Abstract
Background and purpose
Cerebral sinus venous thrombosis (CSVT) is a rare but life-threatening disease and its diagnosis remains challenging. Blood biomarkers, including D-Dimer are currently not recommended in guidelines. Soluble endothelial receptor proteins (sICAM-1, sPECAM-1 and sVCAM-1) have been shown to be promising diagnostic biomarkers in deep vein thrombosis (DVT) and pulmonary embolism (PE). Therefore, we examined endothelial receptor proteins as potential biomarkers for detecting CSVT.
Methods
In this bi-centre, prospective study, we quantified D-Dimer as well as sICAM-1, sPECAM-1 and sVCAM-1 in plasma of patients with clinically suspected CSVT managed in the neurological emergency department (ED) of a tertiary care hospital. All patients underwent cerebral magnetic resonance imaging (MRI) and were followed up after 3, 6 and 12 months to detect thrombus resolution.
Results
Twenty-four out of 75 (32%) patients with clinically suspected CSVT presenting with headache to the ED were diagnosed with acute CSVT. These patients had a mean age of 45 ± 16 years and 78% were female. In patients with CSVT, mean baseline D-dimer (p < 0.001) and sPECAM-1 (p < 0.001) were significantly higher compared to patients without CSVT. The combination of D-Dimer and sPECAM-1 yielded the best ROC-AUC (0.994; < 0.001) with a negative predictive value of 95.7% and a positive predictive value of 95.5%. In addition, higher baseline sPECAM-1 levels (> 198 ng/ml) on admission were associated with delayed venous thrombus resolution at 3 months (AUC = 0.83).
Conclusion
sPECAM-1 in combination with D-Dimer should be used to improve the diagnostic accuracy of acute CSVT and sPECAM-1 may predict long-term outcome of CSVT. Confirmatory results are needed in other settings in order to show their value in the management concept of CSVT patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral sinus venous thrombosis (CSVT) is a rare form of venous thromboembolism (VTE) and is challenging to diagnose due to its highly variable clinical manifestations and the absence of reliable biomarkers. Recent studies have shown that the incidence of CSVT is higher than expected [1, 2], which may be the result of the use and availability of more advanced diagnostic techniques. CSVT frequently occurs in young people, women of childbearing age, and children [3,4,5]. The clinical features of CSVT are variable and unspecific. Up to 80% of CSVT cases have an acute to subacute onset [4]. The leading presenting clinical symptom is headache which can range from thunderclap-like types to migrainous and tension like headaches [6]. Focal neurological symptoms, such as visual loss and sensory motor deficits, are common. In 25% of patients, an isolated headache is the only symptom. Although prognosis is good in most patients, a significant number of people (about 13%) suffer from residual disabilities or death [6, 7].
A frequently discussed biomarker for predicting CSVT is D-Dimer. D-Dimer results from fibrin degradation via fibrinolysis and can predict VTE [8]. However, false‐negative D‐dimers are frequent and lead to unnecessary imaging [9,10,11,12].
PECAM-1, also denoted CD 31, is a cell surface receptor protein expressed on endothelial cells which has important roles in inflammation, angiogenesis, and thrombus resolution [13, 14]. Intracellular adhesion molecule 1 (ICAM-1), also named CD 54, is another adhesion molecule which is involved in thrombosis and the development of post-thrombotic syndromes (PTS) [15, 16]. Vascular cell adhesion molecule 1 (VCAM-1) is essential for leukocyte recruitment and the development of arteriosclerosis. Its role in venous thrombosis is unknown [17, 18]. Soluble endothelial receptor proteins have been shown to be promising biomarkers for deep vein thrombosis (DVT) and pulmonary embolism (PE) [13, 19].
In this study, we aimed to investigate the role of endothelial receptor proteins (PECAM-1; ICAM-1 and VCAM-1) in acute CVST and hypothesised that each biomarker or a combination of these biomarkers can be used to predict acute CSVT and could be higher in CVST patients with delayed thrombus resolution.
