Abstract
Age-related macular degeneration (AMD) is a leading cause of vision loss in the elderly, with dry AMD (d-AMD) leading to geographic atrophy (GA) and significant visual impairment. Multimodal imaging plays a crucial role in d-AMD diagnosis and management, allowing for detailed classification of patient phenotypes and aiding in treatment planning and prognosis determination. Treatment approaches for d-AMD have recently witnessed profound change with the development of specific drugs targeting the complement cascade, with the first anticomplement agents recently approved for GA treatment. Additionally, emerging strategies such as gene therapy and laser treatments may offer potential benefits, though further research is needed to fully establish their efficacy. However, the lack of effective therapies capable of restoring damaged retinal cells remains a major challenge. In the future, genetic treatments aimed at preventing the progression of d-AMD may emerge as a powerful approach. Currently, however, their development is still in the early stages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Multimodal imaging is crucial for diagnosing and managing dry age-related macular degeneration (d-AMD), enabling the detailed classification of patient phenotypes, which aids in treatment planning and determination of prognosis. |
Recent advancements in treatment approaches for d-AMD have been significantly influenced by the development of specific drugs targeting the complement cascade, including the recent approval of the first anticomplement agents for the treatment of geographic atrophy. |
Emerging strategies, such as gene therapy and laser treatments, show potential benefits, although further research is needed to fully ascertain their efficacy. |
Introduction
Age-related macular degeneration (AMD) is a leading cause of visual impairment in elderly people in Western societies [1]. AMD is classified as dry AMD (d-AMD) or neovascular AMD (n-AMD), based on the absence or presence of macular neovascularization (MNV), respectively [2]. Geographic atrophy (GA) represents the late stage of d-AMD and is characterized by the loss of photoreceptors, retinal pigment epithelium, and choriocapillaris [2]. People affected by GA experience progressive irreversible central visual loss, leading to reduced independence and a lower quality of life [3].
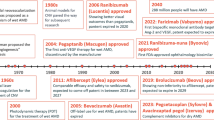
Although for many years no effective treatments were proposed for GA, a deeper understanding of GA pathogenesis has recently led to the development of promising treatment options, which aim to slow the disease progression. In this direction, in 2023, the Food and Drug Administration (FDA) approved the first two drugs for GA [4, 5].
Furthermore, the recent advancements in multimodal imaging have greatly improved the GA phenotype classification. This is crucial for selecting patients for treatment and in determining their prognosis.
The aim of this review is to describe the state of the art of the management of d-AMD. We will focus on the recent multimodal imaging techniques and on the current treatment options. This article is based on previous research; therefore, no approval from the local ethics committee was required.
Multimodal Imaging
Initially, classification systems for d-AMD were based on color fundus photography (CFP) [6]. Subsequently, fundus autofluorescence (FAF) and optical coherence tomography (OCT) emerged as crucial tools in d-AMD classification and management [2, 7]. Supplementary and confirmatory imaging modalities are near-infrared reflectance (NIR), fluorescein angiography (FA), indocyanine green angiography (ICGA), and OCT angiography (OCTA) [2].
Color Fundus Photography
CFP displays a wide spectrum of d-AMD hallmarks, including, for instance, macular drusen and pigmentary abnormalities [2, 6]. Originally, d-AMD classification relied on CFP, involving drusen characteristics (size, consistency, location, and number), presence/absence of pigmentation abnormalities, GA size, and area involved by the GA [6] In 1995, the International Age-Related Maculopathy Epidemiological Study Group agreed on the following criteria to define GA on CFP: sharp demarcation, hypopigmented or depigmented appearance, round or oval shape, increased visibility of the underlying choroidal vessels, and a diameter of at least 175 mm measured using a 30- or 35-mm fundus camera.
However, the various limitations of this examination, such as low resolution of the retina and choroid depth, absence of detailed quantitative information, and low image quality in cases of media opacities or poor mydriasis, have greatly restricted the application of CFP in d-AMD management [7, 8].
Fundus Autofluorescence
FAF is currently considered a valuable technique in detecting atrophic areas and in monitoring its advancement, due to the high-contrast retinal images provided [7]. Different wavelengths can be utilized to excite endogenous fluorophores and capture their autofluorescence. Each specific wavelength targets different fluorophores, thereby providing distinct information.
Blue FAF devices utilize a 488-nm blue wavelength excitation filter to specifically capture the autofluorescence emission from retinal pigment epithelium (RPE) fluorophores (e.g., lipofuscin) [9]. Thus, the signal is severely reduced in correspondence with atrophic lesions, appearing as areas of hypo-autofluorescence on FAF images (Figs. 1–2) [10].
Two types of FAF imaging systems are available: flash fundus camera-based systems and scanning laser ophthalmoscopy (SLO) systems [7].
SLO is characterized by a confocal aperture to remove backscattering light outside the focal plane and offer greater contrast and resolution. However, it cannot be integrated into conventional fundus cameras, requiring additional equipment for image acquisition.
By using the fundus camera, the employment of blue-light excitation for autofluorescence imaging is unfeasible due to technical limitations. Hence, autofluorescence imaging in fundus cameras relies on excitation wavelengths ranging from green to orange (510–610 nm).
The quality of the blue FAF signal is affected by various factors including lens opacities, vitreous floaters, hemorrhages, hard exudates, and pupil dilation [11]. In addition, in the presence of the "diffuse-trickling" GA phenotype (coalescent lobular atrophic lesions appearing rather grayish compared with the black appearance of other GA subtypes) or refractile drusen within atrophic areas, the ability of FAF in discerning between intact retina and atrophic areas is limited [12, 13]. Furthermore, the blocking of blue light by the macular pigment results in reduced signal intensity at the fovea, causing challenges in identifying the atrophic border near the fovea [11]. Different wavelengths (e.g., green light) might reduce macular pigment absorption, maintaining excellent visualization of the atrophic areas in the macular region as well [14].
