Abstract
Background
The benefits and risks of tenecteplase (TNK) versus alteplase (ALT) have recently been assessed in acute ischemic stroke (AIS) patients undergoing mechanical thrombectomy (MT) with diverse results. Due to its high fibrin specificity and lack of excitotoxicity, TNK may have a higher efficacy and safety profile. This study aimed to evaluate the benefits and risks of TNK compared to ALT in AIS patients prior to thrombectomy.
Methods
We systematically searched four key databases, PubMed, Embase, Web of Science and Cochrane Library until January 27, 2024 for clinical studies evaluating the effects of TNK versus ALT in patients with large vessel occlusion undergoing MT. A random-effect meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Results
Ten studies involving 3722 patients receiving TNK (1266 patients) or ALT (2456 patients) were included (age: 69.05 ± 14.95 years; 55.64% male). Compared to ALT-treated patients, TNK-treated patients demonstrated significantly higher rates of early recanalization (odds ratio 2.02, 95%-confidence interval 1.20–3.38, p = 0.008) without increased risk of symptomatic intracerebral hemorrhage (1.06, 0.64–1.76, p = 0.82) or intracerebral hemorrhage (1.21, 0.66–2.25, p = 0.54). TNK-treated patients showed similar rates of functional independence at 90 days (1.13, 0.87–1.46, p = 0.37) as ALT-treated patients, but lower rates of mortality within 90 days (0.65, 0.44–0.96, p = 0.03).
Conclusion
TNK is superior to ALT in achieving early recanalization and is associated with lower mortality within 90 days in AIS patients undergoing MT. Compared with ALT, TNK does not significantly alter functional independence at 90 days, symptomatic intracerebral hemorrhage or intracerebral hemorrhage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Mechanical thrombectomy (MT) has emerged as the treatment of choice for acute ischemic stroke (AIS) with large vessel occlusion, with its effectiveness confirmed in multiple randomized trials [1]. Prior to MT, intravenous thrombolysis is widely recommended. Although some discussion regarding bridging thrombolysis still remains [2,3,4], current evidence increasingly advocates for thrombolytic therapy in MT-eligible patients [5,6,7,8,9]. Alteplase (also: recombinant tissue-plasminogen activator; ALT) is currently the most commonly used and guideline-recommended thrombolytic agent for patients with AIS within 4.5 h after stroke onset [10]. Although the beneficial effects of ALT on recanalization are certain, ALT may induce toxicity and aggravate ischemic brain injury [11, 12], possibly via secondary hemodynamic disturbances [13, 14].
Tenecteplase (TNK) is a promising thrombolytic agent due to its greater fibrin specificity and longer half-life compared with ALT [15, 16]. Although TNK has not been classified as a recommended class I thrombolytic agent in current stroke guidelines [10], increasing numbers of randomized controlled trials (RCTs) confirm the non-inferiority of TNK to ALT [17,18,19,20]. In recent years, studies in AIS started to more systematically compare the efficacy and safety of the two thrombolytic agents [19]. The Australian Stroke Guidelines and the New Zealand Stroke Network recognized TNK, specifically at a dosage of 0.25 mg/kg (maximum 25 mg), as an alternative for ALT in 2019 [21]. By comparing the outcomes of all adult patients treated with ALT in the New Zealand Central Region from January 1 2018 to March 1 2020 with those treated with TNK from March 2 2020 to February 14 2021, it was found that the functional independence at 90 days after switching from ALT to TNK was improved and there was no increase in symptomatic intracranial hemorrhage (ICH, sICH) [21]. In a recent large RCT trial, TNK was non-inferior to ALT in patients with AIS who were eligible for standard intravenous thrombolysis but ineligible for or refused MT [18].
Despite much evidence supporting the use of TNK for thrombolysis in ischemic stroke, there has been limited data on the efficacy and safety of TNK vs. ALT in AIS patients undergoing MT [18, 21, 22]. In a multicenter RCT, the administration of TNK prior to MT was associated with better early recanalization and functional outcome compared with ALT in AIS patients who were treated within 4.5 h of symptom onset [19]. ICH, sICH and mortality in the latter study did not differ between groups. Some studies made similar observations [23,24,25,26], whereas other studies could not confirm these findings [16, 27,28,29,30]. To provide a comprehensive analysis and to deepen the understanding of the benefits and risks of TNK vs. ALT, we performed a meta-analysis comparing the efficacy and safety of both thrombolytics as bridging agents for MT.