Methods
Study population
The study was approved by the local ethical review board (Ethik-Kommission Land Oberösterreich; EK-58-17). Patients with clinically suspected CSVT were screened and enrolled in the outpatient department for neurological emergencies of the Kepler University Hospital and of The Hospital of the Brothers of Saint John of God in Linz, Austria. All patients were referred to one of the hospitals with newly acute (1–2 days) or subacute (up to 10 days) headache, with or without focal neurological disabilities or seizures, and clinically suspected CSVT (headache and additional risk factors for VTE). All patients underwent a detailed neurological examination. Medical history, current medication and risk factors for VTE (previous deep venous thrombosis [DVT] immobilisation, surgery, malignancy, pregnancy, hormone therapy, nicotine, body mass index (BMI), thrombophilia, recent travel, age, and gender) were recorded prospectively in all patients. Recruitment process is presented in Fig. 1. All patients between ages of 18 and 99 with suspected CSVT and newly acute (1–2 days) or subacute (up to 10 days) headache, with or without focal neurological disabilities or seizures were included in the study. Patients with the inability or unwillingness to participate in the study or perform MRI were excluded. Patients without CSVT and clinical stable conditions were released from the hospital. Patients with CSVT were hospitalised.
Chronic headache was defined as headaches for at least 3 months based on ICD-10 criteria [20].
Blood samples
All patients underwent routine blood laboratory analysis including D-Dimer (mg/L) and blood samples for sPECAM-1 (ng/mL), sICAM-1(ng/mL) and sVCAM-1 (ng/mL) at baseline, and, if CSVT was diagnosed, additionally after 3 months (± 4 weeks). Blood samples for quantification of sPECAM-1, sICAM-1 and sVCAM-1 were immediately centrifuged at 4 °C, 3000×g for 10 min and stored at − 80 °C until final analysis. Measurements were then performed using ELISA (sandwich platinum instant ELISA-Kit; Thermo Fisher science, San Diego, USA) according to manufacturer’s instructions.
A D-Dimer cut-off value of > 0.5 mg/L was defined as abnormal [11, 21, 22].
∆D-Dimer, ∆sPECAM-1, ∆sICAM-1 and ∆sVCAM-1 were defined as the difference between baseline and 3 months of follow-up (FU1).
All blood samples were taken in the emergency department before diagnostic and treatment procedures.
Neuroimaging
All patients underwent cranial MRI (1.5 Tesla, SIEMENS MAGNETOM Avanto syngo MR B19) at baseline. An additional scan was done after 3 months in patients with CSVT. All patients with delayed thrombus resolution underwent cranial MRI every 3 months (± 4 weeks) for up to 12 months. Delayed thrombus resolution was defined when residual thrombus was still visible after 3 months brain MRI despite anticoagulant treatment. “Chronic sinus thrombosis” was defined as still significant thrombotic residues after 12 months in neuroimaging.
The standardised protocol included an axial T2w-FLAIR sequence and a high-resolution T1-weighted 3D-MPRAGE sequence as well as T2-weighted, diffusion tensor images, susceptibility weighted imaging and venography.
Treatment
All patients with CSVT were admitted to a stroke unit or neurological intensive care unit and were managed following international guidelines. The initial treatment included adjusted dose of unfractionated heparin (UFH) or weight-based low-molecular-weight-heparin (LMWH; 1 mg/kg twice daily) followed by vitamin K antagonists (or Off-Label Dabigatran 150 mg twice a day), regardless of the presence of intracerebral haemorrhage. One pregnant patient received therapeutic LMWH for 6 months and subsequent prophylactic LMWH. All patients with acute symptomatic seizures were treated with antiepileptic drugs [6, 23].
Statistics
Sample size calculation was based on preliminary data [13] using the sample size calculator provided by the Department of Biometrics, University of Münster, Germany for the primary endpoint sPECAM-1 using a power of 90% at a type I error of 5%. The sample size calculation determined to include 24 patients with CSVT.