Color Fundus Autofluorescence
Color FAF is an imaging technique showing useful findings for retinal diseases, including d-AMD (Fig. 1) [15]. This system, using a 450-nm wavelength in a confocal blue-light FAF device, captures the full-emission spectrum on a color sensor, producing color FAF images that allow the isolation of minor fluorophores (advanced glycation end products [AGEs]; N-retinylidene-N-retinylethanolamine [A2E]; and the oxidized fluorescent form flavin adenine dinucleotide [FAD] of the redox pair FAD-FADH2, and collagen 2, distinct from lipofuscin [15]. The approach, based on emission fluorescence components (EFC), divides the spectrum into short- and long-wavelength components (green, 510–560 nm; red, 560–700 nm) [16]. Areas of atrophy are characterized by the absence or reduction of strong red EFC, while the presence of a residual green EFC signal indicates the presence of drusenoid deposits [16]. Larger longitudinal studies are needed to determine the prognostic implications of these findings.
Multicolor Confocal Scanning Laser Ophthalmoscopy
Multicolor confocal SLO captures composite images simultaneously using confocal scanning lasers at three different wavelengths (blue, green, and near-infrared) (Figs. 1, 2) [17]. Each wavelength penetrates different retinal layers, highlighting details of the inner retina, deeper retinal layers, and structures in the outer retina and choroid, respectively [18]. Unlike color fundus photography, multicolor images are "false-color" or "pseudocolor," composed of information obtained from separate wavelengths. In the context of GA, multicolor imaging can visualize atrophic areas and reveal retinal hemorrhages and fibrotic changes. However, distinguishing subtle hemorrhages and pigmentary lesions may be challenging [17, 18]. The potential advantages of visualizing individual wavelength component images in isolation over a composite image remain to be systematically investigated.
Near-Infrared Reflectance
In the NIR-SLO imaging technique, 750–840-nm wavelengths of light are used [7]. This minimizes discomfort for the patient, as there is minimal interference and absorption by media opacities and the luteal pigment in the macula. Consequently, in contrast to blue-light FAF imaging, the visualization of atrophic areas within the fovea and its direct involvement is facilitated (Figs. 1–2) [19]. Furthermore, NIR images allow for the detection of reticular pseudodrusen (RPD) with high sensitivity [20]. Systematic validation studies for the use of NIR alone in d-AMD are still lacking. However, integrating NIR images in study protocols at all visits, alongside FAF and OCT images, is crucial. Their short capture time has no significant impact on the recording procedure and provides valuable complementary information to other imaging techniques [19].
Optical Coherence Tomography
OCT, providing high-quality, cross-sectional, and en face retinal and choroidal images through depth-resolved segmentation, with a resolution comparable to light microscopy histology, has enabled the segmentation of retina and choroid, obtaining detailed insights into tissue layers and the ability to quantify the loss of specific layers (Fig. 2) [21].
The Classification of Atrophy Meeting (CAM) group proposed OCT as the reference tool to classify atrophy. CAM consensus developed a definition and nomenclature for AMD GA based on spectral domain (SD)-OCT, incorporating other imaging modalities including CFP, FAF, and NIR for additional and confirmatory analyses [8]. This system is based on the observation that photoreceptor (or the outer retinal) atrophy can manifest independently of RPE atrophy, whereas RPE atrophy consistently correlates with thinning or loss of the overlying outer retina [8]. According to the stage and mechanisms of atrophy evolution, the CAM group introduced the following four entities to describe AMD-related atrophy: complete RPE and outer retinal atrophy (cRORA); incomplete RPE and outer retinal atrophy (iRORA); complete outer retinal atrophy (cORA); incomplete outer retinal atrophy (iORA) [8]. Specifically, GA denotes a subset of cRORA with no MNV (present or previous, considering that previous MNV may not be manifest), as evident on CFP, while cRORA encompasses macular atrophy with or without MNV. Nascent GA represents a subset of iRORA without MNV (Fig. 3).
In addition, the CAM group established three specific OCT criteria for cRORA definition: (1) a region with choroidal hyper-transmission measuring at least 250 μm in diameter, (2) an area with RPE attenuation or loss of at least 250 μm in diameter, and (3) evidence of concomitant photoreceptor degeneration above the affected region [8].
Recently, in the fourth CAM report, iRORA is defined by the following criteria: (1) a region of signal hyper-transmission into the choroid; (2) a corresponding zone of attenuation or disruption of the RPE, with or without the persistence of basal laminar deposits; and (3) evidence of overlying photoreceptor degeneration, (i.e., subsidence of the inner nuclear layer [INL] and outer plexiform layer [OPL], presence of a hypo-reflective wedge in the Henle fiber layer [HFL], thinning of the outer nuclear layer [ONL], disruption of the external limiting membrane [ELM], or disintegration of the ellipsoid zone [EZ]), assuming these do not conform to the characteristics of cRORA (Fig. 4) [22]. The designation of iRORA is not applicable when an RPE tear is present [22].
OCT scan of a patient with incomplete retinal pigment epithelium (RPE) and outer retinal atrophy (iRORA) due to age-related macular degeneration. The image shows (1) a region of signal hyper-transmission into the choroid (triangles), (2) a corresponding zone of attenuation or disruption of the RPE, and (3) evidence of overlying photoreceptor degeneration with subsidence of the inner nuclear layer and outer plexiform layer (arrows)
The cases which present some, but not all, signs of iRORA should not yet be defined as iRORA but have risk factors for the progression to iRORA. Several signs, such as hyperreflective foci, heterogeneous internal reflectivity of drusen, RPD, and basal laminar deposits, may be considered as high-risk OCT biomarkers for progression [22].
Furthermore, the CAM 4 report provides evidence that the features of iRORA predict the development of cRORA, using case examples with longitudinal follow-up in eyes with drusen [22].
En face OCT is an innovative approach used to support OCT b-scans for identifying and measuring geographic atrophy. It generates retinal and choroidal coronal section that varies according to image segmentation [23].
Nevertheless, OCT exhibited several limitations: restricted scan field, dependence on image quality for interpretation, difficulties with automated image segmentation, and fewer comprehensive investigations employing SD-OCT in comparison with fundus imaging methods (CFP, FAF) [24].
Optical Coherence Tomography Angiography
Although OCTA has mostly been used to detect neovascularization in AMD, it has also been applied to d-AMD, since alterations in the choriocapillaris may occur at every stage [25].
