Abstract
Background
The long-term prognosis of impulsive compulsive disorders (ICD) remains poorly studied in Parkinson’s disease (PD).
Objective
Evaluating the natural history of ICD and its impact on PD symptoms including cognition and treatment adjustments.
Materials and methods
We assessed PD patients at baseline (BL) with (BL-ICD+) or without (BL-ICD-) ICD despite dopamine agonist (DA) exposure of > 300 mg levodopa-equivalent daily dose for > 12 months at baseline and after more than two years of follow-up. ICD were assessed using the Ardouin’s Scale of Behaviors in PD (ASBPD), cognition using the Mattis scale, and PD symptoms using the UPDRS score. Treatment adjustments, DA withdrawal-associated symptoms, and ICDs social consequences were recorded.
Results
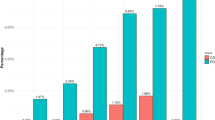
149 patients were included (78 cases and 71 controls), mean duration of follow-up was 4.4 ± 1 years. At baseline, psychiatric disorders were more common among BL-ICD + (42.3 vs 12.3% among BL-ICD-, p < 0.01). At follow-up, 53.8% of BL-ICD + were not ICD-free while 21.1% of BL-ICD- had developed ICD. BL-ICD + more frequently experienced akinesia (21.8 vs 8.5%, p = 0.043) and rigidity worsening (11.5 vs 1.4%, p = 0.019) following therapeutic modifications. Decision to decrease > 50% DA doses (12.8 vs 1.4%, p = 0.019) or to withdraw DA (19.2 vs 5.6%, p = 0.025) was more frequently considered among BL-ICD+ . At follow-up, the prevalence of cognitive decline was lower among BL-ICD + (19.2 vs 37.1%, p = 0.025).
Conclusion
ICDs were associated with increased psychiatric burden at baseline and better cognitive prognosis. Most patients were still showing ICDs at the follow-up visit, suggesting ICD to be considered as a chronic, neuropsychiatric disorder.

Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Potenza MN, Voon V, Weintraub D (2007) Drug Insight: impulse control disorders and dopamine therapies in Parkinson’s disease. Nat Rev Neurol 3:664–672
Weintraub D, Mamikonyan E (2019) Impulse control disorders in Parkinson’s disease. AJP 176:5–11
Voon V, Napier TC, Frank MJ et al (2017) Impulse control disorders and levodopa-induced dyskinesias in Parkinson’s disease: an update. Lancet Neurol 16:238–250
Santangelo G, Barone P, Trojano L et al (2013) Pathological gambling in Parkinson’s disease. A comprehensive review. Parkinsonism Relat Disord 19:645–53
Nirenberg MJ, Waters C (2006) Compulsive eating and weight gain related to dopamine agonist use. Mov Disord 21:524–529
Nakum S, Cavanna AE (2016) The prevalence and clinical characteristics of hypersexuality in patients with Parkinson’s disease following dopaminergic therapy: a systematic literature review. Parkinsonism Relat Disord 25:10–16
Weintraub D, Claassen DO (2017) Impulse Control and Related Disorders in Parkinson’s Disease. Int Rev Neurobiol 133:679–717
Faouzi J, Corvol J-C, Mariani L-L (2021) Impulse control disorders and related behaviors in Parkinson’s disease: risk factors, clinical and genetic aspects, and management. Curr Opin Neurol 34:547–555
Kelly MJ, Baig F, Hu MT-M et al (2020) Spectrum of impulse control behaviours in Parkinson’s disease: pathophysiology and management. J Neurol Neurosurg Psychiatry 91:703–11
Weintraub D, Koester J, Potenza MN et al (2010) Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol 67:589–595
Garcia-Ruiz PJ, Martinez Castrillo JC, Alonso-Canovas A et al (2014) Impulse control disorder in patients with Parkinson’s disease under dopamine agonist therapy: a multicentre study. J Neurol Neurosurg Psychiatry 85:840–844
Corvol J-C, Artaud F, Cormier-Dequaire F et al (2018) Longitudinal analysis of impulse control disorders in Parkinson disease. Neurology 91:e189-201
Siri C, Cilia R, Reali E et al (2015) Long-term cognitive follow-up of Parkinson’s disease patients with impulse control disorders. Mov Disord 30:696–704