Continuous data are presented as mean and standard deviation, categorical data are presented using counts and percentages. Normally distributed continuous variables were compared using the Student’s t-test or the Welch’s t test in case of variance heterogeneity (verification with Levene’s test). The exact Mann–Whitney U test was applied in case of non-normally distributed continuous variables. Assumptions of normal distribution for continuous variables were tested with the Kolmogorov–Smirnov test with Lilliefors correction. For categorical data, the exact chi-square test (n × k tables) or Fisher’s exact test (2 × 2 tables) were used.
Binary logistic regression was used to analyse variables (D-Dimer/sPECAM-1/sICAM-1/sVCAM-1/the combination of these variables) with regard to CSVT status. For various cut-off points, distinct sensitivity/specificity values were calculated, receiver operating characteristic curves (ROC) were constructed, and ROC-AUC (area under curve) including 95% confidence intervals were determined to assess diagnostic value of these biomarkers or the combination of these biomarkers for CSVT diagnosis and chronic progression prediction.
The correlation between continuous variables was assessed by the Bravais–Pearson correlation coefficient (in case of normality) or the Spearman’s rank correlation coefficient (in case of non-normality).
All statistical analyses were performed using R Version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria, URL http://www.R-project.org) and Prism Graph-Pad version 9.01.
The type I error was set to 5% (two-sided). Hence, all inferential results—except for the primary parameter sPECAM-1 for which the sample size estimation was performed—are of descriptive nature only.
Results
Baseline characteristics of study patients (Table 1)
Seventy-five patients with clinically suspected CSVT were included in the study. Mean age was 42.9 ± 17.3 years (range 19–81) and 68% were female. Clinical characteristics, risk factors for CSVT, laboratory parameters and outcome are presented in Table 1. Twenty-four patients (32%) had confirmed CSVT by brain MRI.
Patients with CSVT commonly presented with isolated headache (62.50%), visual impairment (20.83%), seizures and/or focal neurological signs (16.67%). The most common clinical presentation of the control group included headache (78%), dizziness (13.3%) and visual impairment (8%).
Neuroimaging revealed thrombus formation in the sinus transversus (71%), sinus sigmoideus (58%), sinus sagittalis superior (37.5%) and with extension to the jugular vein (37.5%). In 67% of patients, more than one sinus were occluded and 25% of patients suffered from cortical deep vein thrombosis. Intracranial haemorrhage was present in 21% of patients already on admission as revealed by neuroimaging.
In 51 patients with clinically suspected CSVT, neuroimaging results were negative. Clinical characteristics, risk factors for CSVT and routine laboratory parameters did not differ from patients with proven CSVT (Table 1). Headache was the predominant complaint in patients without CSVT, and none of the patients presented with seizures or focal neurological signs on admission. Interestingly, neuroimaging revealed acute ischaemic stroke in two patients without evidence for CSVT and no focal neurological deficits.
Thrombus resolution at 3 months and outcome
Of the 24 patients with acute CSVT, one-third (8/24, 33%) had complete resolution of CSVT after 3 months. Further thrombus resolution was observed in additionally 8 patients (33%) by 12 months, leaving one-third of all CSVT patients with neuroimaging signs of CSVT beyond 12 months.
Other relevant complications included symptomatic epilepsy (N = 3/24) and chronic headache (N = 6/24). In those patients, thrombus persistence was higher than in patients without chronic headache (4/6 versus 2/18, p = 0.01; Odds ratio [OR] 7.0; Relative risk [RR] 3.0 95% CI 0.11–0.80).