Recently, the concept of "signal flow voids" that are easily measured at the choriocapillaris level as dark hypo-reflective areas in intermediate/late stages of d-AMD has received considerable interest [25, 26]. These findings may indicate areas of progressive non-perfusion or loss of perfusion within the characteristic capillary network of the choroid (“ischemic choroidopathy”) [24,25,26]. It has been suggested that evidence on OCTA of flow voids might predispose to late-stage AMD. Furthermore, OCTA could detect non-exudative or inactive type 1 NV in eyes that might be otherwise classified as non-neovascular AMD [25].
Fluorescein Angiography
Fluorescein angiography is useful for identifying characteristic features of d-AMD, such as drusen and GA [27]. Drusen typically exhibit staining, while atrophic regions may display early hyper-fluorescence or a "window defect" secondary to RPE atrophy [27, 28]. Several drawbacks of this diagnostic approach should be highlighted: FA is an invasive and dye-requiring exam with potential risk of allergic reactions, prolonged image acquisition times, and limitations in depth-resolved segmentation and lateral resolution [29].
Treatment
So far, numerous clinical trials have been carried out to satisfy the unfulfilled needs of d-AMD treatment. The status of research on potential treatments for d-AMD will be examined in the following section, dividing the section into treatment for (a) early/intermediate d-AMD, (b) late d-AMD (GA), and (c) early/intermediate/late d-AMD.
-
(a)
Early/intermediate dry AMD
Nutritional Supplementation
Nutraceutical products have garnered significant attention due to their therapeutic potential. The Age-Related Eye Disease Study (AREDS) trial demonstrated the protective effects of antioxidant and mineral supplements in delaying the progression from intermediate to late d-AMD in patients with intermediate AMD. [30]. In the phase III AREDS (AREDS2) trial, lutein, zeaxanthin, and omega-3 long-chain polyunsaturated fatty acids took the place of β-carotene, due to an increased risk of lung cancer in smokers [31]. Notably, lutein and zeaxanthin exhibited antioxidant properties as pigments of human macula. However, no evidence confirmed higher efficacy of the AREDS2 formulation than the first AREDS supplementation, despite a safer profile [32]. Nevertheless, AREDS2 formulation remains widely used in d-AMD management.
Laser Therapy
In the 1990s, thermal laser photocoagulation using a continuous-wave (CW) laser beam was employed in d-AMD to induce drusen regression. However, thermal damage to the photoreceptor and inner retina, subretinal fibrosis, and choroidal neovascularization were reported [33]. Therefore, different strategies have been studied to avoid these collateral effects.
Nanosecond and Subthreshold Laser Treatment
The 3-ns pulsed laser, delivering a subvisible threshold laser spot with a shorter pulse than conventional CW lasers, selectively modulates pigmented tissues, sparing retinal neurons and minimizing thermal damage [34]. Studies suggest its potential in reducing drusen load in patients with AMD while minimizing collateral damage associated with CW laser treatment, such as new vessel growth and Bruch’s membrane disruption. However, further investigations are needed to determine the safety, specific effects, and mechanisms of nanosecond laser treatment in patients with AMD [34].
Another potential laser treatment for preventing d-AMD progression is the yellow subthreshold laser treatment [35]. A pilot study reported no side effects in patients with RPD, and ongoing randomized clinical trials aim to evaluate its efficacy further [35].
Conversely, the LEAD Study highlighted that subthreshold nanosecond laser (SNL) treatment did not significantly slow the progression to d-AMD in patients initially diagnosed with intermediate AMD [36]. However, a potential beneficial effect was noted in patients without reticular pseudo-drusen over a 24-month follow-up, suggesting the need for further trials to assess SNL’s potential in slowing intermediate AMD progression [36].
Photobiomodulation
Photobiomodulation (PBM) is designed to enhance electron transport chain activity through cytochrome C oxidase to reduce oxidative stress [37]. The Valeda Light Delivery System (LumiThera, Inc., USA) is a PBM device that emits light primarily in the yellow (590 nm), red (660 nm), and near-infrared (850 nm) wavelengths. Numerous clinical studies have investigated the effects of PBM on AMD [37].
The TORPA 1 trial demonstrated an increase in visual acuity and contrast sensitivity, with no change in fixation stability over 12 months. Phase II of the TORPA 2 trial reported a visual gain of +5.14 letters at 3 months, along with a significant decrease in drusen volume and central thickness [38].
The LIGHTSITE I study underscored the effectiveness of PBM in enhancing vision and reducing drusen in patients with d-AMD, recording a four-letter improvement in visual acuity. Following these results, the LIGHTSITE II study, a randomized multicenter trial, commenced to assess the outcomes of repeated PBM treatments on 53 eyes at baseline and 4 and 8 months. This trial noted a four-letter enhancement in best-corrected visual acuity (BCVA) at month 9 for participants receiving PBM treatments [37, 38].
-
(b)
Late dry AMD (GA)
Anti-Inflammatory Therapy
Pathophysiology and progression of d-AMD are significantly influenced by inflammation, particularly by complement cascade [39, 40]. While anticomplement factors have shown encouraging results as GA therapeutic strategy, other molecules are still under investigation.
Anticomplement Factors
This innate immunity system consists of serum and membrane-bound proteins that are activated through three biochemical pathways (classical, alternative, lectin), all converging to C3 activation. Briefly, C3 cleaves into C3a and C3b, which activates C5, which then splits into C5a and C5b, thus developing membrane attack complex (MAC) and inflammasome [41]. Strong evidence suggests that the onset and progression of d-AMD and GA are related to complement cascade imbalance. Since complement factors have been observed to accumulate in drusen, complement cascade is believed to play a role in both drusen production and choriocapillaris loss [42]. Several therapies (e.g., pegcetacoplan) have targeted C3, aiming at blocking MAC and inflammasomes, although a reduction of their beneficial anti-infective and anti-inflammatory effects. As an alternative, downstream components such as C5, have been investigated (e.g., avacincaptad pegol, eculizumab), so that host defense could be preserved, still preventing the activation of MAC and inflammasomes [43].
