Abstract
ELEVATE (Study 410; NCT03288129) is the first prospective, multicenter, open-label, Phase IV study of perampanel as monotherapy or first adjunctive therapy in patients aged ≥ 4 years with focal-onset seizures or generalized tonic–clonic seizures in the United States. The study included Screening, Titration (≤ 13 weeks), Maintenance (39 weeks), and Follow-up (4 weeks) Periods. During Titration, perampanel was initiated at 2 mg/day and up-titrated to 4 mg/day at Week 3. Depending on response and tolerability, optional up-titrations to a maximum of 12 mg/day occurred. The primary endpoint was retention rate; additional endpoints included seizure-freedom rate, 50% responder rate, and incidence of treatment-emergent adverse events (TEAEs). At baseline, 10 (18.5%) patients were assigned to the monotherapy group and 44 (81.5%) patients to the first adjunctive therapy group. However, due to the addition of an anti-seizure medication along with perampanel on the first day of treatment, one patient was excluded from the monotherapy subgroup analyses. The mean perampanel exposure duration was 39.8 weeks and 32 (59.3%) patients completed the study. Retention rate at 12 months (or study completion) was 63.0% (monotherapy, 77.8%; first adjunctive therapy, 59.1%). Seizure-freedom rate during the Maintenance Period was 32.7% (monotherapy, 44.4%; first adjunctive therapy, 29.5%) and the 50% responder rate was 78.7% (monotherapy, 85.7%; first adjunctive therapy, 76.9%). TEAEs and serious TEAEs were reported by 88.9% (n = 48/54) and 7.4% (n = 4/54) of patients, respectively. Overall, the efficacy and safety of perampanel as monotherapy or first adjunctive therapy support the use of perampanel as early-line treatment for epilepsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early response to anti-seizure medication (ASM) is associated with improved prognosis in patients with epilepsy [1]. The failure to control seizures with two or more ASMs (administered as monotherapy or adjunctive therapy) reduces the likelihood of achieving seizure control with subsequent ASMs [2, 3]; however, polytherapy can increase treatment-emergent adverse events (TEAEs), drug interactions between ASMs, psychiatric and behavioral side effects (such as depression), and noncompliance [4,5,6]. In addition, polytherapy may increase seizure frequency and therefore impact patient quality of life (QoL) [7]. There are limited data regarding the efficacy or effectiveness of ASMs administered as monotherapy in adults with focal-onset seizures (FOS) or generalized tonic–clonic seizures (GTCS) [8]. To improve the QoL and prognosis for patients with FOS and/or GTCS, further research is required to explore the efficacy of ASMs administered as monotherapy or early adjunctive therapy.
Perampanel, a selective, non-competitive α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist, is a once-daily oral ASM for FOS and GTCS [9, 10]. In the United States (US), perampanel is approved for the treatment of FOS (adjunctive and monotherapy), with or without focal to bilateral tonic–clonic seizures (FBTCS), in patients aged ≥ 4 years, and as adjunctive treatment of GTCS in patients aged ≥ 12 years [9]. The approval of perampanel as monotherapy was based on the US Food and Drug Administration’s regulatory pathway for monotherapy use, whereby efficacy and safety data were extrapolated from three Phase III studies of adjunctive perampanel in patients with treatment-resistant FOS, with or without FBTCS [11,12,13]. In a recent pooled analysis of data from 44 real-world studies of perampanel (PERMIT), it was found that fewer previous ASMs or concomitant ASMs at baseline were associated with improved seizure control and reduced likelihood of adverse events (AEs) [14]. In addition, emerging data from open-label and real-world studies investigating the efficacy and safety of perampanel as monotherapy [15,16,17,18,19] or first adjunctive treatment [19,20,21] support its use as an early-line treatment for patients who are treatment naïve or have less refractory epilepsy. However, further research regarding the clinical use of perampanel as monotherapy or first adjunctive therapy is required to improve the management of epilepsy in patients.
To address this, we report the efficacy and safety results from ELEVATE (Study 410; NCT03288129), the first prospective study of perampanel administered as monotherapy or first adjunctive therapy in a clinical setting in patients aged ≥ 4 years with FOS, with or without FBTCS, or GTCS in the US.
Methods
Study design
ELEVATE was a multicenter, open-label, Phase IV study of oral perampanel (tablets) as monotherapy or first adjunctive therapy in patients aged ≥ 4 years with FOS, with or without FBTCS, or with GTCS conducted at 14 sites in the US between August 23, 2017 and April 27, 2021. The study consisted of a Screening Period (up to 6 weeks prior to the first perampanel dose), Titration Period (up to 13 weeks), Maintenance Period (39 weeks), and Follow-up Period (4 weeks; Fig. 1).