Methods
Search strategy
This meta-analysis follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (see Supplementary File 1). We systematically searched four key databases, PubMed, Embase, Web of Science and the Cochrane Library up to January 27 2024. We conducted a comprehensive search of titles and abstracts using a precise combination of keywords and MeSH terms. These included “Alteplase”, “Tenecteplase”, “Ischemic Stroke”, “Ischaemic Stroke”, “Cerebral Infarction”, “Middle Cerebral Artery Infarction”, “Middle Cerebral Artery Stroke” and “Thrombectomy”, by using the “AND” and “OR” logical operators in conjunction with each other (see Supplementary File 2).
Inclusion criteria and exclusion criteria
The inclusion criteria were: (1) RCT or cohort study or observational study, (2) conducted in AIS patients over 18 years of age who underwent MT, (3) comparison groups include TNK and ALT groups, (4) study includes indicators of efficacy or safety, (5) study published as full-text manuscript or conference abstract in English, and (6) sample size > 5 per group. Exclusion criteria were: Review, case series, meta-analysis, protocol, letter, editorial, basic research in cells or animals, duplicate database sources, lack of specific numbers for any indicator observed.
Data extraction
Two experienced researchers (NW and TRD) independently screened the articles and extracted data of the eligible studies, including publication types, study design, country and occluded site, total number of patients, age, sex, thrombolytic dose, outcomes, covariates and main results. Any discrepancies were resolved by discussion among all authors. Primary outcomes were early successful recanalization, defined as successful vascular reperfusion (more than 50% restoration of blood flow in the involved territory) observed at the time of the initial angiographic assessment (modified Thrombolysis in Cerebral Infarction (mTICI) or expanded Thrombolysis in Cerebral Infarction (eTICI) score 2b-3), and safety indicators included sICH and ICH. ICH was defined as any type of ICH assessed by imaging or hemorrhage-related rating scale, sICH was defined as ICH that was temporally related to and directly contributing to the deterioration of the neurological condition [30]. Secondary outcomes were functional independence at 90 days defined as mRS score of 0–2 and all-cause mortality within 90 days.
Quality assessment
Considering that most of the included studies were non-randomized, we used the Newcastle–Ottawa scale (NOS) for cohort studies to assess the quality of the included studies. Two investigators (NW and TRD) independently assessed the included studies in three ways: cohort selection, cohort comparability, and assessment of outcomes [31]. Any discrepancies were resolved by discussion among all authors. A study with 3 or fewer, 4 to 6, or 7 to 9 stars can be considered low quality, moderate quality, or high quality respectively. Additionally, funnel plots were used to detect potential publication bias and asymmetry was considered present if p < 0.10 (Egger’s test) [32].
Statistical analysis
In this meta-analysis, outcome data were analyzed using random-effects models. We calculated odds ratios (OR) with corresponding 95% confidence intervals (CI) as measures of effect size. Inter-study heterogeneity was assessed via the I2 statistic with thresholds set at ≤ 25% (possible/unclear), > 25% to ≤ 50% (low), > 50% to ≤ 75% (moderate), and > 75% (high) heterogeneity. To address the presence of heterogeneity in treatment outcomes, we conducted subgroup analyses based on several variables: the nature of the trial (randomized vs. non-randomized), its design (double-blind vs. non-blind), its approach (prospective vs. retrospective), the infarction location (anterior circulation vs. posterior circulation vs. anterior + posterior circulation), NOS study quality (high vs. medium) and review system (peer-review vs. no peer-review). Apart from making comparisons between subgroups, we also performed a pooled analysis of only peer-reviewed articles in order to compare them with the overall pooled results, since peer-reviewed articles are considered to have a more rigorous quality review. Additionally, we performed sensitivity analyses excluding single studies to further explore inter-study heterogeneity. Statistical analyses were performed using Review Manager software (RevMan, version 5.4).