Pegcetacoplan
Pegcetacoplan (APL-2, Apellis Pharmaceuticals, Waltham, MA, USA) is a synthetic C3 inhibitor approved by the FDA in February 2023 as first anticomplement agent for AMD GA treatment. The phase II FILLY study (NCT02503332) evaluated its efficacy and safety over 12 months. To qualify for participation, individuals needed to be at least 60 years old, exhibit AMD-related geographic atrophy (if the total GA area was 2.5–17.5 mm2 or—if multifocal—at least one lesion measured 1.25 mm2 or larger on FAF imaging), and have BCVA of 20/320 or better in the study eye. The primary endpoint was the size of the geographic atrophy lesion, measured by FAF. Briefly, patients were randomly assigned to 15 mg pegcetacoplan intravitreal injections either monthly or every other month (EOM), or sham intravitreal injections [42]. A significant reduction in GA growth was reported by both treatment arms (monthly group: −29% vs. EOM group: −20%). However, no significant differences in best-corrected visual acuity (BCVA) were observed between groups [42]. Adverse events were reported in the pegcetacoplan group, including two cases of infectious endophthalmitis and a higher rate of new-onset investigator-determined n-AMD (monthly group: 20.9%, EOM group: 8.9% vs. sham group: 1.2%) [42]. Post hoc analysis of n-AMD cases revealed no temporal clustering of onset. All n-AMD cases were successfully treated with anti-vascular endothelial growth factor (VEGF) intravitreal injections [44]. Another post hoc analysis showed lower progression from iRORA to cRORA in treated patients, suggesting a potential effect in the early GA [45].
The phase III DERBY (NCT03525600) and OAKS (NCT03525613) trials evaluated the FILLY study’s treatment arms and outcomes over 24 months. Both studies confirmed a significant reduction in GA growth, mostly over 18–24 months, with a comparable impact on foveal and extrafoveal lesions [4]. Interestingly, n-AMD at month 24 was found at a lower rate than in the FILLY trial (< 12%). The phase III GALE trial, which is still ongoing, will assess the long-term safety and efficacy of pegcetacoplan in patients who completed DERBY and OAKS. Notably, the report from the American Society of Retina Specialists (ASRS) Research and Safety in Therapeutics Committee noted that 14 eyes from 13 patients developed retinal vasculitis following their first intravitreal injection of pegcetacoplan [46]. Most cases (79%) were characterized by occlusive retinal vasculopathy, with symptoms generally manifesting around 10.5 days post-injection. Clinical assessment revealed prevalent inflammation in the anterior chamber and vitritis in 86% of cases [46]. The involvement was primarily observed in retinal veins (100%), with a significant number of cases (86%) also presenting with retinal hemorrhages. Visual acuity was notably affected, decreasing from a median baseline of 20/60 to 20/300 at the time of vasculitis presentation, and slightly improving to 20/200 at the last follow-up [46]. The etiology of the vasculitis remains unidentified, and optimal treatment strategies are yet to be established. The report recommends against the continued use of pegcetacoplan in affected patients and suggests considering infectious etiologies and corticosteroid treatments to expedite resolution of inflammatory symptoms [46].
Avacincaptad Pegol
Avacincaptad pegol (ACP) (Zimura, Iveric Bio, Inc., Parsippany, NJ, USA) is a synthetic C5 inhibitor that achieved FDA approval for treatment of AMD GA in August 2023 [5, 47]. The efficacy and safety of ACP were assessed in the phase II–III GATHER1 trial, where monthly ACP intravitreal injections (2 mg or 4 mg) were randomized to sham intravitreal injections (2 mg or 4 mg) over 18 months. To qualify for participation, individuals needed to be at least 50 years old, exhibit AMD-related geographic atrophy that did not involve the center point (with the total GA area ranging from 2.5 to 17.5 mm2, or—if multifocal—at least one lesion measuring 1.25 mm2 or larger on FAF imaging), and have best-corrected visual acuity ranging from 20/25 to 20/320 in the study eye. The primary endpoint was the size of the geographic atrophy lesion, measured by FAF.
After 18 months, 4 mg ACP demonstrated a slightly higher reduction in mean growth of GA than 2 mg ACP (28.1% vs. 30%, respectively) [5]. Safety analysis yielded promising results, with no endophthalmitis or intraocular inflammation, and low-rate conversion to n-AMD (ACP cohorts: < 15.7% vs. sham group: < 2.7%) [5, 47]. In the phase III GATHER2, 2 mg ACP was evaluated over a 24-month period. The ACP group reported considerably slower GA progression rate than the sham group [5]. The safety profile was excellent, and the n-AMD rate was comparable between the two groups (ACP cohort: 5% vs. sham cohort: 3%) [5].
Other Anticomplement Factors
The remaining anticomplement drugs that have been studied and have shown no efficacy in treating GA are listed in Table 1 [48] [49].
Other Anti-Inflammatory Therapy
Other anti-inflammatory drugs have been studied and have shown no benefits in the treatment of GA (Table 1) [50] [51].
-
(c)
Early/intermediate/late dry-AMD
Neuroprotective Agents
Neuroprotection emerged to delay photoreceptor degradation by retinal injury in advanced d-AMD [52]. This approach encompasses different strategies, including neurotrophic factors activation, oxidative stress reduction, apoptosis prevention, inflammation suppression, and delivery of umbilical-derived cells [53].
Ciliary Neurotrophic Factor
Ciliary neurotrophic factor (CNTF) is an IL-6 cytokine that is prominently expressed by Schwann cells [54]. CNTF showed protective effects on both RPE and photoreceptors, since damaged RPE and Müller cells release this molecule to regenerate retinal neurons [55]. To prevent systemic side effects, CNTF has been released intravitreally by encapsulated cell technology (ECT), consisting of encapsulating cells within a semipermeable polymer capsule implant (NT-501) [53, 55]. Notably, ECT resulted in a dose-dependent increase in retinal thickness and a preserved vision at 12-month follow-up [53].
Brimonidine
Brimonidine is an alpha-2-adrenergic agonist that is commonly prescribed as an intraocular pressure-lowering drug. Interestingly, it is thought to display neuroprotective properties, as well [54]. The Brimonidine Drug Delivery System (Brimo DDS), created by Allergan (part of AbbVie), introduces an intravitreal implant designed to administer brimonidine into the vitreous body for a prolonged duration [56]. This system was evaluated in a blinded, randomized, placebo-controlled phase II trial involving 113 participants (NCT00658619), where Brimo DDS showed promise in decelerating the expansion of GA [56]. Subsequently, a more extensive study, the phase IIb BEACON trial (n = 303; NCT02087085), reported a decrease of 10% in the enlargement of GA areas after 2 years [57].