During the Titration Period, perampanel was initiated at 2 mg/day and up-titrated to 4 mg/day at Week 3; additional up-titrations (to a maximum of 12 mg/day in 2-mg increments at intervals of ≥ 2 weeks) were optional based on clinical response and tolerability. Patients who concomitantly received enzyme-inducing ASMs (phenytoin, carbamazepine, oxcarbazepine, or eslicarbazepine) could be up-titrated in increments of 2 mg at 1-week intervals. During the Maintenance Period, patients continued to receive the perampanel dose that was administered at the end of the Titration Period; perampanel dose could be adjusted (to a maximum of 12 mg/day) during the Maintenance Period depending on clinical response and tolerability. Any patients unable to tolerate 4 mg/day by the end of the Titration Period or during the Maintenance Period were discontinued from the study.
Patients
Patients were eligible for the study if they had experienced either two unprovoked (or reflex) seizures > 24 h apart or had experienced one unprovoked (or reflex) seizure with electroencephalogram evidence of seizures, and were either treatment naïve or required adjunctive therapy following failure to control seizures with ASM monotherapy. It was recommended that patients who received perampanel as first adjunctive therapy be on a stable dose of one concomitant ASM for ≥ 8 weeks prior to perampanel initiation and throughout the 13-week Titration Period. Dose and administration of the concomitant ASM could be modified during the Maintenance Period as per the investigator’s judgment. The main exclusion criteria were previous or current treatment with perampanel, presence or history of Lennox-Gastaut syndrome, and presence of non-motor focal aware seizures only.
Efficacy assessments
The primary endpoint of the study was retention rate at 3, 6, 9, and 12 months (or study completion) in the Safety Analysis Set (SAS), defined as all patients who received at least one dose of perampanel and had at least one post-dose safety assessment. The retention rate refers to the number of patients who remained on perampanel (either as monotherapy or first adjunctive therapy) at the above specified time-points. The secondary endpoints included seizure freedom (proportion of patients who achieve seizure-free status for FOS, FBTCS, and GTCS during the Maintenance Period) in the Full Analysis Set (FAS), defined as patients who received at least one dose of perampanel and had at least one post-dose seizure measurement. Exploratory endpoints included 50% responder rate (patients who have ≥ 50% reduction in seizure frequency relative to baseline) during the Maintenance Period and median percent change in seizure frequency per 28 days during the Titration and Maintenance Periods relative to baseline; assessed in a subset of the FAS with sufficient baseline seizure frequency data. Seizure frequency was based on both prospective counts (seizure counts at baseline and thereafter in seizure diaries) and retrospective counts (seizure counts during the 12-week period prior to first perampanel dose). Last observation carried forward (LOCF) type imputation was employed to handle missing data for seizure-related efficacy endpoints. Up to 2 months of titration data were used for imputation for patients who dropped out early in the Maintenance Period.
Safety and tolerability assessments
Secondary endpoints included the assessment of the incidence of TEAEs, treatment-related TEAEs, serious TEAEs, TEAEs leading to perampanel dose adjustment, and most common TEAEs. In addition, discontinuation from treatment, prior and concomitant ASM, cognition, suicidality or depressive symptoms, perampanel dosage and exposure, and compliance were monitored throughout the study. The incidence of suicidal behavior and suicidal ideation (i.e., suicidality) were monitored using the Columbia-Suicide Severity Rating Scale (C-SSRS) in patients aged ≥ 6 years. C-SSRS scores were evaluated by the investigator and an isolated suicidality rating scale response was classified as a TEAE per the investigator’s judgment. Patients under 6 years old were clinically monitored for suicidality.
Exploratory endpoints
Additional exploratory endpoints investigated the proportion of patients with cognitive impairment relative to baseline. Cognition was assessed using the EpiTrack® screening tool (or EpiTrack® Junior in patients aged ≥ 6 to 16 years), scored by the investigator, to clinically monitor adverse cognitive effects associated with ASMs [22]. To aid the interpretation of the cognition score, depression was assessed using the Beck Depression Inventory-II (BDI-II) [23]. Additionally, change in subjective sleep quality was assessed using the retrospective sleep quality instrument, the Pittsburgh Sleep Quality Index (PSQI) [24], and QoL was assessed using the epilepsy-specific QoL in Epilepsy Inventory-31 [QOLIE-31] survey. The details of each assessment and scoring parameters are presented in Supplementary Table 1.
Post hoc analysis of patients with a history of psychiatric and behavioral events
Psychiatric and behavioral side effects have been associated with the use of ASMs in patients with epilepsy, and evidence suggests that patients with a history of psychiatric events may be predisposed to these side effects [25,26,27]. Therefore, a post hoc analysis was performed to assess safety and the mean change from baseline in cognition (EpiTrack®) scores at Week 52 in a subgroup of patients with a history of psychiatric and behavioral events (as defined by Medical Dictionary for Regulatory Activities [MedDRA]) from ELEVATE.
Statistical analysis
As this study did not have a control arm, only descriptive statistics were performed. Efficacy and safety outcomes are summarized by treatment group (overall population, monotherapy group [patients who either received no ASMs at baseline or received other ASMs and converted to perampanel only at baseline], and first adjunctive therapy group) and by seizure type (FOS [patients with FOS only, with or without FBTCS] and GTCS [patients with GTCS only]).
