168–1174. https://doi.org/10.1001/archophthalmol.2009.198
Sunness JS, Rubin GS, Applegate CA, Bressler NM, Marsh MJ, Hawkins BS, Haselwood D (1997) Visual function abnormalities and prognosis in eyes with age-related geographic atrophy of the macula and good visual acuity. Ophthalmology 104:1677–1691. https://doi.org/10.1016/s0161-6420(97)30079-7
Brader HS, Ying GS, Martin ER, Maguire MG, Complications of Age-Related Macular Degeneration Prevention Trial (CAPT) Research Group (2013) Characteristics of incident geographic atrophy in the complications of age-related macular degeneration prevention trial. Ophthalmology 120:1871–1879. https://doi.org/10.1016/j.ophtha.2013.01.049
Wang J, Ying GS (2021) Growth rate of geographic atrophy secondary to age-related macular degeneration: a meta-analysis of natural history studies and implications for designing future trials. Ophthalmic Res 64:205–215. https://doi.org/10.1159/000510507
Colijn JM, Liefers B, Joachim N, Verzijden T, Meester-Smoor MA, Biarnes M, Mones J, de Jong P, Vingerling JR, Mitchell P, Sanchez CI, Wang JJ, Klaver CCW, Eye NEDRC, Consortium E-R (2021) Enlargement of geographic atrophy from first diagnosis to end of life. JAMA Ophthalmol 139:743–750. https://doi.org/10.1001/jamaophthalmol.2021.1407
Lindner M, Boker A, Mauschitz MM, Gobel AP, Fimmers R, Brinkmann CK, Schmitz-Valckenberg S, Schmid M, Holz FG, Fleckenstein M, Fundus Autofluorescence in Age-Related Macular Degeneration Study Group (2015) Directional kinetics of geographic atrophy progression in age-related macular degeneration with foveal sparing. Ophthalmology 122:1356–1365. https://doi.org/10.1016/j.ophtha.2015.03.027
Heier JS, Pieramici D, Chakravarthy U, Patel SS, Gupta S, Lotery A, Lad EM, Silverman D, Henry EC, Anderesi M, Tschosik EA, Gray S, Ferrara D, Guymer R, Chroma and Spectri Study Investigators (2020) Visual function decline resulting from geographic atrophy: results from the Chroma and Spectri phase 3 trials. Ophthalmol Retina 4:673–688. https://doi.org/10.1016/j.oret.2020.01.019
You JI, Kim DG, Yu SY, Kim ES, Kim K (2021) Correlation between topographic progression of geographic atrophy and visual acuity changes. Korean J Ophthalmol 35:448–454. https://doi.org/10.3341/kjo.2021.0037
Sunness JS, Rubin GS, Zuckerbrod A, Applegate CA (2008) Foveal-sparing scotomas in advanced dry age-related macular degeneration. J Vis Impair Blind 102:600–610
Sunness JS, Rubin GS, Broman A, Applegate CA, Bressler NM, Hawkins BS (2008) Low luminance visual dysfunction as a predictor of subsequent visual acuity loss from geographic atrophy in age-related macular degeneration. Ophthalmology 115: 1480–1488, 1488 e1481–1482. https://doi.org/10.1016/j.ophtha.2008.03.009
Chakravarthy U, Bailey CC, Scanlon PH, McKibbin M, Khan RS, Mahmood S, Downey L, Dhingra N, Brand C, Brittain CJ, Willis JR, Venerus A, Muthutantri A, Cantrell RA (2020) Progression from early/intermediate to advanced forms of age-related macular degeneration in a large UK cohort: rates and risk factors. Ophthalmol Retina 4:662–672. https://doi.org/10.1016/j.oret.2020.01.012
Sunness JS, Gonzalez-Baron J, Bressler NM, Hawkins B, Applegate CA (1999) The development of choroidal neovascularization in eyes with the geographic atrophy form of age-related macular degeneration. Ophthalmology 106:910–919. https://doi.org/10.1016/S0161-6420(99)00509-6