Laboratory parameters
D-Dimer
D-Dimer levels were above the cut-off of 0.5 mg/L in 35/75 patients (46%) including 16 patients without CSVT. In 4 patients with CSVT, D-Dimer levels were not elevated (16%), as in patients with ischaemic stroke (N = 2). Mean baseline D-Dimer-levels were significantly higher in the CSVT group compared to the control group without CSVT (p value < 0.001; Table 2). D-Dimer levels > 0.5 mg/L had a diagnostic sensitivity of 83% and specificity of 73% for CSVT. D-Dimer levels did correlate with sPECAM-1 baseline levels [r = 0.50 (95% CI 0.24–0.70); p value < 0.001] but not with creatinine clearance, sICAM-1 and sVCAM-1 levels and the number of affected sinuses.
sPECAM-1
Mean baseline sPECAM-1 levels were significantly higher in patients with CSVT compared to patients without CSVT (p value < 0.001; Table 2). Elevated sPECAM-1 levels above 198.7 ng/mL had a diagnostic sensitivity of 95.7% and a specificity of 90.9% for detecting CSVT. (FPR: N = 3, 4%; FNR: N = 2, 9%).
Baseline sPECAM-1 levels correlated with D-Dimer (see above) and sICAM-1 levels [r = 0.44 (95% CI 0.16–0.66); p value = 0.002]. There was no difference in the number of affected cerebral sinus veins or in isolated cortical vein and sinus vein thrombosis and receptor protein levels.
sICAM-1
There was no difference in sICAM-1 levels between patients with or without CSVT (p value = 0.09; Table 2). sICAM-1 levels only correlated with sPECAM-1 at baseline (p = 0.02). There was no difference in the number of affected cerebral sinus veins or in isolated cortical vein and sinus vein thrombosis.
Baseline sICAM-1 levels were significantly higher in patients with persistent neuroimaging signs of cerebral sinus vein thrombosis beyond 12 months (846 vs 1069 ng/ml; p value < 0.001).
sVCAM-1
There was no difference in sVCAM-1 levels between patients with or without CSVT (p = 0.25; Table 2). sVCAM-1 levels didn’t correlate with any other parameter at baseline and follow-up.
D-Dimer, sPECAM-1, sICAM-1 and sVCAM-1 in predicting CSVT (Table 3)
Using ROC analyses, sPECAM-1 and D-Dimer had the highest AUC (0.89 and 0.86, respectively) in identifying patients with CSVT (Fig. 2, Table 3). Logistic regression analysis revealed that the combination of D-Dimer and sPECAM-1 yielded the best AUC (0.994; 95% CI 0.98–1.000; p < 0.001) with a negative predictive value (NPV) of 95.65% and a positive predictive value (PPV) of 95.45% (Table 3). The combination of D-Dimer (cut-point > 0.5 mg/L) and sPECAM-1 (cut-off > 198.7 ng/mL) resulted in a reduction of patients with “false-negative” D-Dimer levels from 4 to 0 cases.
In patients with isolated headache and without focal neurological signs and symptoms (n = 15), sPECAM-1 > 198.7 ng/mL had an AUC of 0.94 (95% CI 0.86–1.000)). Again, the combination of sPECAM-1 and D-Dimer increased the AUC to 0.99 (95% CI 0.97–1.000).
D-Dimer, sPECAM-1, sICAM-1 and sVCAM-1 in predicting delayed thrombus resolution (Table 3)
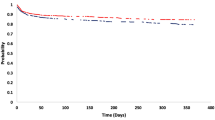
ROC curves in predicting delayed thrombus resolution and chronification are shown in Figs. 3 and 4. AUC was 0.61, 0.83 and 0.80 for D-Dimer, sPECAM-1 and sICAM-1, respectively.
Discussion
In this study, we found that (1) baseline sPECAM-1 levels were significantly higher in patients with CSVT compared to patients without CSVT, and (2) sPECAM-1 may qualify as a reliable biomarker not only for predicting CSVT, but also for predicting delayed radiologic thrombus resolution. To our knowledge, this is the first study to investigate the role of endothelial receptor proteins in CSVT. Interestingly, the combination of D-Dimer and sPECAM-1 plasma levels was better in predicting chronic CSVT compared to each biomarker alone, especially D-Dimer.