Results
Patients
The study enrolled 68 patients; two (2.9%) patients were aged 4 to < 12 years, seven (10.3%) were aged 12 to < 18 years, 53 (77.9%) were aged 18 to 64 years, and six (8.8%) were aged > 64 years. Of these, 54 patients aged ≥ 12 years were treated with perampanel and included in the SAS, and 52 patients were included in the FAS. Patient demographics and clinical characteristics during baseline are presented in Table 1. The mean (standard deviation [SD]) age of patients in the overall population was 38.5 (17.3) years; four (7.4%) patients were aged 12 to < 18 years, 44 (81.5%) patients were aged 18 to 64 years, and six (11.1%) patients were aged > 64 years. The mean (SD) time since epilepsy diagnosis in the overall population was 6.2 (9.1) years.
Out of the 54 patients in the SAS, 10 (18.5%) patients were assigned to the perampanel monotherapy group (Fig. 2). However, due to the addition of an ASM along with perampanel on the first day of treatment, one patient was excluded from the monotherapy subgroup analyses; the other nine patients in the monotherapy group were treatment naïve prior to perampanel initiation. The remaining 44 (81.5%) patients in the SAS received perampanel as first adjunctive therapy at baseline (Fig. 2); the most common concomitant ASMs at baseline were levetiracetam (54.5% [n = 24/44]) and lamotrigine (13.6% [n = 6/44]). During the study, four (44.4%) patients in the monotherapy group added another ASM and five (55.6%) patients remained on perampanel monotherapy for the entire study; 10 (22.7%) patients in the first adjunctive therapy group converted to monotherapy and 34 (77.3%) remained on perampanel as first adjunctive therapy. In total, 32 (59.3%) patients completed the study; the most common reason for discontinuation was AEs (18.5% [n = 10/54]) (Fig. 2). No patient who completed the study reported developing drug-resistant epilepsy.
Patient disposition. a One patient did not take perampanel. b Patients who either received no ASMs at baseline or received another ASM and converted to perampanel only at baseline. One patient was excluded from the monotherapy subgroup analyses due to the addition of an ASM along with perampanel on the first day of treatment. c FOS includes patients with FOS only (with or without FBTCS); GTCS includes patients with GTCS only; FOS + GTCS includes patients with mixed FOS and GTCS. d Patients who received perampanel as first adjunctive therapy at baseline. ASM anti-seizure medication, Excl exclusion criteria, FBTCS focal to bilateral tonic–clonic seizures, FOS focal-onset seizures, GTCS generalized tonic–clonic seizures, Incl inclusion criteria
The mean (SD) duration of exposure to perampanel was 39.8 (20.0) weeks in the overall population. In the perampanel monotherapy and first adjunctive therapy groups, the mean (SD) duration of exposure to perampanel was 45.1 (17.0) weeks and 38.3 (20.6) weeks, respectively. The median (minimum, maximum) daily dose of perampanel was 4.0 (4, 12) mg in the monotherapy group and 6.0 (4, 11) mg in the first adjunctive therapy group during the Maintenance Period.
Efficacy outcomes
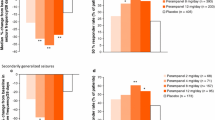
Retention rates at 3, 6, 9, and 12 months (or study completion) are presented by treatment group and seizure type in Fig. 3. Retention rate in the overall population at 12 months (or study completion) was 63.0% (n = 34/54; monotherapy group, 77.8% [n = 7/9]; first adjunctive therapy group, 59.1% [n = 26/44]). The seizure-freedom rate and 50% responder rate are presented in Fig. 4 by treatment group and seizure type; seizure freedom was achieved by 32.7% (n = 17/52) of patients and 50% response was achieved by 78.7% (n = 37/47) of patients in the overall population during the Maintenance Period. Seizure freedom and 50% response was achieved by 44.4% (n = 4/9) and 85.7% (n = 6/7) of patients in the monotherapy group, respectively; similarly, 29.5% (n = 13/44) of patients in the first adjunctive therapy group achieved seizure freedom and 76.9% (n = 30/39) of patients achieved 50% response. The median (minimum, maximum) reduction in total-seizure frequency per 28 days during the Titration and Maintenance Periods in the overall population was 86.8% (-2700.0, 100.0) and 77.9% (-2700.0, 100.0), respectively (Supplementary Fig. 1).