Results
Literature retrieval
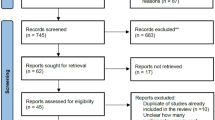
We retrieved a total of 2076 publications, including articles and conference abstracts, from the four key databases: PubMed (335), EMBASE (1461), Web of Science (238), and the Cochrane Library (143). Of these, 394 articles were duplicates and the remaining 1783 articles were screened by title, abstract or full text. After reviewing all the screened literature, a total of ten studies were suitable for inclusion in this meta-analysis. The selection process is summarized in Fig. 1, which provides a detailed flowchart of the literature screening methodology.
Characteristics of included studies
This meta-analysis included ten studies [16, 19, 23,24,25,26,27,28,29,30] with a total of 3722 AIS patients who received MT. Of these, 1266 patients were treated with TNK and 2456 with ALT for thrombolysis. Table 1 shows characteristics of the included studies. Regarding study design, this meta-analysis included two RCTs [19, 30] and eight non-randomized, non-blind cohort studies [16, 23,24,25,26,27,28,29]. Eight used a prospective approach [16, 19, 23,24,25, 27, 28, 30], while two were based on a retrospective approach [26, 29]. Regarding review system, six were peer-reviewed studies [19, 23,24,25, 28, 30] and four were non-peer-reviewed studies [16, 26, 27, 29]. The largest sample was included in the study by Checkouri et al. (n = 1865) [28], while Marín et al. [29] had the smallest (n = 18). Six studies [16, 19, 23, 26, 27, 29] included both anterior and posterior circulation occlusion, three studies [25, 28, 30] focused on anterior circulation occlusion, and one [24] on posterior circulation occlusion. Thrombolytic doses were highly consistent across studies, using intravenous TNK (0.25 mg/kg) and ALT (0.9 mg/kg). Of note, in one study [24] a broader dose application was introduced in TNK treatment, allowing for 0.25 mg/kg or 0.4 mg/kg.
Quality assessment
Eight studies [16, 19, 23,24,25, 27, 28, 30] were classified as high quality (NOS scores 7–9), while two studies [26, 29] were medium quality (NOS scores 4–6). Detailed NOS scores for each study are shown in Table 2.
Risk of bias
Funnel plots (Supplementary File 3) suggested no significant publication bias for the outcome of early recanalization, sICH, ICH and functional independence at 90 days and mortality within 90 days (all p > 0.1, Egger’s test).
Overall analysis of primary outcomes
Nine studies evaluated early recanalization, eight investigated sICH rates and six evaluated ICH rates, as shown in Fig. 2. TNK treatment achieved a significantly higher early recanalization rate (20.2%) compared to ALT (13.1%) (weighted OR 2.02; 95%-CI 1.20–3.38, p = 0.02, Fig. 2A), with moderate heterogeneity (I2 = 73%, Fig. 2A). However, the rate of sICH (TNK + MT 4.8%, ALT + MT 7.0%; 1.06, 0.64–1.76, p = 0.82, Fig. 2B) and ICH (TNK + MT 29.4%, ALT + MT 33.6%; 1.21, 0.66–2.25, p = 0.54, Fig. 2C) was not significantly different, with no and low heterogeneity (I2 = 0% and 41%, respectively).
Overall analysis of secondary outcomes
Six studies analyzed functional independence at 90 days and five analyzed mortality within 90 days. TNK treatment exhibited comparable functional independence at 90 days to ALT (1.13, 0.87–1.46, p = 0.37, Fig. 3A), with no heterogeneity (I2 = 0%, Fig. 3A). Yet, TNK treatment was associated with lower mortality within 90 days (0.65, 0.44–0.96, p = 0.03, Fig. 3B), with no heterogeneity across the studies (I2 = 0%, Fig. 3B).
Subgroup analyses
Regarding early recanalization, our findings indicate a uniform treatment effect across all subgroups. The trend of the results consistently favored TNK treatment across all subgroups, although the ORs for TNK treatment were not significant in randomized trials, double-blind studies and patients with occlusion of the intracranial anterior circulation (Fig. 4).