Mamikonyan E, Siderowf AD, Duda JE et al (2008) Long-term follow-up of impulse control disorders in Parkinson’s disease. Mov Disord 23:75–80
Bastiaens J, Dorfman BJ, Christos PJ et al (2013) Prospective cohort study of impulse control disorders in Parkinson’s disease. Mov Disord 28:327–333
Averbeck BB, O’Sullivan SS, Djamshidian A (2014) Impulsive and compulsive behaviors in Parkinson’s disease. Annu Rev Clin Psychol 10:553–580
Cormier-Dequaire F, Bekadar S, Anheim M et al (2018) Suggestive association between OPRM1 and impulse control disorders in Parkinson’s disease. Mov Disord 33:1878–1886
Tomlinson CL, Stowe R, Patel S et al (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25:2649–2653
Goetz CG, Tilley BC, Shaftman SR et al (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23:2129–2170
Schmidt R, Freidl W, Fazekas F et al (1994) The Mattis dementia rating scale: normative data from 1,001 healthy volunteers. Neurology 44:964–964
Dubois B, Burn D, Goetz C et al (2007) Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord 22:2314–2324
Ardouin C, Chéreau I, Llorca P-M et al (2009) Assessment of hyper- and hypodopaminergic behaviors in Parkinson’s disease. Rev Neurol (Paris) 165:845–856
Weiss HD, Marsh L (2012) Impulse control disorders and compulsive behaviors associated with dopaminergic therapies in Parkinson disease. Neurol Clin Pract 2:267–274
Nirenberg MJ (2013) Dopamine agonist withdrawal syndrome: implications for patient care. Drugs Aging 30:587–592
Grant JE, Odlaug BL, Chamberlain SR et al (2013) A proof of concept study of tolcapone for pathological gambling: relationships with COMT genotype and brain activation. Eur Neuropsychopharmacol 23:1587–1596
Kurtis MM, Rajah T, Delgado LF et al (2017) The effect of deep brain stimulation on the non-motor symptoms of Parkinson’s disease: a critical review of the current evidence. npj Parkinson’s Dis 3:1–12
deS Santin MN, Voulleminot P, Vrillon A et al (2021) Impact of subthalamic deep brain stimulation on impulse control disorders in Parkinson’s disease: a prospective study. Mov Disord. 36:750–7
Liang J, Groves M, Shanker VL (2015) Clozapine treatment for impulse control disorders in Parkinson’s disease patients: a case series. Mov Disord Clin Pract 2:283–285
Béreau M, Van Waes V, Servant M et al (2023) Apathy in Parkinson’s disease: clinical patterns and neurobiological basis. Cells 12:1599
Scott BM, Eisinger RS, Burns MR et al (2020) Co-occurrence of apathy and impulse control disorders in Parkinson disease. Neurology 95:e2769–e2780
Sierra M, Carnicella S, Strafella AP et al (2015) Apathy and impulse control disorders: Yin & Yang of dopamine dependent behaviors. J Parkinsons Dis 5:625–636
Marín-Lahoz J, Sampedro F, Martinez-Horta S et al (2019) Depression as a risk factor for impulse control disorders in Parkinson disease. Ann Neurol 86:762–769
Aarsland D, Påhlhagen S, Ballard CG et al (2011) Depression in Parkinson disease–epidemiology, mechanisms and management. Nat Rev Neurol 8:35–47
Aracil-Bolaños I, Strafella AP (2016) Molecular imaging and neural networks in impulse control disorders in Parkinson’s disease. Parkinsonism Relat Disord 22(Suppl 1):S101-105
Cowen PJ (2008) Serotonin and depression: pathophysiological mechanism or marketing myth? Trends Pharmacol Sci 29:433–436
Kraemmer J, Smith K, Weintraub D et al (2016) Clinical-genetic model predicts incident impulse control disorders in Parkinson’s disease. J Neurol Neurosurg Psychiatry 87:1106–1111
Zhi Y, Yuan Y, Si Q et al (2019) The association between DRD3 Ser9Gly polymorphism and depression severity in Parkinson’s disease. Parkinson’s Dis 2019:e1642087
Peciña M, Karp JF, Mathew S et al (2019) Endogenous opioid system dysregulation in depression: implications for new therapeutic approaches. Mol Psychiatry 24:576–587
Funding
None.