Elamipretide
Elamipretide is a peptide enhancing mitochondrial function. Although the phase I ReCLAIM trial showed positive effects on d-AMD, the subsequent ReCLAIM-2 study failed to meet primary endpoints related to visual acuity and GA progression [58].
Tandospirone
Tandospirone is a neuroprotective serotoninergic receptor agonist (5-HT1a) [59]. Despite promising preclinical results, this molecule did not show a significant reduction in GA growth [59].
Palucorcel
Palucorcel (CNTO-2476) is an in vitro preparation of human umbilical-derived cells that have been injected subretinally in patients with GA [60]. However, this formulation resulted in retinal complications, with no improvements in GA progression or visual acuity [60].
Vision Cycle Modulation
Elevated energy demand of photoreceptors results in large amounts of metabolic waste (e.g., lipofuscin), with potential harmful effects [61]. Visual cycle modulators, such as ALK-100, emixustat, and fenretinide, attempted to target phototransduction enzymes to mitigate cytotoxic by-products, despite unconvincing results [61,62,63]. ALK-001 (Alkeus Pharmaceuticals) is a modified-vitamin A compound that disrupts the visual cycle by producing toxic by-products at a slower rate [61]. Its impact on d-AMD is still under evaluation in the phase III SAGA study (NCT03845582) [64]. Emixustat is a retinoid isomerohydrolase inhibitor, whereas fenretinide is a retinol-binding protein (RBP) antagonist [62]. Both these molecules showed no significant effect on GA growth [62]. Furthermore, fenretinide raised concerns due to the increased risk of dyschromatopsia and impaired dark adaptation [63].
Gene Therapy
Over 100 loci involved in multiple pathways (e.g., lipid metabolism, complement cascade) have been implicated in AMD pathogenesis, with different associated risk of d-AMD [65]. As complement cascade concerns, the complement factor H (CFH) 402H allele has been linked to an increased susceptibility to GA, whereas complement factor B (CFB) haplotypes (R32Q and R32Q/IVS10) showed protective effects [66]. Interestingly, several complement factors have been targeted as intravitreal adeno-associated viral vector-based gene therapy with promising results.
Hemera Biosciences has designed HMR59, a gene therapy employing an adeno-associated viral (AAV) vector to produce sCD59 [66]. This substance interacts with CD59, a key protein in the assembly of the MAC. Initial outcomes from a phase I clinical study (NCT03144999) indicated the absence of significant adverse effects alongside a statistically meaningful reduction in the size of GA [65, 66].
In this direction, GT005 represents another gene therapy approach, also based on an AAV vector, designed to inhibit the formation of MAC [65, 66]. This therapy is currently undergoing assessment in terms of both its safety and effectiveness across several clinical trials, including phase 1/2 (FOCUS) as well as phase 2 studies (HORIZON and EXPLORE).
The most recent investigation, LIGHTSITE III, a randomized, double-masked, multicenter clinical trial, evaluated the efficacy of PBM across 148 eyes. This study demonstrated significant enhancements in BCVA, with a 5.5-letter increase from baseline at month 13 during the initial four treatment series [67].
Conclusion
This review summarizes the most commonly used techniques in multimodal imaging, as well as the current treatments and future therapeutic prospects. The advancements in multimodal imaging have enabled early diagnosis and provided highly reliable prognostic biomarkers. Additionally, while treatment for d-AMD has long been unsatisfactory, the emergence of the first promising drugs marks a significant development. However, the lack of effective therapies capable of restoring damaged retinal cells remains a major challenge. Nevertheless, it appears that initial steps are being taken towards the development of successful treatments, including gene therapy. In the future, genetic treatments aimed at preventing the progression of d-AMD may emerge as a powerful approach. Currently, however, their development is still in the early stages.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Wong WL, Su X, Li X, Cheung CMG, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014. https://doi.org/10.1016/S2214-109X(13)70145-1.
Ferris FL, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–51. https://doi.org/10.1016/J.OPHTHA.2012.10.036.
Sacconi R, Corbelli E, Querques L, Bandello F, Querques G. A review of current and future management of geographic atrophy. Ophthalmol Ther. 2017;6:69–77. https://doi.org/10.1007/S40123-017-0086-6.
Heier JS, Lad EM, Holz FG, Rosenfeld PJ, Guymer RH, Boyer D, et al. Pegcetacoplan for the treatment of geographic atrophy secondary to age-related macular degeneration (OAKS and DERBY): two multicentre, randomised, double-masked, sham-controlled, phase 3 trials. Lancet. 2023;402:1434–48. https://doi.org/10.1016/S0140-6736(23)01520-9.
Khanani AM, Patel SS, Staurenghi G, Tadayoni R, Danzig CJ, Eichenbaum DA, et al. Efficacy and safety of avacincaptad pegol in patients with geographic atrophy (GATHER2): 12-month results from a randomised, double-masked, phase 3 trial. Lancet. 2023;402:1449–58. https://doi.org/10.1016/S0140-6736(23)01583-0.
The age-related eye disease study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: The age-related eye disease study report number 6. Am J Ophthalmol 2001;132:668–81. https://doi.org/10.1016/S0002-9394(01)01218-1.
Holz FG, Sadda SVR, Staurenghi G, Lindner M, Bird AC, Blodi BA, et al. Imaging protocols in clinical studies in advanced age-related macular degeneration: recommendations from classification of atrophy consensus meetings. Ophthalmology. 2017;124:464–78. https://doi.org/10.1016/J.OPHTHA.2016.12.002.
Sadda SR, Guymer R, Holz FG, Schmitz-Valckenberg S, Curcio CA, Bird AC, et al. Consensus definition for atrophy associated with age-related macular degeneration on OCT: classification of atrophy report 3. Ophthalmology. 2018;125:537–48. https://doi.org/10.1016/J.OPHTHA.2017.09.028.
Pilotto E, Guidolin F, Convento E, Spedicato L, Vujosevic S, Cavarzeran F, et al. Fundus autofluorescence and microperimetry in progressing geographic atrophy secondary to age-related macular degeneration. Br J Ophthalmol. 2013;97:622–6. https://doi.org/10.1136/BJOPHTHALMOL-2012-302633.