Holz FG, Sadda SR, Staurenghi G, Lindner M, Bird AC, Blodi BA, Bottoni F, Chakravarthy U, Chew EY, Csaky K, Curcio CA, Danis R, Fleckenstein M, Freund KB, Grunwald J, Guymer R, Hoyng CB, Jaffe GJ, Liakopoulos S, Mones JM, Oishi A, Pauleikhoff D, Rosenfeld PJ, Sarraf D, Spaide RF, Tadayoni R, Tufail A, Wolf S, Schmitz-Valckenberg S, CAM group (2017) Imaging protocols in clinical studies in advanced age-related macular degeneration: recommendations from classification of atrophy consensus meetings. Ophthalmology 124:464–478. https://doi.org/10.1016/j.ophtha.2016.12.002
Heesterbeek TJ, Lores-Motta L, Hoyng CB, Lechanteur YTE, den Hollander AI (2020) Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol Opt 40:140–170. https://doi.org/10.1111/opo.12675
AREDS Research Group, Chew EY, Clemons T, SanGiovanni JP, Danis R, Domalpally A, McBee W, Sperduto R, Ferris FL (2012) The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology 119:2282–2289. https://doi.org/10.1016/j.ophtha.2012.05.027
Sadda SR, Guymer R, Holz FG, Schmitz-Valckenberg S, Curcio CA, Bird AC, Blodi BA, Bottoni F, Chakravarthy U, Chew EY, Csaky K, Danis RP, Fleckenstein M, Freund KB, Grunwald J, Hoyng CB, Jaffe GJ, Liakopoulos S, Mones JM, Pauleikhoff D, Rosenfeld PJ, Sarraf D, Spaide RF, Tadayoni R, Tufail A, Wolf S, Staurenghi G (2018) Consensus definition for atrophy associated with age-related macular degeneration on OCT: classification of atrophy report 3. Ophthalmology 125:537–548. https://doi.org/10.1016/j.ophtha.2017.09.028
Guymer RH, Rosenfeld PJ, Curcio CA, Holz FG, Staurenghi G, Freund KB, Schmitz-Valckenberg S, Sparrow J, Spaide RF, Tufail A, Chakravarthy U, Jaffe GJ, Csaky K, Sarraf D, Mones JM, Tadayoni R, Grunwald J, Bottoni F, Liakopoulos S, Pauleikhoff D, Pagliarini S, Chew EY, Viola F, Fleckenstein M, Blodi BA, Lim TH, Chong V, Lutty J, Bird AC, Sadda SR (2020) Incomplete retinal pigment epithelial and outer retinal atrophy in age-related macular degeneration: classification of atrophy meeting report 4. Ophthalmology 127:394–409. https://doi.org/10.1016/j.ophtha.2019.09.035
Joachim N, Mitchell P, Kifley A, Rochtchina E, Hong T, Wang JJ (2013) Incidence and progression of geographic atrophy: observations from a population-based cohort. Ophthalmology 120:2042–2050. https://doi.org/10.1016/j.ophtha.2013.03.029
Nassisi M, Fan W, Shi Y, Lei J, Borrelli E, Ip M, Sadda SR (2018) Quantity of intraretinal hyperreflective foci in patients with intermediate age-related macular degeneration correlates with 1-year progression. Invest Ophthalmol Vis Sci 59:3431–3439. https://doi.org/10.1167/iovs.18-24143
Veerappan M, El-Hage-Sleiman AM, Tai V, Chiu SJ, Winter KP, Stinnett SS, Hwang TS, Hubbard GB 3rd, Michelson M, Gunther R, Wong WT, Chew EY, Toth CA, Age-related Eye Disease Study 2 Ancillary Spectral Domain Optical Coherence Tomography Study G (2016) Optical coherence tomography reflective drusen substructures predict progression to geographic atrophy in age-related macular degeneration. Ophthalmology 123:2554–2570. https://doi.org/10.1016/j.ophtha.2016.08.047
Tan ACS, Pilgrim MG, Fearn S, Bertazzo S, Tsolaki E, Morrell AP, Li M, Messinger JD, Dolz-Marco R, Lei J, Nittala MG, Sadda SR, Lengyel I, Freund KB, Curcio CA (2018) Calcified nodules in retinal drusen are associated with disease progression in age-related macular degeneration. Sci Transl Med 10. https://doi.org/10.1126/scitranslmed.aat4544
Laiginhas R, Shi Y, Shen M, Jiang X, Feuer W, Gregori G, Rosenfeld PJ (2022) Persistent hypertransmission defects detected on en face swept source optical computed tomography images predict the formation of geographic atrophy in age-related macular degeneration. Am J Ophthalmol 237:58–70. https://doi.org/10.1016/j.ajo.2021.11.001