Retention rate of perampanel at 3, 6, 9, and 12 months (or study completion) by treatment group ([a] perampanel monotherapy, [b] perampanel first adjunctive therapy, and [c] overall population) and seizure type (Safety Analysis Set). a Patients who either received no ASMs at baseline or received another ASM and converted to perampanel only at baseline. One patient was excluded from the monotherapy subgroup analyses due to the addition of an ASM along with perampanel on the first day of treatment. b FOS includes patients with FOS only (with or without FBTCS); GTCS includes patients with GTCS only. The “Total” column includes patients with FOS only, GTCS only, and mixed FOS and GTCS; therefore, the total number of patients is greater than the sum of patients with FOS only or GTCS only. c Patients who received perampanel as first adjunctive therapy at baseline. d Patients who either received perampanel monotherapy or perampanel as first adjunctive therapy at baseline. ASM anti-seizure medication, FBTCS focal to bilateral tonic–clonic seizures, FOS focal-onset seizures, GTCS generalized tonic–clonic seizures
a Seizure-freedom rate and b 50% responder rate during the entire Maintenance Period (LOCF) by treatment group and seizure type (Full Analysis Set). Percentages are based on the total number of patients with non-missing values. a FOS includes patients with FOS only (with or without FBTCS); GTCS includes patients with GTCS only. The “Total” column includes patients with FOS only, GTCS only, and mixed FOS and GTCS; therefore, the total number of patients is greater than the sum of patients with FOS only or GTCS only. b Patients who either received no ASMs at baseline or received another ASM and converted to perampanel only at baseline. One patient was excluded from the monotherapy subgroup analyses due to the addition of an ASM along with perampanel on the first day of treatment. c Patients who received perampanel as first adjunctive therapy at baseline. d Patients who either received perampanel monotherapy or perampanel as first adjunctive therapy at baseline. ASM anti-seizure medication, FBTCS focal to bilateral tonic–clonic seizures, FOS focal-onset seizures, GTCS generalized tonic–clonic seizures, LOCF last observation carried forward
Safety outcomes
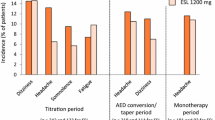
An overview of TEAEs and most common TEAEs by treatment group and seizure type is presented in Table 2. TEAEs were reported by 88.9% (n = 48/54) of patients in the overall population (monotherapy group, 77.8% [n = 7/9]; first adjunctive therapy group, 90.9% [n = 40/44]). The incidence of TEAEs was lower during the Maintenance Period compared with the Titration Period across seizure types (Supplementary Table 2); in total, 87.0% (n = 47/54) of patients reported TEAEs during the Titration Period and 54.5% (n = 24/44) of patients reported TEAEs during the Maintenance Period.
TEAEs leading to perampanel dose adjustment occurred in 13 (24.1%) patients in the overall population (monotherapy group, 22.2% [n = 2/9]; first adjunctive therapy group, 25.0% [n = 11/44]). The most common TEAEs were dizziness (overall population, 27.8% [n = 15/54]; monotherapy group, 11.1% [n = 1/9]; first adjunctive therapy group, 31.8% [n = 14/44]) and fatigue (overall population, 16.7% [n = 9/54]; monotherapy group, 22.2% [n = 2/9]; first adjunctive therapy group, 13.6% [n = 6/44]). The incidence of TEAEs leading to perampanel dose adjustment in patients taking concomitant levetiracetam or lamotrigine (the most common concomitant ASMs in the first adjunctive group) was 29.0% (n = 9/31).
Serious TEAEs were reported in four (7.4%) patients (Table 2); these were sudden unexpected death in epilepsy (SUDEP; n = 1 [FOS]), worsening depression and suicidal ideation (n = 1 [GTCS]), transient ischemic attack (n = 1 [FOS]), and mental status changes (n = 1, [GTCS]). The event of SUDEP was reported in a patient receiving perampanel as first adjunctive therapy with concomitant oxcarbazepine and was not related to perampanel. The events of worsening depression and suicidal ideation were reported in a patient receiving perampanel monotherapy (after transitioning from other ASMs to perampanel monotherapy at baseline); these events led to discontinuation and were considered to be related to perampanel. This patient had a history of ongoing major depression disorder and was receiving concomitant anti-depressant medication.
In the overall population, 33.3% (n = 18/54) of patients reported psychiatric AEs. Three (5.6%) patients (FOS, n = 2 [one with FBTCS]; GTCS, n = 1) experienced on-treatment suicidal ideation, as indicated by ≥ 1 positive response for suicidal ideation on the C-SSRS. Two of these patients had a history of suicidal ideation at baseline; both patients recovered without dose adjustment, and their suicidal ideation TEAEs resolved. The third patient (mentioned in the above paragraph) discontinued perampanel treatment; following discontinuation, the patient recovered, and the suicidal ideation resolved. No patients experienced on-treatment suicidal behavior.
Exploratory endpoints
Additional exploratory endpoints are presented by seizure type in Table 3. In the overall population, there was a negative change in total EpiTrack® (n = 28), BDI-II (n = 29), and PSQI (n = 30) scores at 12 months compared to baseline (mean [SD] change from baseline in total score: EpiTrack® -0.4 [3.3], BDI-II -1.2 [7.9], PSQI -0.2 [3.9]) and an improvement in QOLIE (n = 29) total score at 12 months compared to baseline (mean [SD] change from baseline in total score, 2.4 [9.5]). However, these changes in total score were not clinically significant (Supplementary Table 1). The PSQI and QOLIE-31 individual domain results are presented in Supplementary Table 3.