D-Dimer is the only routinely used diagnostic biomarker in patients with suspected VTE [12, 24], and PE or DVT can be safely ruled out in low risk situations (Wells score < 4) without the need for further imaging [9, 25, 26]. In contrast, data about the diagnostic accuracy of D-Dimer in patients with CSVT remain controversial. Two systematic reviews showed that patients with clinical isolated headaches or symptom duration > 7 days have often negative D-Dimer [9, 11]. In our study, D-Dimer (cut-off 0.5 mg/L) had a sensitivity of 82.61% and a specificity of 72.73%, which confirms that isolated D-Dimer levels are not useful as a diagnostic approach. Interestingly, D-Dimer levels > 0.5 had a sensitivity of 100% and a specificity of 52% in patients with visual (diplopia or anopia) or other focal neurological disorders (in our case focal paresis or hemiparesis), which is in line with previous studies (mean sensitivity between 93 and 97.8% respectively) [9, 11]. The high rate of false positive results of D-dimer may be explained by elevated levels observed in advanced age, during pregnancy, smoking, heart disease or acute inflammation [27, 28].
Therefore, D-Dimer measurement is not recommended in the guidelines for the diagnosis of cerebral sinus vein thrombosis to avoid unnecessary imaging. D-Dimer is often influenced by pro-inflammatory condition in contrast to sPECAM-1 [13, 19] We think additional sPECAM-1 measurement in patients with suspected CSVT can be helpful especially in patients with isolated headache or patients with unspecific inflammatory conditions (pregnancy or neoplastic disease) due to its robustness against pro-inflammatory cytokines. Another finding in our study is that sPECAM-1 and sICAM-1 were associated with thrombus persistence. Negative parameters of sPECAM-1 could contribute to save costs and avoid unnecessary imaging.
sPECAM-1 and sICAM-1 have been previously described to predict chronic VTE [13, 15, 16]. Though its role in CSVT is still unclear due to the unique anatomical particularities of the dural sinus, the dural sinus veins contain no muscular tissue, possess no valves and the walls are composed of dura mater lined by endothelium [29, 30], which could explain the high rate of chronification in CSVT. In our study, sPECAM-1 and sICAM-1 were associated with thrombus persistence.
Further neuropathological examinations could help identify the role of sPECAM-1 and sICAM-1 in thrombus resolution at the side of sinus veins thrombosis.
Our patients had anticoagulant treatment for at least 3 months. Although high rates of recanalization were detected in the first 3 months after the index event in large and small cohorts of patients with CSVT, about two-third in our cohort had not resolved the thrombus after 3 months [31, 32]. Recent guidelines do not give a clear recommendation for the ideal duration of oral anticoagulation, which often limits young patients in daily activities and sports. Longitudinal studies about monitoring the thrombus development and resolution of CSVT are rare [31]. We found that sPECAM-1 is able to identify patients with risk of delayed thrombus and may be beneficial and helpful to individually decide the ideal duration of oral anticoagulation of the patient and reduce the rate of chronic sinus vein thrombosis.
The present study has important limitations. First, the study is based on a relatively small sample size. But sample size calculation based on data in Kellermair et al. [13] showed a sufficient power of 90% at a type I error of 5%.
Second, we didn’t include pregnant women, patients with postpartal CSVT and patients with septic sinus vein thrombosis, which limits generalizability of our results. Third, although there is evidence that lysis of the thrombus and recanalization of venous segments are typically observed in the first weeks, [33] the definition of thrombus persistence or delayed thrombus resolution is arbitrarily chosen. The definition of “chronic sinus thrombosis” is also not clearly standardised and chosen similar to the definition of chronic deep vein thrombosis (thrombotic residues after 6–12 months) [34, 35] as still significant thrombotic residues after 12 months in neuroimaging. Fourth, we did not include a control group of patients without headache.
In conclusion, sPECAM-1, sICAM-1 and D-Dimer can be helpful parameters for the acute diagnosis of CSVT and can predict delayed thrombus resolution and therefore could help identify the ideal duration of anticoagulation and avoid excessive imaging checks.