Pfau M, von der Emde L, de Sisternes L, Hallak JA, Leng T, Schmitz-Valckenberg S, Holz FG, Fleckenstein M, Rubin DL (2020) Progression of photoreceptor degeneration in geographic atrophy secondary to age-related macular degeneration. JAMA Ophthalmol 138:1026–1034. https://doi.org/10.1001/jamaophthalmol.2020.2914
Reiter GS, Told R, Schranz M, Baumann L, Mylonas G, Sacu S, Pollreisz A, Schmidt-Erfurth U (2020) Subretinal drusenoid deposits and photoreceptor loss detecting global and local progression of geographic atrophy by SD-OCT imaging. Invest Ophthalmol Vis Sci 61:11. https://doi.org/10.1167/iovs.61.6.11
Shen LL, Sun M, Grossetta Nardini HK, Del Priore LV (2020) Progression of unifocal versus multifocal geographic atrophy in age-related macular degeneration: a systematic review and meta-analysis. Ophthalmol Retina 4:899–910. https://doi.org/10.1016/j.oret.2020.03.020
Domalpally A, Danis RP, White J, Narkar A, Clemons T, Ferris F, Chew E (2013) Circularity index as a risk factor for progression of geographic atrophy. Ophthalmology 120:2666–2671. https://doi.org/10.1016/j.ophtha.2013.07.047
Shen LL, Sun M, Khetpal S, Grossetta Nardini HK, Del Priore LV (2020) Topographic variation of the growth rate of geographic atrophy in nonexudative age-related macular degeneration: a systematic review and meta-analysis. Invest Ophthalmol Vis Sci 61:2. https://doi.org/10.1167/iovs.61.1.2
Holz FG, Bindewald-Wittich A, Fleckenstein M, Dreyhaupt J, Scholl HP, Schmitz-Valckenberg S, FAM-Study Group (2007) Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol 143:463–472. https://doi.org/10.1016/j.ajo.2006.11.041
Fritsche LG, Chen W, Schu M, Yaspan BL, Yu Y, Thorleifsson G, Zack DJ, Arakawa S, Cipriani V, Ripke S, Igo RP Jr, Buitendijk GH, Sim X, Weeks DE, Guymer RH, Merriam JE, Francis PJ, Hannum G, Agarwal A, Armbrecht AM, Audo I, Aung T, Barile GR, Benchaboune M, Bird AC, Bishop PN, Branham KE, Brooks M, Brucker AJ, Cade WH, Cain MS, Campochiaro PA, Chan CC, Cheng CY, Chew EY, Chin KA, Chowers I, Clayton DG, Cojocaru R, Conley YP, Cornes BK, Daly MJ, Dhillon B, Edwards AO, Evangelou E, Fagerness J, Ferreyra HA, Friedman JS, Geirsdottir A, George RJ, Gieger C, Gupta N, Hagstrom SA, Harding SP, Haritoglou C, Heckenlively JR, Holz FG, Hughes G, Ioannidis JP, Ishibashi T, Joseph P, Jun G, Kamatani Y, Katsanis N, C NK, Khan JC, Kim IK, Kiyohara Y, Klein BE, Klein R, Kovach JL, Kozak I, Lee CJ, Lee KE, Lichtner P, Lotery AJ, Meitinger T, Mitchell P, Mohand-Said S, Moore AT, Morgan DJ, Morrison MA, Myers CE, Naj AC, Nakamura Y, Okada Y, Orlin A, Ortube MC, Othman MI, Pappas C, Park KH, Pauer GJ, Peachey NS, Poch O, Priya RR, Reynolds R, Richardson AJ, Ripp R, Rudolph G, Ryu E, Sahel JA, Schaumberg DA, Scholl HP, Schwartz SG, Scott WK, Shahid H, Sigurdsson H, Silvestri G, Sivakumaran TA, Smith RT, Sobrin L, Souied EH, Stambolian DE, Stefansson H, Sturgill-Short GM, Takahashi A, Tosakulwong N, Truitt BJ, Tsironi EE, Uitterlinden AG, van Duijn CM, Vijaya L, Vingerling JR, Vithana EN, Webster AR, Wichmann HE, Winkler TW, Wong TY, Wright AF, Zelenika D, Zhang M, Zhao L, Zhang K, Klein ML, Hageman GS, Lathrop GM, Stefansson K, Allikmets R, Baird PN, Gorin MB, Wang JJ, Klaver CC, Seddon JM, Pericak-Vance MA, Iyengar SK, Yates JR, Swaroop A, Weber BH, Kubo M, Deangelis MM, Leveillard T, Thorsteinsdottir U, Haines JL, Farrer LA, Heid IM, Abecasis GR, AMD Gene Consortium (2013) Seven new loci associated with age-related macular degeneration. Nat Genet 45(4):433–439, 439e1–2. https://doi.org/10.1038/ng.2578