Sato T, Suzuki M, Ooto S, Spaide RF. Multimodal imaging findings and multimodal vision testing in neovascular age-related macular degeneration. Retina. 2015;35:1292–302. https://doi.org/10.1097/IAE.0000000000000505.
Forte R, Querques G, Querques L, Leveziel N, Benhamou N, Souied EH. Multimodal evaluation of foveal sparing in patients with geographicatrophy due to age-related macular degeneration. Retina. 2013;33:482–9. https://doi.org/10.1097/IAE.0B013E318276E11E.
Spaide RF. Outer retinal atrophy after regression of subretinal drusenoid deposits as a newly recognized form of late age-related macular degeneration. Retina. 2013;33:1800–8. https://doi.org/10.1097/IAE.0B013E31829C3765.
Saksens NTM, Fleckenstein M, Schmitz-Valckenberg S, Holz FG, den Hollander AI, Keunen JEE, et al. Macular dystrophies mimicking age-related macular degeneration. Prog Retin Eye Res. 2014;39:23–57. https://doi.org/10.1016/J.PRETEYERES.2013.11.001.
Wolf-Schnurrbusch UEK, Wittwer VV, Ghanem R, Niederhaeuser M, Enzmann V, Framme C, et al. Blue-light versus green-light autofluorescence: lesion size of areas of geographic atrophy. Invest Ophthalmol Vis Sci. 2011;52:9497–502. https://doi.org/10.1167/IOVS.11-8346.
Borrelli E, Lei J, Balasubramanian S, Uji A, Cozzi M, Sarao V, et al. Green emission fluorophores in eyes with atrophic age-related macular degeneration: a colour fundus autofluorescence pilot study. Br J Ophthalmol. 2018;102:827–32. https://doi.org/10.1136/BJOPHTHALMOL-2017-310881.
Vujosevic S, Toma C, Sarao V, Veritti D, Brambilla M, Muraca A, et al. Color fundus autofluorescence to determine activity of macular neovascularization in age-related macular degeneration. Transl Vis Sci Technol. 2021;10:1–10. https://doi.org/10.1167/TVST.10.2.33.
Tan ACS, Fleckenstein M, Schmitz-Valckenberg S, Holz FG. Clinical application of multicolor imaging technology. Ophthalmologica. 2016;236:8–18. https://doi.org/10.1159/000446857.
Pang CE, Freund KB. Ghost maculopathy: an artifact on near-infrared reflectance and multicolor imaging masquerading as chorioretinal pathology. Am J Ophthalmol. 2014. https://doi.org/10.1016/J.AJO.2014.03.003.
Lindner M, Böker A, Mauschitz MM, Göbel AP, Fimmers R, Brinkmann CK, et al. Directional kinetics of geographic atrophy progression in age-related macular degeneration with foveal sparing. Ophthalmology. 2015;122:1356–65. https://doi.org/10.1016/J.OPHTHA.2015.03.027.
Wu Z, Ayton LN, Luu CD, Baird PN, Guymer RH. Reticular pseudodrusen in intermediate age-related macular degeneration: prevalence, detection, clinical, environmental, and genetic associations. Invest Ophthalmol Vis Sci. 2016;57:1310–6. https://doi.org/10.1167/IOVS.15-18682.
Mrejen S, Spaide RF. Optical coherence tomography: imaging of the choroid and beyond. Surv Ophthalmol. 2013;58:387–429. https://doi.org/10.1016/j.survophthal.2012.12.001.
Guymer RH, Rosenfeld PJ, Curcio CA, Holz FG, Staurenghi G, Freund KB, et al. Incomplete retinal pigment epithelial and outer retinal atrophy in age-related macular degeneration: classification of atrophy meeting report 4. Ophthalmology. 2020;127:394–409. https://doi.org/10.1016/J.OPHTHA.2019.09.035.
Zhou H, Bacci T, Freund KB, Wang RK. Three-dimensional segmentation and depth-encoded visualization of choroidal vasculature using swept-source optical coherence tomography. Exp Biol Med (Maywood). 2021;246:2238–45. https://doi.org/10.1177/15353702211028540.
Laíns I, Wang JC, Cui Y, Katz R, Vingopoulos F, Staurenghi G, et al. Retinal applications of swept source optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA). Prog Retin Eye Res. 2021. https://doi.org/10.1016/J.PRETEYERES.2021.100951.
Cicinelli MV, Rabiolo A, Sacconi R, Carnevali A, Querques L, Bandello F, et al. Optical coherence tomography angiography in dry age-related macular degeneration. Surv Ophthalmol. 2018;63:236–44. https://doi.org/10.1016/J.SURVOPHTHAL.2017.06.005.
Lupidi M, Cerquaglia A, Chhablani J, Fiore T, Singh SR, Cardillo Piccolino F, et al. Optical coherence tomography angiography in age-related macular degeneration: The game changer. Eur J Ophthalmol. 2018;28:349–57. https://doi.org/10.1177/1120672118766807.
Garrity ST, Sarraf D, Freund KB, Sadda SR. Multimodal imaging of nonneovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2018;59:48–64. https://doi.org/10.1167/IOVS.18-24158.
Fleckenstein M, Mitchell P, Freund KB, Sadda SV, Holz FG, Brittain C, et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125:369–90. https://doi.org/10.1016/J.OPHTHA.2017.08.038.
Kornblau IS, El-Annan JF. Adverse reactions to fluorescein angiography: a comprehensive review of the literature. Surv Ophthalmol. 2019;64:679–93. https://doi.org/10.1016/j.survophthal.2019.02.004.
Kassoff A, Kassoff J, Buehler J, Eglow M, Kaufman F, Mehu M, et al. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–36. https://doi.org/10.1001/ARCHOPHT.119.10.1417.
Chew EY, Clemons TE, SanGiovanni JP, Danis R, Ferris FL, Elman M, et al. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–15. https://doi.org/10.1001/JAMA.2013.4997.
Chew EY, Clemons TE, SanGiovanni JP, Danis RP, Ferris FL, Elman MJ, et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014;132:142–9. https://doi.org/10.1001/JAMAOPHTHALMOL.2013.7376.
Dorin G. Evolution of retinal laser therapy: minimum intensity photocoagulation (MIP). Can the laser heal the retina without harming it? Semin Ophthalmol. 2004;19:62–8. https://doi.org/10.1080/08820530490884173.