Post hoc analysis of patients with a history of psychiatric and behavioral events
There were 24 patients with a history of psychiatric or behavioral events, based on clinical history, included in the post hoc analysis. Five (20.8%) patients received perampanel monotherapy and 19 (79.2%) patients received perampanel as first adjunctive therapy. The most common concomitant ASM during the study was levetiracetam (37.5% [n = 9/24]). The results of the post hoc analysis are presented in Supplementary Table 4. The incidence of any TEAE in this subgroup was 95.8% (n = 23/24); 41.7% (n = 10/24) reported psychiatric TEAEs, in comparison to 33.3% (n = 18/54) in the overall population. Four (44.4%) patients receiving concomitant levetiracetam reported a psychiatric TEAE. With respect to cognition, the mean (SD) change in EpiTrack® total score from baseline at 12 months was − 1.1 (3.1) in patients with a history of psychiatric or behavioral events; this was consistent with the mean change in the overall population (− 0.4 [3.3]).
Discussion
In this analysis of data from ELEVATE, perampanel monotherapy or first adjunctive therapy was associated with retention rates of 63.0%, median reductions in seizure frequency of 77.9%, and was generally well tolerated. Efficacy and safety data were similar between seizure types. Cognitive function, sleep quality, and QoL were not negatively impacted following perampanel monotherapy or first adjunctive therapy. Only one patient had de novo suicidal ideation which was reported as a TEAE, and no one experienced suicidal behavior. These findings are consistent with the known safety profile of perampanel. No new safety signals were observed [9]. Additionally, more than one in five patients (22.7%) in the first adjunctive therapy group were able to convert to perampanel monotherapy, thereby reducing medication burden.
Results observed in ELEVATE are in line with clinical evidence from other open-label and real-world studies assessing the efficacy and safety of perampanel in patients with epilepsy administered as monotherapy [15,16,17,18,19] or first adjunctive therapy [20, 21]. In ELEVATE, the retention rate at 12 months (or study completion) was 77.8% among patients receiving perampanel monotherapy, which is comparable to retention rates of 50.0–71.4% at 12 months (or study completion) in other real-world studies investigating efficacy of perampanel monotherapy [16,17,18,19]. Patients receiving perampanel as first adjunctive therapy in ELEVATE had a 50% responder rate of 76.9%, which is consistent with the 50% responder rate (80.0%) reported in the Phase IV Study 412 that evaluated the efficacy and safety of perampanel as a first adjunctive therapy in patients with FOS, with or without FBTCS [20].
In ELEVATE, 44.4% of patients receiving perampanel monotherapy achieved seizure-free status during the Maintenance Period, which is lower than that observed in FREEDOM (Study 342; NCT03201900), an open-label study that evaluated perampanel monotherapy among patients with newly diagnosed FOS, with or without FBTCS, or in patients who had relapsed [15]. In FREEDOM, seizure-freedom rates were 63.0% (4 mg/day) and 74.0% (4 or 8 mg/day) during the Maintenance Period. The higher seizure-freedom rate in the FREEDOM study could have been due to the shorter duration of the Maintenance Period (FREEDOM, 26 weeks; ELEVATE, 39 weeks) and the greater number of patients receiving perampanel monotherapy (FREEDOM, n = 89; ELEVATE, n = 9). Furthermore, the FREEDOM study only assessed patients with FOS, with or without FBTCS and included a greater proportion of patients who were treatment naïve or newly diagnosed which could contribute to the higher seizure-freedom rate in this study.
The safety profile reported for perampanel administered as monotherapy or first adjunctive therapy in ELEVATE was comparable to that described in other studies that evaluated perampanel either as monotherapy or adjunctive therapy including the FREEDOM study [15], Phase III studies of adjunctive perampanel [11,12,13], and the PERMIT study; a large, pooled analysis of real-world data [14]. Moreover, the incidence of serious TEAEs was low in patients receiving perampanel monotherapy (11.1%) or first adjunctive therapy (6.8%) in ELEVATE, which was again consistent with the findings of previous studies of perampanel monotherapy (0.0–10.1%) [15,16,17,18,19] or first adjunctive therapy (0.0–7.8%) [20, 21].
The safety profile of early-line perampanel reported here is comparable to that reported for other ASMs, such as lacosamide and cenobamate [28, 29]. Despite differences in retention rate (63% with perampanel in the overall population at 12 months (or study completion) and 79% with cenobamate at 12 months), the incidence of serious TEAEs was low with early-line perampanel (7.4%) and similar to that reported with adjunctive lacosamide (6.6%) and adjunctive cenobamate (8.1%). The differences in study design may explain the differences in retention rate between perampanel and cenobamate. In ELEVATE, patients who could not tolerate 4 mg/day perampanel by the end of the Titration Period or during the Maintenance Period were required, per study protocol, to withdraw from the study, likely accounting for the 10 patients who discontinued treatment due to AEs. In addition, the study design only allowed for one concomitant ASM, along with conversion to perampanel monotherapy or dose adjustment only, which could have resulted in discontinuations due to a lack of efficacy. Whereas in the phase 3 trial assessing the efficacy of of long-term adjunctive cenobamate, a high proportion of patients were receiving ≥ 2 concomitant ASMs (n = 1098, 82.0%) and, could remove, add, or adjust the dose of concomitant ASM as clinically required except for those receiving concomitant phenytoin or phenobarbital [29].