Subgroup analyses for primary outcomes. A Early recanalization, B sICH and C ICH in the pooled data analysis stratified by study characteristics. Data are odds ratios (ORs) with 95% confidence intervals (CIs). n represents the number of events analyzed in the group, while N represents the total patient size of the group
For the outcome of ICH, our analysis did not reveal any treatment effect heterogeneity among the subgroups study approach, occluded site, and NOS study quality, and review system. Notably, the comparison between double-blind randomized trials and non-randomized, non-blind trials showed a significant difference in ICH rates. In double-blind randomized trials, ICH rates did not significantly differ between TNK and ALT treatments, whereas in non-randomized, non-blind trials, the ICH rate was significantly higher for ALT compared to TNK (Fig. 4).
Regarding mortality within 90 days, our analysis did not reveal any treatment effect heterogeneity across all examined subgroups except for the subgroup concerning publication status. Overall, the TNK group exhibited lower mortality within 90 days, although differences in mortality were not significant among non-peer-reviewed studies (Fig. 5).
Subgroup analyses for secondary outcomes. A Functional independence at 90 days and B mortality within 90 days in the pooled data analysis stratified by study characteristics. Data are odds ratios (ORs) with 95% confidence intervals (CIs). n represents the number of events analysed in the group, while N represents the total patient size the group
For the outcomes of sICH and functional independence at 90 days, our analyses did not reveal any treatment effect heterogeneity across all examined subgroups, as represented in Figs. 4 and 5, respectively.
When we performed a meta-analyses including only data from published peer-reviewed articles (that is excluding conference abstracts) and compared the results with the overall meta-analysis results included all ten studies (peer-reviewed articles plus conference abstracts without peer-review), we obtained similar pooled results for the primary and secondary outcomes, as shown in Supplementary File 4 and Supplementary File 5, with the heterogeneity of results on early recanalization and ICH being slightly increased. TNK treatment was again associated with a higher early recanalization rate compared to ALT treatment (1.98; 1.00–3.91, p = 0.05, Supplementary File 4A) and reduced mortality within 90 days (0.66, 0.44–0.97, p = 0.04, Supplementary File 5B). Compared to ALT, TNK exhibited comparable rates of sICH (1.15, 0.66–2.02, p = 0.62, Supplementary File 4B), ICH (1.16, 0.58–2.31, p = 0.68, Supplementary File 4C) and functional independence at 90 days (1.11, 0.86–1.45, p = 0.42, Supplementary File 5A).
Sensitivity analyses
Considering inter-study heterogeneity, we subsequently conducted sensitivity analyses by excluding single studies to evaluate the individual impact of each study on the pooled overall effect size of early recanalization and ICH. In these analyses, we found that the pooled effects for early recanalization remained stable following single study removal from analyses.
While pooled analyses showed no statistically significant difference in ICH rates between TNK and ALT treatment, the study by Bala et al. [30] was the main source of heterogeneity affecting the ICH results: Excluding this study significantly reduced heterogeneity from 41 to 0%, and altered the results from no statistically significant difference between the two groups to a significantly higher ICH rate in the ALT group than in the TNK group. This may be due to the fact that the hemorrhage rates in the TNK and ALT groups in the study by Bala et al. were comparable and accounted for 22.5% in the weighted analyses, partially offsetting the difference between the two groups. It is noteworthy that 31.2% of patients in the study by Bala et al. [30] were treated for carotid artery lesions by stenting during thrombolysis bridging to MT, which could also potentially affect the ICH rate between the two groups. The authors recommended that carotid stenting timing needs to be further investigated (see Supplementary File 6).
Discussion
This meta-analysis investigated the efficacy and safety of TNK vs. ALT in patients with large vessel occlusion undergoing MT, aiming to provide more comprehensive information for clinical decision-making. Our results revealed that intravenous TNK more frequently achieved successful than ALT, which was the primary efficacy outcome, without increasing the risk of sICH and ICH, which were the primary safety outcomes. Regarding the secondary outcomes, TNK was associated with reduced mortality within 90 days and exhibited comparable functional independence at 90 days. The enhanced recanalization potentially explains the reduced mortality, albeit direct impacts of the improved recanalization on sICH, ICH and functional independence were not apparent. To ensure uniformity in the evaluation process, we applied the NOS assessment used for cohort studies [31], which might have led to a degree of underestimation of quality for the retrospective studies [26, 29]. Overall, all the included literature was of medium to high quality.