Author information
Authors and Affiliations
Contributions
TW: Conception, execution of the study, analysis of the data, writing of the first draft, and review of the manuscript. TG: Conception, execution of the study, and analysis of the data. SB: Execution of the study, review, and critique of the manuscript. ER: Execution of the study, review, and critique of the manuscript. LLM: Execution of the study, review, and critique of the manuscript. MV: Execution of the study, review, and critique of the manuscript. AMB: Execution of the study, review, and critique of the manuscript. DG: Execution of the study, review, and critique of the manuscript. NM: Execution of the study, review, and critique of the manuscript. AP: Execution of the study, review, and critique of the manuscript. OR: Execution of the study, review, and critique of the manuscript. CB-C: Execution of the study, review, and critique of the manuscript. FO: Execution of the study, review, and critique of the manuscript. CA: Execution of the study, review, and critique of the manuscript. PK: Execution of the study, review, and critique of the manuscript. AM: Execution of the study, review, and critique of the manuscript. ML: Execution of the study, review, and critique of the manuscript. JLH: Execution of the study, review, and critique of the manuscript. PK: Execution of the study, review, and critique of the manuscript. AC: Execution of the study, review, and critique of the manuscript. VF: Execution of the study, review, and critique of the manuscript. DM: Execution of the study, review, and critique of the manuscript. LD: Execution of the study, review, and critique of the manuscript. AK: Execution of the study, review, and critique of the manuscript. CT: Conception, execution of the study, review, and critique of the manuscript. NM: Conception, execution of the study, analysis of the data, review, and critique of the manuscript. MA: Conception, execution, organization of the study, analysis of the data, review, and critique of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
T.W. received grants from the Revue Neurologique, the France Parkinson, the Fondation Planiol and the APTES organizations, honorarium from Abbvie, Ipsen and travels funding from LVL medical, Abbvie, and the Movement disorders society, E.R. received honorarium for speech from orkyn aguettant, elivie and for participating in an advisory board from allergan, research support from Merz-Pharma, Orkyn, Aguettant, Elivie, Ipsen, Allergan, Everpharma, Fondation Desmarest, AMADYS, ADCY5.org, Agence Nationale de la Recherche, Societé Française de Médecine Esthétique, Dystonia Medical Research Foundation. The rest of the authors declares no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
415_2023_12170_MOESM1_ESM.docx
Supplementary file1 (DOCX 13 KB) Detailed comparisons of current and history of psychiatric disorders between cases and controls at baseline. *p value <0.05
415_2023_12170_MOESM3_ESM.pdf
Supplementary file3 (PDF 80 KB) Forest plot showing the outcome of logistic regression determining the factors associated with ICD resolution among patients showing ICD at baseline. LEDD: levodopa-equivalent daily doses, COMTI: catechol ortho-methyl transferase inhibitor OR: Odd Ratio, CI: Confidence Interval. Odd ratios are given with their confidence intervals and their respective p-values
415_2023_12170_MOESM4_ESM.docx
Supplementary file4 (DOCX 13 KB) Motor consequences following therapeutic modifications in the whole cohort. *p value <0.05
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wirth, T., Goetsch, T., Corvol, JC. et al. Prognosis of impulse control disorders in Parkinson’s disease: a prospective controlled study. J Neurol 271, 2412–2422 (2024). https://doi.org/10.1007/s00415-023-12170-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-12170-7