Cognitive function and QoL in patients with epilepsy can be negatively affected by poor sleep quality. The use of some ASMs can lead to disruptions in sleep quality, such as daytime sleepiness, which can worsen seizure control [30]. Here, we demonstrated that perampanel did not adversely affect sleep quality. In addition, cognitive function and QoL were not negatively impacted following perampanel monotherapy or first adjunctive therapy. These findings are supported by previous evidence that suggests perampanel can have a positive effect on sleep quality [30, 31].
In the overall ELEVATE population, 33.3% (n = 18/54) of patients reported psychiatric TEAEs. This result is in line with previous real-world studies of perampanel in which 15.4–28.5% of patients reported psychiatric TEAEs [14, 18, 19]. Among the 24 patients who had a history of psychiatric or behavioral events in ELEVATE, the incidence of psychiatric TEAEs was 41.7% (n = 10/24), which was numerically greater compared with the overall study population. These observations are in line with the evidence that patients with a history of psychiatric and behavioral events may be predisposed to psychiatric AEs [25, 26]. Moreover, psychiatric TEAEs are frequently reported with the use of perampanel, levetiracetam, and topiramate [32, 33]. In ELEVATE, 37.5% (n = 9/24) of patients with pre-existing psychiatric or behavioral conditions were receiving concomitant levetiracetam, of whom four (44.4%) reported psychiatric TEAEs. However, results from a post hoc analysis of pooled data (N = 1038) from four phase III trials assessing the effects of perampanel in patients already receiving 1–3 concomitant ASMs demonstrated that concomitant treatment with levetiracetam had no significant effect on the occurrence of psychiatric AEs in this patient population [34]. Interpretation of these data from ELEVATE may be limited due to the small number of patients with a history of psychiatric or behavioral events included in this post hoc analysis. Overall, this post hoc analysis showed that perampanel was well tolerated in patients with a history of psychiatric and behavioral events, however, all patients should be monitored for signs of psychiatric AEs and perampanel dose reductions may be considered to manage symptoms [26].
There are some limitations of ELEVATE that should be considered when interpretating the results. Firstly, this was an open-label study of perampanel as monotherapy or first adjunctive therapy without a placebo-control arm. A placebo group is deemed unethical for studies of ASM monotherapy because patients in this group would not receive any ASMs to regulate seizures which could be critical for epilepsy management [35]. In addition, the study faced recruitment challenges due to the COVID-19 pandemic, which severely limited the recruitment for more than a year. At this point, it was decided to stop enrollment and analyze the data; thus, the number of patients enrolled in ELEVATE was smaller than intended. Furthermore, the study was not designed with enrollment stratification to ensure a certain number of patients in each patient cohort, thus patient numbers in the subgroups were small, particularly in the monotherapy treatment group and across seizure types.
Conclusion
ELEVATE is the first prospective study of perampanel administered as monotherapy or first adjunctive therapy in patients aged ≥ 4 years with FOS, with or without FBTCS, or GTCS in the US. Perampanel as monotherapy and as first adjunctive therapy was generally well tolerated and outcomes were consistent with the known safety profile of perampanel, with no new safety signals observed [9]. The data observed in the ELEVATE study contribute to emerging data that perampanel can be introduced as an early line treatment in patients with FOS or GTCS, rather than administrating after a patient has failed several ASMs.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Kwan P, Brodie MJ (2000) Early identification of refractory epilepsy. N Engl J Med 342:314–319
Kwan P, Schachter SC, Brodie MJ (2011) Drug-resistant epilepsy. N Engl J Med 365:919–926
Brodie MJ, Barry SJE, Bamagous GA, Norrie JD, Kwan P (2012) Patterns of treatment response in newly diagnosed epilepsy. Neurology 78:1548–1554
St Louis EK, Rosenfeld WE, Bramley T (2009) Antiepileptic drug monotherapy: the initial approach in epilepsy management. Curr Neuropharmacol 7:77–82
Cramer JA, Mintzer S, Wheless J, Mattson RH (2010) Adverse effects of antiepileptic drugs: a brief overview of important issues. Expert Rev Neurother 10:885–891
Gambardella A, Tinuper P, Acone B, Bonanni P, Coppola G, Perucca E (2021) Selection of antiseizure medications for first add-on use: A consensus paper. Epilepsy Behav 122:108087