Li M, Atmaca-Sonmez P, Othman M, Branham KE, Khanna R, Wade MS, Li Y, Liang L, Zareparsi S, Swaroop A, Abecasis GR (2006) CFH haplotypes without the Y402H coding variant show strong association with susceptibility to age-related macular degeneration. Nat Genet 38:1049–1054. https://doi.org/10.1038/ng1871
Sofat R, Casas JP, Webster AR, Bird AC, Mann SS, Yates JR, Moore AT, Sepp T, Cipriani V, Bunce C, Khan JC, Shahid H, Swaroop A, Abecasis G, Branham KE, Zareparsi S, Bergen AA, Klaver CC, Baas DC, Zhang K, Chen Y, Gibbs D, Weber BH, Keilhauer CN, Fritsche LG, Lotery A, Cree AJ, Griffiths HL, Bhattacharya SS, Chen LL, Jenkins SA, Peto T, Lathrop M, Leveillard T, Gorin MB, Weeks DE, Ortube MC, Ferrell RE, Jakobsdottir J, Conley YP, Rahu M, Seland JH, Soubrane G, Topouzis F, Vioque J, Tomazzoli L, Young I, Whittaker J, Chakravarthy U, de Jong PT, Smeeth L, Fletcher A, Hingorani AD (2012) Complement factor H genetic variant and age-related macular degeneration: effect size, modifiers and relationship to disease subtype. Int J Epidemiol 41:250–262. https://doi.org/10.1093/ije/dyr204
Johnson LV, Ozaki S, Staples MK, Erickson PA, Anderson DH (2000) A potential role for immune complex pathogenesis in drusen formation. Exp Eye Res 70:441–449. https://doi.org/10.1006/exer.1999.0798
Loyet KM, Deforge LE, Katschke KJ Jr, Diehl L, Graham RR, Pao L, Sturgeon L, Lewin-Koh SC, Hollyfield JG, van Lookeren CM (2012) Activation of the alternative complement pathway in vitreous is controlled by genetics in age-related macular degeneration. Invest Ophthalmol Vis Sci 53:6628–6637. https://doi.org/10.1167/iovs.12-9587
Mullins RF, Schoo DP, Sohn EH, Flamme-Wiese MJ, Workamelahu G, Johnston RM, Wang K, Tucker BA, Stone EM (2014) The membrane attack complex in aging human choriocapillaris: relationship to macular degeneration and choroidal thinning. Am J Pathol 184:3142–3153. https://doi.org/10.1016/j.ajpath.2014.07.017
Reynolds R, Hartnett ME, Atkinson JP, Giclas PC, Rosner B, Seddon JM (2009) Plasma complement components and activation fragments: associations with age-related macular degeneration genotypes and phenotypes. Invest Ophthalmol Vis Sci 50:5818–5827. https://doi.org/10.1167/iovs.09-3928
Liao DS, Grossi FV, El Mehdi D, Gerber MR, Brown DM, Heier JS, Wykoff CC, Singerman LJ, Abraham P, Grassmann F, Nuernberg P, Weber BHF, Deschatelets P, Kim RY, Chung CY, Ribeiro RM, Hamdani M, Rosenfeld PJ, Boyer DS, Slakter JS, Francois CG (2020) Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: a randomized phase 2 trial. Ophthalmology 127:186–195. https://doi.org/10.1016/j.ophtha.2019.07.011
Apellis announces top-line results from phase 3 derby DERBY and OAKS studies in geographic atrophy (GA) and plans to submit NDA to FDA in the first half of 2022. http://investors.apellis.com/news-releases/news-release-details/apellis-announces-top-line-results-phase-3-derby-and-oaks. Accessed 13 May 2022
Boyer D, Steinle N, Wykoff C, Heier H, Singh R, Monés J, Staurenghi G, Holz FG, Bliss C, Deschatelets P, Grossi F, Francois C, Ribeiro R (2021) Safety of intravitreal pegcetacoplan in geographic atrophy: results from the DERBY and OAKS trials. Presented at: American Society of Retina Specialists; October 11, 2021; San Antonio, TX. http://investors.apellis.com/static-files/a9edaac8-e228-4ec5-a27d-f22e4d78f6c4. Accessed 4 Nov 2022