For the outcome of early recanalization, subgroup analyses showed a high degree of consistency across included studies. Sensitivity analyses which excluded single studies, further affirmed the robustness of the meta-analysis results for the outcome of early recanalization. However, the study by Bala et al. [30] reported an observation different from the other studies with a higher early recanalization rate in the ALT than in the TNK cohort, although this did not reach statistical significance (18.6% vs. 8%, p = 0.21). This deviation may be attributed to the concurrent administration of emergency carotid stenting. The higher fibrin specificity and longer plasma half-life of TNK compared to ALT [11] might provide a better thrombolytic capacity and thus improve early recanalization. Besides, TNK offers the advantage of single-bolus dosing [33], which not only facilitates a more timely and convenient administration but may also accelerate the onset of recanalization compared to ALT. ALT, in contrast, requires a 1–3-h infusion period [33], potentially delaying recanalization. Such differences in administration speed could explain the delay in recanalization rates observed with ALT when compared to TNK, underscoring the practical benefits of TNK in clinical settings.
Evidence from previous meta-analyses in AIS patients consistently suggests that the efficacy and safety of TNK is not inferior to ALT [22, 34,35,36]. However, given the substantial impact of MT on AIS outcome [2], many studies have intentionally excluded patients undergoing MT from their study cohorts [22, 34,35,36]. As a result, patients receiving MT were underrepresented in meta-analyses. Nonetheless, bridging thrombolysis is a common treatment in AIS. Therefore, it is clinically important to assess the efficacy and safety of the use of TNK and ALT in MT patients. Our meta-analysis, despite detecting no significant impact on functional independence at 90 days, showed that TNK treatment was associated with a lower mortality within 90 days than ALT treatment. The lower mortality rate observed in the TNK group compared to the ALT group can be attributed primarily to higher recanalization rates with similar risks of hemorrhage. Additional RCTs will need to replicate this finding.
Although the pooled analysis revealed no significant difference in rates of sICH and overall ICH following thrombolysis with TNK and ALT, the subgroup analysis for ICH within four non-randomized trials demonstrated a higher ICH incidence in the ALT than TNK group. Several factors could influence the risk of brain hemorrhage. Firstly, TNK's higher fibrin specificity may reduce hemorrhage risks by limiting circulating plasminogen activation and degradation of fibrinogen [15, 33]. Additionally, the absence of data on antiplatelet use during or after MT could also impact ICH rates. As a potential mechanism, ALT might elevate pro-inflammatory signals in the ischemic brain, thereby contributing to ICH via blood–brain barrier disruption [37]. The occurrence of ICH remains a significant concern in ALT treated AIS patients [38]. As a genetically modified ALT variant, TNK represents a potential alternative [39]. The mechanisms underlying the safety of TNK deserves attention in AIS patients undergoing MT.
Besides TNK, reteplase has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of ST-segment elevation myocardial infarction [40]. Although TNK has been increasingly studied in ischemic stroke, studies of the safety and efficacy of reteplase in ischemic stroke remain limited. Compared with TNK and ALT, the fibrinolytic speed of reteplase is slower, and the risk of ICH may be lower [41]. The results of a recently published phase II RCT showed similar mortality, sICH and functional outcome at 90 days in AIS patients for reteplase and ALT [42], but the sample size was small. Further studies on the benefits and risks of reteplase in larger trials are needed.