Data availability
Data available on reasonable request.
References
Devasagayam S, Wyatt B, Leyden J, Kleinig T (2016) Cerebral venous sinus thrombosis incidence is higher than previously thought: a retrospective population-based study. Stroke 47(9):2180–2182
Silvis SM, de Sousa DA, Ferro JM, Coutinho JM (2017) Cerebral venous thrombosis. Nat Rev Neurol 13(9):555–565
Bousser MG, Ferro JM (2007) Cerebral venous thrombosis: an update. Lancet Neurol 6(2):162–170
Ferro JM, Canhao P (2014) Cerebral venous sinus thrombosis: update on diagnosis and management. Curr Cardiol Rep 16(9):523
Behrouzi R, Punter M (2018) Diagnosis and management of cerebral venous thrombosis. Clin Med (Lond) 18(1):75–79
Saposnik G, Barinagarrementeria F, Brown RD Jr et al (2011) Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American heart association/American stroke association. Stroke 42(4):1158–1192
Varga EA, Moll S (2004) Cardiology patient pages. Prothrombin 20210 mutation (factor II mutation). Circulation 110(3):e15-18
Lee AY, Ginsberg JS (1997) The role of D-dimer in the diagnosis of venous thromboembolism. Curr Opin Pulm Med 3(4):275–279
Dentali F, Squizzato A, Marchesi C, Bonzini M, Ferro JM, Ageno W (2012) D-dimer testing in the diagnosis of cerebral vein thrombosis: a systematic review and a meta-analysis of the literature. J Thromb Haemost 10(4):582–589
Tanislav C, Siekmann R, Sieweke N et al (2011) Cerebral vein thrombosis: clinical manifestation and diagnosis. BMC Neurol 11:69
Alons IM, Jellema K, Wermer MJ, Algra A (2015) D-dimer for the exclusion of cerebral venous thrombosis: a meta-analysis of low risk patients with isolated headache. BMC Neurol 15:118
Lalive PH, de Moerloose P, Lovblad K, Sarasin FP, Mermillod B, Sztajzel R (2003) Is measurement of D-dimer useful in the diagnosis of cerebral venous thrombosis? Neurology 61(8):1057–1060
Kellermair J, Redwan B, Alias S et al (2013) Platelet endothelial cell adhesion molecule 1 deficiency misguides venous thrombus resolution. Blood 122(19):3376–3384
Wimmer I, Tietz S, Nishihara H et al (2019) PECAM-1 stabilizes blood-brain barrier integrity and favors paracellular T-cell diapedesis across the blood-brain barrier during neuroinflammation. Front Immunol 10:711
Obi AT, Andraska E, Kanthi Y et al (2017) Endotoxaemia-augmented murine venous thrombosis is dependent on TLR-4 and ICAM-1, and potentiated by neutropenia. Thromb Haemost 117(2):339–348
Rabinovich A, Cohen JM, Cushman M et al (2015) Inflammation markers and their trajectories after deep vein thrombosis in relation to risk of post-thrombotic syndrome. J Thromb Haemost 13(3):398–408
Ley K, Huo Y (2001) VCAM-1 is critical in atherosclerosis. J Clin Invest 107(10):1209–1210
Koning GA, Schiffelers RM, Storm G (2002) Endothelial cells at inflammatory sites as target for therapeutic intervention. Endothelium 9(3):161–171
Kellermair J, Fellner A, Bittinger A et al (2020) Soluble platelet endothelial cell adhesion molecule 1 (sPECAM-1) improves diagnostic accuracy of D-Dimer in patients with suspected deep vein thrombosis (DVT). J Thromb Thrombolysis 50(2):380–385
Headache Classification Committee of the International Headache S (2013) The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 33(9):629–808
van Belle A, Buller HR, Huisman MV et al (2006) Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 295(2):172–179