Thomas SV, Koshy S, Nair CR, Sarma SP (2005) Frequent seizures and polytherapy can impair quality of life in persons with epilepsy. Neurol India 53:46–50
Glauser T, Ben-Menachem E, Bourgeois B, Cnaan A, Guerreiro C, Kälviäinen R, Mattson R, French JA, Perucca E, Tomson T (2013) Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia 54:551–563
Food and Drug Administration (FDA) (December 2021) FYCOMPA® Prescribing Information. https://www.fycompa.com/media/media/Files/Fycompa/Fycompa_Prescribing_Information.pdf. Accessed January 5, 2023
European Medicines Agency (EMA) (May 2023) FYCOMPA® Annex I: Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/fycompa-epar-product-information_en.pdf. Accessed November 16, 2023
Krauss GL, Serratosa JM, Villanueva V, Endziniene M, Hong Z, French J, Yang H, Squillacote D, Edwards HB, Zhu J, Laurenza A (2012) Randomized phase III study 306: adjunctive perampanel for refractory partial-onset seizures. Neurology 78:1408–1415
French JA, Krauss GL, Biton V, Squillacote D, Yang H, Laurenza A, Kumar D, Rogawski MA (2012) Adjunctive perampanel for refractory partial-onset seizures: Randomized phase III study 304. Neurology 79:589–596
French JA, Krauss GL, Steinhoff BJ, Squillacote D, Yang H, Kumar D, Laurenza A (2013) Evaluation of adjunctive perampanel in patients with refractory partial-onset seizures: Results of randomized global phase III study 305. Epilepsia 54:117–125
Villanueva V, D’Souza W, Goji H, Kim DW, Liguori C, McMurray R, Najm I, Santamarina E, Steinhoff BJ, Vlasov P, Wu T, Trinka E, PERMIT pooled analysis participants (2022) PERMIT study: a global pooled analysis study of the effectiveness and tolerability of perampanel in routine clinical practice. J Neurol 269:1957–1977
Yamamoto T, Lim SC, Ninomiya H, Kubota Y, Shin WC, Kim DW, Shin DJ, Hoshida T, Iida K, Ochiai T, Matsunaga R, Higashiyama H, Hiramatsu H, Kim JH (2020) Efficacy and safety of perampanel monotherapy in patients with focal-onset seizures with newly diagnosed epilepsy or recurrence of epilepsy after a period of remission: The open-label Study 342 (FREEDOM Study). Epilepsia Open 5:274–284
Chinvarun Y (2022) A retrospective, real-world experience of perampanel monotherapy in patient with first new onset focal seizure: A Thailand experience. Epilepsia Open 7:67–74
Gil-Nagel A, Burd S, Toledo M, Sander JW, Lebedeva A, Patten A, Laurenza A, Study 504 investigator group (2018) A retrospective, multicentre study of perampanel given as monotherapy in routine clinical care in people with epilepsy. Seizure 54:61–66
Toledano Delgado R, García-Morales I, Parejo-Carbonell B, Jiménez-Huete A, Herrera-Ramirez D, González-Hernández A, Ayuga Loro F, Santamarina E, Toledo M, Ojeda J, Poza JJ, Molins A, Giner P, Estévez María JC, Castro-Vilanova MD, Zurita J, Saiz-Diaz RA, Gómez-Ibañez A, Rodriguez-Uranga J, Gil-Nagel A, Campos D, Sánchez-Larsen Á, Aguilar-Amat Prior MJ, Mauri Llerda JA, Huertas González N, García-Barragán N (2020) Effectiveness and safety of perampanel monotherapy for focal and generalized tonic-clonic seizures: Experience from a national multicenter registry. Epilepsia 61:1109–1119
Wechsler RT, Wheless J, Zafar M, Huesmann GR, Lancman M, Segal E, Chez M, Aboumatar S, Patten A, Salah A, Malhotra M (2022) PROVE: Retrospective, non-interventional, Phase IV study of perampanel in real-world clinical care of patients with epilepsy. Epilepsia Open 7:293–305
Kim JH, Kim DW, Lee SK, Seo DW, Lee JW, Park HJ, Lee SA (2020) First add-on perampanel for focal-onset seizures: An open-label, prospective study. Acta Neurol Scand 141:132–140
Abril Jaramillo J, Estévez María JC, Girón Úbeda JM, Vega López Ó, Calzado Rivas ME, Pérez Díaz H, García Martín G, Vila Herrero E, Chamorro-Muñoz M, Vázquez F, De la Fuente C, Redondo L, Peláez N, Santágueda P, Rodríguez Uranga JJ (2020) Effectiveness and safety of perampanel as early add-on treatment in patients with epilepsy and focal seizures in the routine clinical practice: Spain prospective study (PERADON). Epilepsy Behav 102:106655
Lähde N, Basnyat P, Lehtinen H, Rainesalo S, Rosti-Otajärvi E, Peltola J (2021) EpiTrack is a feasible tool for assessing attention and executive functions in patients with refractory epilepsy. Epilepsy Behav 115:107691
Button KS, Kounali D, Thomas L, Wiles NJ, Peters TJ, Welton NJ, Ades AE, Lewis G (2015) Minimal clinically important difference on the Beck Depression Inventory–II according to the patient’s perspective. Psychol Med 45:3269–3279
Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28:193–213
Chen B, Choi H, Hirsch LJ, Legge A, Buchsbaum R, Detyniecki K (2018) Cross-sensitivity of psychiatric and behavioral side effects with antiepileptic drug use. Seizure 62:38–42
Kanner AM, Patten A, Ettinger AB, Helmstaedter C, Meador KJ, Malhotra M (2021) Does a psychiatric history play a role in the development of psychiatric adverse events to perampanel and to placebo? Epilepsy Behav 125:108380