Limitations
This meta-analysis has some limitations: First, to get the most comprehensive information, conference abstracts were included in this meta-analysis because there were limited peer-reviewed studies available. This approach has a major advantage, since it reduces publication bias, which favors studies with beneficial over those with neutral or detrimental findings to be published. However, we also performed a meta-analysis restricted to peer-reviewed articles, which confirmed that TNK was not inferior in efficacy and safety to ALT. Second, while nine of the included studies reported consistent TNK doses (0.25 mg/kg), there is still a possibility of some heterogeneity related to drug dosing, as one study [24] included a higher TNK dose (0.4 mg/kg). Third, when comparing the recanalization rates between the TNK and rt-PA groups, it is noteworthy that although the TNK group still exhibited a higher recanalization rate in RCTs and studies on anterior circulation strokes, it did not reach statistical significance. The absence of significant differences in the RCTs can be attributed to the small number of RCTs, which had limited sample sizes. Only one observational study was specifically performed in anterior circulation strokes. Additional RCTs will have to provide more definitive insights into the comparative effects of TNK vs. rt-PA in AIS patients eligible for MT.
Conclusions
In AIS patients undergoing MT, TNK demonstrates superior ability over ALT in achieving early recanalization, which is associated with lower mortality within 90 days. Compared with ALT, TNK does not significantly alter functional independence at 90 days, sICH rate, or ICH rate when administered as bridging agent prior to MT.
Data availability
The data included into this study are accessible to researchers upon reasonable request.
References
Jadhav AP, Desai SM, Jovin TG (2021) Indications for mechanical thrombectomy for acute ischemic stroke: current guidelines and beyond. Neurology 97(20 Suppl 2):S126-s136. https://doi.org/10.1212/wnl.0000000000012801
Jang KM, Choi HH, Jang MJ, Cho YD (2022) Direct endovascular thrombectomy alone vs. bridging thrombolysis for patients with acute ischemic stroke: a meta-analysis. Clin Neuroradiol 32(3):603–613. https://doi.org/10.1007/s00062-021-01116-z
Sattari SA, Antar A, Sattari AR et al (2023) Endovascular thrombectomy versus endovascular thrombectomy preceded by intravenous thrombolysis: a systematic review and meta-analysis. World Neurosurg 177:39–58. https://doi.org/10.1016/j.wneu.2023.05.033
Ryu JC, Kwon B, Song Y et al (2024) Effect of intravenous thrombolysis prior to mechanical thrombectomy according to the location of M1 occlusion. J Stroke 26(1):75–86. https://doi.org/10.5853/jos.2023.01529
Maïer B, Finitsis S, Mazighi M et al (2023) Thrombectomy with or without intravenous thrombolytics in basilar artery occlusion. Ann Neurol 94(3):596–604. https://doi.org/10.1002/ana.26720
D’anna L, Foschi M, Russo M et al (2023) Endovascular thrombectomy with or without intravenous thrombolysis for anterior circulation large vessel occlusion in the imperial college London thrombectomy registry. J Clin Med. https://doi.org/10.3390/jcm12031150
Fan L, Zang L, Liu X et al (2021) Outcomes of mechanical thrombectomy with pre-intravenous thrombolysis: a systematic review and meta-analysis. J Neurol 268(7):2420–2428. https://doi.org/10.1007/s00415-020-09778-4
Cronin CA (2023) Acute ischemic stroke: don’t skip the thrombolytics before transfer for thrombectomy. Neurology 100(14):643–644. https://doi.org/10.1212/WNL.0000000000206841
Fischer U, Kaesmacher J, Strbian D et al (2022) Thrombectomy alone versus intravenous alteplase plus thrombectomy in patients with stroke: an open-label, blinded-outcome, randomised non-inferiority trial. Lancet 400(10346):104–115. https://doi.org/10.1016/s0140-6736(22)00537-2
Berge E, Whiteley W, Audebert H et al (2021) European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. https://doi.org/10.1177/2396987321989865