Perrier A, Roy PM, Sanchez O et al (2005) Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med 352(17):1760–1768
Weimar C, Kurth T et al (2018) Zerebrale Venen- und Sinusthrombose, S2k-Leitlinie, 2018. Deutsche Gesellschaft für Neurologie (Hrsg.), Leitlinien für Diagnostik und Therapie in der Neurologie. Online: www.dgn.org/leitlinien. Published 2018. Accessed 01 June 2018
Linkins LA, Takach LS (2017) Review of D-dimer testing: good, bad, and ugly. Int J Lab Hematol 39(Suppl 1):98–103
Scarvelis D, Wells PS (2006) Diagnosis and treatment of deep-vein thrombosis. CMAJ 175(9):1087–1092
Agnelli G, Becattini C (2010) Acute pulmonary embolism. N Engl J Med 363(3):266–274
Schwameis M, Steiner MM, Schoergenhofer C et al (2015) D-dimer and histamine in early stage bacteremia: a prospective controlled cohort study. Eur J Intern Med 26(10):782–786
Rodelo JR, De la Rosa G, Valencia ML et al (2012) D-dimer is a significant prognostic factor in patients with suspected infection and sepsis. Am J Emerg Med 30(9):1991–1999
Adeeb N, Mortazavi MM, Tubbs RS, Cohen-Gadol AA (2012) The cranial dura mater: a review of its history, embryology, and anatomy. Childs Nerv Syst 28(6):827–837
Kilic T, Akakin A (2008) Anatomy of cerebral veins and sinuses. Front Neurol Neurosci 23:4–15
Rezoagli E, Martinelli I, Poli D et al (2018) The effect of recanalization on long-term neurological outcome after cerebral venous thrombosis. J Thromb Haemost 16(4):718–724
Kellermair L, Zeller MWG, Kulyk C, Tomasits J, von Oertzen TJ, Vosko MR (2022) Dabigatran in cerebral sinus vein thrombosis and thrombophilia. Life (Basel). 12(7):970
Killewich LA, Macko RF, Cox K et al (1997) Regression of proximal deep venous thrombosis is associated with fibrinolytic enhancement. J Vasc Surg 26(5):861–868
Killewich LA, Bedford GR, Beach KW, Strandness DE Jr (1989) Spontaneous lysis of deep venous thrombi: rate and outcome. J Vasc Surg 9(1):89–97
van Ramshorst B, van Bemmelen PS, Hoeneveld H, Faber JA, Eikelboom BC (1992) Thrombus regression in deep venous thrombosis. Quantification of spontaneous thrombolysis with duplex scanning. Circulation 86(2):414–419
Funding
Open access funding provided by Johannes Kepler University Linz.
Author information
Authors and Affiliations
Contributions
Research Project: (1) A Conception, B Organization, C Execution. Statistical Analysis: (2) A Design, B Execution, C Review and Critique. Manuscript: (3) A Writing of the first draft, B Review and Critique. LK: 1A, 1B, 1C, 2A, 2B, 3A; CH, MWGZ: 1C, 2C, 3B; CK, SW: 1B, 2C, 3B; DB, JK, RH: 2C, 3B; TF: 2A, 2B, 3B; MRV: 1A, 1C, 2C, 3B.
Corresponding author
Ethics declarations
Conflicts of interest
Lukas Kellermair has nothing to declare Christoph Höfer has nothing to declare Matthias W.G. Zeller: has nothing to declare Christa Kubasta: has nothing to declare; Bandke, Dave: has nothing to declare Serge Weis: has nothing to declare; Jörg Kellermair: has nothing to declare; Thomas Forstner: has nothing to declare Raimund Helbok: has nothing to declare Milan R. Vosko: has nothing to declare.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kellermair, L., Höfer, C., Zeller, M.W.G. et al. Endothelial receptor proteins in acute venous thrombosis and delayed thrombus resolution in cerebral sinus vein thrombosis. J Neurol (2024). https://doi.org/10.1007/s00415-024-12225-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00415-024-12225-3