Chidlow G, Plunkett M, Casson RJ, Wood JPM. Investigations into localized re-treatment of the retina with a 3-nanosecond laser. Lasers Surg Med. 2016;48:602–15. https://doi.org/10.1002/LSM.22506.
Querques G, Sacconi R, Gelormini F, Borrelli E, Prascina F, Zucchiatti I, et al. Subthreshold laser treatment for reticular pseudodrusen secondary to age-related macular degeneration. Sci Rep. 2021. https://doi.org/10.1038/S41598-021-81810-7.
Guymer RH, Chen FK, Hodgson LAB, Caruso E, Harper CA, Wickremashinghe SS, et al. Subthreshold nanosecond laser in age-related macular degeneration: observational extension study of the LEAD clinical trial. Ophthalmol Retina. 2021;5:1196–203. https://doi.org/10.1016/J.ORET.2021.02.015.
Markowitz SN, Devenyi RG, Munk MR, Croissant CL, Tedford SE, Rückert R, et al. A double-masked, randomized, sham-controlled, single-center study with photobiomodulation for the treatment of dry age-related macular degeneration. Retina. 2020;40:1471–82. https://doi.org/10.1097/IAE.0000000000002632.
Fantaguzzi F, Tombolini B, Servillo A, Zucchiatti I, Sacconi R, Bandello F, et al. Shedding light on photobiomodulation therapy for age-related macular degeneration: a narrative review. Ophthalmol Ther. 2023;12:2903–15. https://doi.org/10.1007/S40123-023-00812-Y.
Datta S, Cano M, Ebrahimi K, Wang L, Handa JT. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog Retin Eye Res. 2017;60:201–18. https://doi.org/10.1016/J.PRETEYERES.2017.03.002.
Xu H, Chen M. Targeting the complement system for the management of retinal inflammatory and degenerative diseases. Eur J Pharmacol. 2016;787:94–104. https://doi.org/10.1016/J.EJPHAR.2016.03.001.
Sarma JV, Ward PA. The complement system. Cell Tissue Res. 2011;343:227–35. https://doi.org/10.1007/S00441-010-1034-0.
Liao DS, Grossi FV, El Mehdi D, Gerber MR, Brown DM, Heier JS, et al. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: a randomized phase 2 trial. Ophthalmology. 2020;127:186–95. https://doi.org/10.1016/J.OPHTHA.2019.07.011.
Jaffe GJ, Westby K, Csaky KG, Monés J, Pearlman JA, Patel SS, et al. C5 inhibitor avacincaptad pegol for geographic atrophy due to age-related macular degeneration: a randomized pivotal phase 2/3 trial. Ophthalmology. 2021;128:576–86. https://doi.org/10.1016/J.OPHTHA.2020.08.027.
Wykoff CC, Rosenfeld PJ, Waheed NK, Singh RP, Ronca N, Slakter JS, et al. Characterizing new-onset exudation in the randomized phase 2 FILLY trial of complement inhibitor pegcetacoplan for geographic atrophy. Ophthalmology. 2021;128:1325–36. https://doi.org/10.1016/J.OPHTHA.2021.02.025.
Nittala MG, Metlapally R, Ip M, Chakravarthy U, Holz FG, Staurenghi G, et al. Association of pegcetacoplan with progression of incomplete retinal pigment epithelium and outer retinal atrophy in age-related macular degeneration: a post hoc analysis of the FILLY randomized clinical trial. JAMA Ophthalmol. 2022;140:243–9. https://doi.org/10.1001/JAMAOPHTHALMOL.2021.6067.
Witkin AJ, Jaffe GJ, Srivastava SK, Davis JL, Kim JE. Retinal vasculitis after intravitreal pegcetacoplan: report from the ASRS research and safety in therapeutics (ReST) committee. J Vitreoretin Dis. 2023;8:9–20. https://doi.org/10.1177/24741264231220224.
Patel SS, Lally DR, Hsu J, Wykoff CC, Eichenbaum D, Heier JS, et al. Avacincaptad pegol for geographic atrophy secondary to age-related macular degeneration: 18-month findings from the GATHER1 trial. Eye (Lond). 2023;37:3551–7. https://doi.org/10.1038/S41433-023-02497-W.
Yehoshua Z, Alexandre De Amorim Garcia Filho C, Nunes RP, Gregori G, Penha FM, Moshfeghi AA, et al. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration: the COMPLETE study. Ophthalmology 2014;121:693–701. https://doi.org/10.1016/J.OPHTHA.2013.09.044.
Holz FG, Sadda SR, Busbee B, Chew EY, Mitchell P, Tufail A, et al. Efficacy and safety of lampalizumab for geographic atrophy due to age-related macular degeneration: chroma and spectri phase 3 randomized clinical trials. JAMA Ophthalmol. 2018;136:666–77. https://doi.org/10.1001/JAMAOPHTHALMOL.2018.1544.
Khanani AM, Hershberger VS, Pieramici DJ, Khurana RN, Brunstein F, Ma L, et al. Phase 1 study of the anti-HtrA1 antibody-binding fragment FHTR2163 in geographic atrophy secondary to age-related macular degeneration. Am J Ophthalmol. 2021;232:49–57. https://doi.org/10.1016/J.AJO.2021.06.017.
Bahrami F, L. Morris D, H. Pourgholami M. Tetracyclines: drugs with huge therapeutic potential. Mini Rev Med Chem 2012;12:44–52. https://doi.org/10.2174/138955712798868977.
Pardue MT, Allen RS. Neuroprotective strategies for retinal disease. Prog Retin Eye Res. 2018;65:50–76. https://doi.org/10.1016/J.PRETEYERES.2018.02.002.
Zhang K, Hopkins JJ, Heier JS, Birch DG, Halperin LS, Albini TA, et al. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc Natl Acad Sci U S A. 2011;108:6241–5. https://doi.org/10.1073/PNAS.1018987108.
Ju WK, Lee MY, Hofmann HD, Kirsch M, Chun MH. Expression of CNTF in Müller cells of the rat retina after pressure-induced ischemia. NeuroReport. 1999;10:419–22. https://doi.org/10.1097/00001756-199902050-00038.