Wang YF, Tsirka SE, Strickland S et al (1998) Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med 4(2):228–231. https://doi.org/10.1038/nm0298-228
Tsirka SE, Gualandris A, Amaral DG, Strickland S (1995) Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature 377(6547):340–344. https://doi.org/10.1038/377340a0
Kilic E, Kilic U, Matter CM et al (2005) Aggravation of focal cerebral ischemia by tissue plasminogen activator is reversed by 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor but does not depend on endothelial NO synthase. Stroke 36(2):332–336. https://doi.org/10.1161/01.STR.0000152273.24063.f7
Kilic E, Bähr M, Hermann DM (2001) Effects of recombinant tissue plasminogen activator after intraluminal thread occlusion in mice: role of hemodynamic alterations. Stroke 32(11):2641–2647. https://doi.org/10.1161/hs1101.097381
Warach SJ, Dula AN, Milling TJ (2020) Tenecteplase thrombolysis for acute ischemic stroke. Stroke 51(11):3440–3451. https://doi.org/10.1161/STROKEAHA.120.029749
Cruz Culebras A, Lorenzo Barreto P, Garcia Madrona S et al (2021) Comparative safety and efficacy of tenecteplase versus alteplase in acute ischemic stroke before thrombectomy. Eur Stroke J 6(1 SUPPL):309. https://doi.org/10.1177/23969873211034932
Menon BK, Buck BH, Singh N et al (2022) Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet 400(10347):161–169. https://doi.org/10.1016/s0140-6736(22)01054-6
Wang YJ, Li SY, Pan YS et al (2023) Tenecteplase versus alteplase in acute ischaemic cerebrovascular events (TRACE-2): a phase 3, multicentre, open-label, randomised controlled, non-inferioritytrial. Lancet 401(10377):645–654. https://doi.org/10.1016/s0140-6736(22)02600-9
Campbell BCV, Mitchell PJ, Churilov L et al (2018) Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med 378(17):1573–1582. https://doi.org/10.1056/NEJMoa1716405
Li S, Pan Y, Wang Z et al (2022) Safety and efficacy of tenecteplase versus alteplase in patients with acute ischaemic stroke (TRACE): a multicentre, randomised, open label, blinded-endpoint (PROBE) controlled phase II study. Stroke Vasc Neurol 7(1):47–53. https://doi.org/10.1136/svn-2021-000978
Mahawish K, Gommans J, Kleinig T et al (2021) Switching to tenecteplase for stroke thrombolysis: real-world experience and outcomes in a regional stroke network. Stroke 52(10):e590–e593. https://doi.org/10.1161/strokeaha.121.035931
Ma P, Zhang Y, Chang L et al (2022) Tenecteplase vs. alteplase for the treatment of patients with acute ischemic stroke: a systematic review and meta-analysis. J Neurol 269(10):5262–5271. https://doi.org/10.1007/s00415-022-11242-4
Hendrix P, Collins MK, Griessenauer CJ et al (2023) Tenecteplase versus alteplase before mechanical thrombectomy: experience from a US healthcare system undergoing a system-wide transition of primary thrombolytic. J NeuroInterventional Surg 15(e2):E277–E281. https://doi.org/10.1136/jnis-2022-019662
Alemseged F, Ng FC, Williams C et al (2021) Tenecteplase vs alteplase before endovascular therapy in basilar artery occlusion. Neurology 96(9):E1272–E1277. https://doi.org/10.1212/WNL.0000000000011520
Marnat G, Lapergue B, Gory B et al (2023) Intravenous thrombolysis with tenecteplase versus alteplase combined with endovascular treatment of anterior circulation tandem occlusions: a pooled analysis of ETIS and TETRIS. Eur Stroke J. https://doi.org/10.1177/23969873231206894
Vetra J, Teivane A, Jurjans K et al (2022) The comparison of revascularization rate in stroke with large vessel oclussion using tenectaplase vs alteplase. Eur Stroke J 7(1 SUPPL):167–168. https://doi.org/10.1177/23969873221087559
Ainz Gomez L, Baena P, Cabezas Rodríguez JA et al (2021) Shorter times to recanalization using tnk compared to RTPA: real-life experience analysis. Eur Stroke J 6(1 SUPPL):129–130. https://doi.org/10.1177/23969873211034932
Checkouri T, Gerschenfeld G, Seners P et al (2023) Early recanalization among patients undergoing bridging therapy with tenecteplase or alteplase. Stroke 54(10):2491–2499. https://doi.org/10.1161/strokeaha.123.042691