Kaiser PK, Jaffe GJ, Holz FG, Heier JS, Sadda SV, Khanani AM (2022) Considerations on the management of macular neovascularization in patients with geographic atrophy enrolled in clinical trials. Retina Today. March 2022 supplement
Iveric Bio Announces Positive Topline Data from Zimura® GATHER2 Phase 3 clinical trial in geographic atrophy. http://investors.ivericbio.com/news-releases/news-release-details/iveric-bio-announces-positive-topline-data-zimurar-gather2-phase. Accessed October 19, 2022
Heier JS, Khanani AM, Eichenbaum DA, Wykoff CC, Hsu J, Monés J, Nielsen JS, Holz F, Tufail A, D’Amico DJ, Sheth VS, Kaiser PK, Ehlers JP, Jaffe GJ, Tang J, Zhu L, Patel H, Clark J (2022) GATHER2 Pivotal Phase 3 study results: safety of intravitreal avacincaptad pegol in geographic atrophy. Presented at: American Academy of Ophthalmology Annual Meeting; September 30 – October 3, 2022; Chicago, IL. http://ivericmedical.com/static/AAO%202022%20G2%20Safety-480581c4521bd9855d702148a9aff2c4.pdf. Accessed 4 Nov 2022
Nittala MG, Metlapally R, Ip M, Chakravarthy U, Holz FG, Staurenghi G, Waheed N, Velaga SB, Lindenberg S, Karamat A, Koester J, Ribeiro R, Sadda S (2022) Association of pegcetacoplan with progression of incomplete retinal pigment epithelium and outer retinal atrophy in age-related macular degeneration: a post hoc analysis of the FILLY randomized clinical trial. JAMA Ophthalmol 140:243–249. https://doi.org/10.1001/jamaophthalmol.2021.6067
Riedl S, Vogl WD, Mai J, Reiter GS, Lachinov D, Grechenig C, McKeown A, Scheibler L, Bogunovic H, Schmidt-Erfurth U (2022) The effect of pegcetacoplan treatment on photoreceptor maintenance in geographic atrophy monitored by artificial intelligence-based OCT analysis. Ophthalmol Retina. https://doi.org/10.1016/j.oret.2022.05.030
Lally DR (2021) Nascent GA and intermediate AMD progression in the GATHER1 clinical trial: post hoc analysis of 18 month data. Presented at: American Academy of Ophthalmology; November 13–16, 2021; New Orleans, LA. http://ivericmedical.com/static/AAO%202021_Lally_Presentation-ead46ab17c93ff62d513105fc54248b2.pdf. Accessed 4 Nov 2022
Ehlers JP (2021) Exploration of machine learning–enhanced compartmental retinal integrity assessment for progression risk and treatment response in the GATHER1 study. Presented at: Macula Society; June 8–11, 2022; Berlin, Germany. http://ivericmedical.com/static/Ehlers%202022%20Mac%20Soc-0421268f5d894a98be3a117455b25d81.pdf. Accessed 4 Nov 2022
Funding
The medical writing support was provided by IMPRINT Science, New York, NY, USA and funded by Iveric Bio.
Author information
Authors and Affiliations
Contributions
Conceptualization: Anat Loewenstein, Omer Trivizki; Writing—review and editing: Anat Loewenstein, Omer Trivizki.
Corresponding author
Ethics declarations
Ethical approval
This review article does not contain any studies with human participants or animals performed by any of the others.
Conflict of interest
Anat Loewenstein is a consultant to Iveric Bio, Beyeonics, Bayer, WebMD, Allergan, Roche, Novartis, Notal Vision. Omer Trivizki declares no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Loewenstein, A., Trivizki, O. Future perspectives for treating patients with geographic atrophy. Graefes Arch Clin Exp Ophthalmol 261, 1525–1531 (2023). https://doi.org/10.1007/s00417-022-05931-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-022-05931-z