Piedad J, Rickards H, Besag FM, Cavanna AE (2012) Beneficial and adverse psychotropic effects of antiepileptic drugs in patients with epilepsy: a summary of prevalence, underlying mechanisms and data limitations. CNS Drugs 26:319–335
Vossler DG, Knake S, O’Brien TJ, Watanabe M, Brock M, Steiniger-Brach B, Williams P, Roebling R, on behalf of the SP0982 co-investigators (2020) Efficacy and safety of adjunctive lacosamide in the treatment of primary generalised tonic-clonic seizures: a double-blind, randomised, placebo-controlled trial. J Neurol Neurosurg Psychiatry 91:1067–1075
Sperling MR, Klein P, Aboumatar S, Gelfand M, Halford JJ, Krauss GL, Rosenfeld WE, Vossler DG, Wechsler R, Borchert L, Kamin M (2020) Cenobamate (YKP3089) as adjunctive treatment for uncontrolled focal seizures in a large, phase 3, multicenter, open-label safety study. Epilepsia 61:1099–1108
Carvalho BMS, Chaves J, da Silva AM (2022) Effects of antiepileptic drugs on sleep architecture parameters in adults. Sleep Sci 15:224–244
Rocamora R, Alvarez I, Chavarria B, Principe A (2020) Perampanel effect on sleep architecture in patients with epilepsy. Seizure 76:137–142
Hansen CC, Ljung H, Brodtkorb E, Reimers A (2018) Mechanisms Underlying Aggressive Behavior Induced by Antiepileptic Drugs: Focus on Topiramate, Levetiracetam, and Perampanel. Behav Neurol 2018:2064027
Stephen LJ, Wishart A, Brodie MJ (2017) Psychiatric side effects and antiepileptic drugs: Observations from prospective audits. Epilepsy Behav 71:73–78
Chung S, Williams B, Dobrinsky C, Patten A, Yang H, Laurenza A (2017) Perampanel with concomitant levetiracetam and topiramate: Post hoc analysis of adverse events related to hostility and aggression. Epilepsy Behav 75:79–85
Mintzer S, French JA, Perucca E, Cramer JA, Messenheimer JA, Blum DE, Rogawski MA, Baulac M (2015) Is a separate monotherapy indication warranted for antiepileptic drugs? Lancet Neurol 14:1229–1240
Acknowledgements
The authors would like to thank the study participants. Medical writing support, under the direction of the authors, was provided by Clare Campbell, PhD, of CMC Affinity, a division of IPG Health Medical Communications, funded by Eisai Inc., in accordance with Good Publication Practice (GPP 2022) guidelines.
Funding
Study 410 was funded by Eisai Inc.
Author information
Authors and Affiliations
Contributions
Vineet Punia was involved in the acquisition and interpretation of the study data. Pavel Klein and Temenuzhka Mihaylova were involved in the acquisition of the study data. Victor Biton was involved with the acquisition, analysis, and interpretation of the study data. Omar Samad, Dinesh Kumar, and Manoj Malhotra were involved in the analysis and interpretation of the study data. Leock Y Ngo was involved in the interpretation of the study data. All authors critically reviewed the manuscript and approved the final version for submission.
Corresponding author
Ethics declarations
Conflicts of interest
Vineet Punia has carried out consultancy and/or been on an advisory board for Catalyst Pharmaceuticals, Eisai Inc., and UNEEG Medical. Pavel Klein has served as a consultant for Abbott, Angelini, Arvelle Therapeutics, Neurelis, SK Life Science, and UniQure; as a consultant, advisory board member, and speaker to Aquestive, Eisai Inc., Sunovion, and UCB Pharma; is a member of the Medical Advisory Board of Stratus and of the Scientific Advisory Board of OB Pharma; is the CEO of PrevEp; has received speaker’s honoraria from Eisai Inc.; and has received research support from CURE/Department of Defense and National Institute of Health. Temenuzhka Mihaylova and Victor Biton have no real or apparent conflicts of interest to disclose. Omar Samad and Dinesh Kumar are employees of Eisai Inc. Leock Y Ngo and Manoj Malhotra are former employees of Eisai Inc.
Ethics approval
The study protocol was approved by Cleveland Clinic Institutional Review Board (study number: 18–045) and had therefore been performed in accordance with the ethical standards laid down in the Declaration of Helsinki.
Informed consent
All participants provided written informed consent before participation in the study and after the study procedures had been fully explained. The informed consent form included a disclaimer that data will be anonymized and published.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Punia, V., Klein, P., Mihaylova, T. et al. Perampanel as monotherapy or first adjunctive therapy in pediatric and adult patients with epilepsy: the first United States-based phase IV open-label ELEVATE study. J Neurol 271, 4587–4598 (2024). https://doi.org/10.1007/s00415-024-12399-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-024-12399-w