Marín AS, Madrona SG, Barreto PL et al (2023) Safety of tenecteplase vs alteplase in stent implantation in the acute ischemic stroke. Eur Stroke J 8(2):500. https://doi.org/10.1177/23969873231169660
Bala F, Almekhlafi M, Singh N et al (2023) Safety and efficacy of tenecteplase versus alteplase in stroke patients with carotid tandem lesions: results from the AcT trial. Int J Stroke. https://doi.org/10.1177/17474930231205208
Okekunle AP, Jones S, Adeniji O et al (2023) Stroke in Africa: a systematic review and meta-analysis of the incidence and case-fatality rates. Int J Stroke 18(6):634–644. https://doi.org/10.1177/17474930221147164
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634. https://doi.org/10.1136/bmj.315.7109.629
Van De Werf FJ (1999) The ideal fibrinolytic: can drug design improve clinical results? Eur Heart J 20(20):1452–1458. https://doi.org/10.1053/euhj.1999.1659
Burgos AM, Saver JL (2019) Evidence that tenecteplase is noninferior to alteplase for acute ischemic stroke: meta-analysis of 5 randomized trials. Stroke 50(8):2156–2162. https://doi.org/10.1161/strokeaha.119.025080
Katsanos AH, Psychogios K, Turc G et al (2022) Off-label use of tenecteplase for the treatment of acute ischemic stroke: a systematic review and meta-analysis. JAMA Netw Open 5(3):e224506. https://doi.org/10.1001/jamanetworkopen.2022.4506
Rose D, Cavalier A, Kam W et al (2023) Complications of intravenous tenecteplase versus alteplase for the treatment of acute ischemic stroke: a systematic review and meta-analysis. Stroke 54(5):1192–1204. https://doi.org/10.1161/strokeaha.122.042335
Cheng G, Zhao W, Xin Y et al (2021) Effects of ML351 and tissue plasminogen activator combination therapy in a rat model of focal embolic stroke. J Neurochem 157(3):586–598. https://doi.org/10.1111/jnc.15308
Qiu L, Cai Y, Geng Y et al (2022) Mesenchymal stem cell-derived extracellular vesicles attenuate tPA-induced blood-brain barrier disruption in murine ischemic stroke models. Acta Biomater 154:424–442. https://doi.org/10.1016/j.actbio.2022.10.022
Alemseged F, Campbell BCV (2021) Tenecteplase thrombolysis in posterior circulation stroke. Front Neurol 12:678887. https://doi.org/10.3389/fneur.2021.678887
Rashedi S, Greason CM, Sadeghipour P et al (2024) Fibrinolytic agents in thromboembolic diseases: historical perspectives and approved indications. Semin Thromb Hemost. https://doi.org/10.1055/s-0044-1781451
Yang Y, Gu B, Xu XY (2023) In silico study of different thrombolytic agents for fibrinolysis in acute ischemic stroke. Pharmaceutics. https://doi.org/10.3390/pharmaceutics15030797
Li S, Wang X, Jin A et al (2024) Safety and efficacy of reteplase versus alteplase for acute ischemic stroke: a phase 2 randomized controlled trial. Stroke 55(2):366–375. https://doi.org/10.1161/strokeaha.123.045193
Acknowledgements
The authors thank Drs. Antonio Cruz-Culebras (University Hospital Ramón y Cajal, Madrid, Spain), Francisco Moniche (University Hospital Virgen del Rocio, Seville, Spain), Jānis Vētra (Riga Stradiņš University, Riga, Latvia), Leire Ainz Gomez (University Hospital Virgen del Rocio, Seville, Spain) and Philipp Hendrix (Geisinger Health System, Danville, USA) for generously providing data from their studies for this meta-analysis.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
NW and DMH designed the study. NW and TRD reviewed the relevant articles to select eligible studies and collected data. NW and JG performed the statistical analyses, evaluated the study quality and risk of bias. DMH and JG gave important advice on the interpretation of the data. NW drafted the manuscript and prepared the figures. All authors corrected it and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, N., Doeppner, T.R., Hermann, D.M. et al. Efficacy and safety of intravenous tenecteplase compared to alteplase before mechanical thrombectomy in acute ischemic stroke: a meta-analysis. J Neurol (2024). https://doi.org/10.1007/s00415-024-12445-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00415-024-12445-7