Li R, Wen R, Banzon T, Maminishkis A, Miller SS. CNTF mediates neurotrophic factor secretion and fluid absorption in human retinal pigment epithelium. PLoS One. 2011. https://doi.org/10.1371/JOURNAL.PONE.0023148.
Kuppermann BD, Patel SS, Boyer DS, Augustin AJ, Freeman WR, Kerr KJ, et al. Phase 2 study of the safety and efficacy of brimonidine drug delivery system (BRIMO DDS) generation 1 in patients with geographic atrophy secondary to age-related macular degeneration. Retina. 2021;41:144–55. https://doi.org/10.1097/IAE.0000000000002789.
Freeman WR, Bandello F, Souied E, Guymer RH, Garg SJ, Chen FK, et al. Randomized phase IIb study of brimonidine drug delivery system generation 2 for geographic atrophy in age-related macular degeneration. Ophthalmol Retina. 2023;7:573–85. https://doi.org/10.1016/J.ORET.2023.03.001.
Lin JB, Murakami Y, Miller JW, Vavvas DG. Neuroprotection for age-related macular degeneration. Ophthalmol Sci. 2022. https://doi.org/10.1016/J.XOPS.2022.100192.
Jaffe GJ, Schmitz-Valckenberg S, Boyer D, Heier J, Wolf-Schnurrbusch U, Staurenghi G, et al. Randomized trial to evaluate tandospirone in geographic atrophy secondary to age-related macular degeneration: the GATE study. Am J Ophthalmol. 2015;160:1226–34. https://doi.org/10.1016/J.AJO.2015.08.024.
Heier JS, Ho AC, Samuel MA, Chang T, Riemann CD, Kitchens JW, et al. Safety and efficacy of subretinally administered palucorcel for geographic atrophy of age-related macular degeneration: phase 2b study. Ophthalmol Retina. 2020;4:384–93. https://doi.org/10.1016/J.ORET.2019.11.011.
Brunk UT, Terman A. Lipofuscin: Mechanisms of age-related accumulation and influence on cell function. Free Radic Biol Med. 2002;33:611–9. https://doi.org/10.1016/S0891-5849(02)00959-0.
Rosenfeld PJ, Dugel PU, Holz FG, Heier JS, Pearlman JA, Novack RL, et al. Emixustat hydrochloride for geographic atrophy secondary to age-related macular degeneration: a randomized clinical trial. Ophthalmology. 2018;125:1556–67. https://doi.org/10.1016/J.OPHTHA.2018.03.059.
Mata NL, Lichter JB, Vogel R, Han Y, Bui TV, Singerman LJ. Investigation of oral fenretinide for treatment of geographic atrophy in age-related macular degeneration. Retina. 2013;33:498–507. https://doi.org/10.1097/IAE.0B013E318265801D.
Radu RA, Han Y, Bui TV, Nusinowitz S, Bok D, Lichter J, et al. Reductions in serum vitamin A arrest accumulation of toxic retinal fluorophores: a potential therapy for treatment of lipofuscin-based retinal diseases. Invest Ophthalmol Vis Sci. 2005;46:4393–401. https://doi.org/10.1167/IOVS.05-0820.
Khanani AM, Thomas MJ, Aziz AA, Weng CY, Danzig CJ, Yiu G, et al. Review of gene therapies for age-related macular degeneration. Eye (Lond). 2022;36:303–11. https://doi.org/10.1038/S41433-021-01842-1.
Dreismann AK, McClements ME, Barnard AR, Orhan E, Hughes JP, Lachmann PJ, et al. Functional expression of complement factor I following AAV-mediated gene delivery in the retina of mice and human cells. Gene Ther. 2021;28:265–76. https://doi.org/10.1038/S41434-021-00239-9.
Boyer D, Hu A, Warrow D, Xavier S, Gonzalez V, Lad E, et al. LIGHTSITE III: 13-month efficacy and safety evaluation of multiwavelength photobiomodulation in nonexudative (Dry) age-related macular degeneration using the lumithera valeda light delivery system. Retina. 2024. https://doi.org/10.1097/IAE.0000000000003980.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. No funding or sponsorship was received for the publication of this article.
Author information
Authors and Affiliations
Contributions
Andrea Servillo, Riccardo Sacconi, Gloria Oldoni, Eugenio Barlocci, Beatrice Tombolini, Marco Battista, Federico Fantaguzzi, Federico Rissotto, Cecilia Mularoni, Mariacristina Parravano, Ilaria Zucchiatti, Lea Querques, Francesco Bandello, and Giuseppe Querques all contributed to the conception or design of the work; the acquisition, analysis, and interpretation of data; drafting of the work; and revising it critically for important intellectual content. Each coauthor has seen and agrees with how his or her name is listed.
Corresponding author
Ethics declarations
Conflict of Interest
The authors report there are no competing interests to declare. This report does not contain any personal information that could lead to the identification of the patients. Riccardo Sacconi has the following disclosures: Abbvie, Bayer, Medivis, Novartis, Roche, and Zeiss. Francesco Bandello has the following disclosures: Allergan, Bayer, Boehringer-Ingelheim, Fidia Sooft, Hofmann La Roche, Novartis, Ntc Pharma, Sifi, Thrombogenics, Zeiss. Giuseppe Querques has the following disclosures: Alimera Sciences, Allergan Inc, Amgen, Heidelberg, KBH, LEH Pharma, Lumithera, Novartis, Bayer Shering-Pharma, Sandoz, Sifi, Soof-Fidia, Zeiss. Giuseppe Querques is an Editorial Board member of Ophthalmology and Therapy. Giuseppe Querques was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Andrea Servillo, Gloria Oldoni, Eugenio Barlocci, Beatrice Tombolini, Marco Battista, Federico Fantaguzzi, Federico Rissotto, Cecilia Mularoni, Mariacristina Parravano, Ilaria Zucchiatti, and Lea Querques have nothing to declare.
Ethical Approval
This article is based on previous research; therefore, no approval from the local ethics committee was required.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Servillo, A., Sacconi, R., Oldoni, G. et al. Advancements in Imaging and Therapeutic Options for Dry Age-Related Macular Degeneration and Geographic Atrophy. Ophthalmol Ther (2024). https://doi.org/10.1007/s40123-024-00970-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40123-024-00970-7