Abstract
Background
Caring for a partner or family member with Parkinson’s disease (PD) negatively affects the caregiver’s own physical and emotional well-being, especially those caring for people with advanced PD (APD). This study was designed to examine the impact of APD on caregiver perceived burden, quality of life (QoL), and health status.
Methods
Dyads of people with PD and their primary caregivers were identified from the Adelphi Parkinson’s Disease Specific Program (DSP™) using real-world data from the United States, Japan and five European countries. Questionnaires were used to capture measures of clinical burden (people with PD) and caregiver burden (caregivers).
Results
Data from 721 patient-caregiver dyads in seven countries were captured. Caregivers had a mean age 62.6 years, 71.6% were female, and 70.4% were a spouse. Caregivers for people with APD had a greater perceived burden, were more likely to take medication and had lower caregiver treatment satisfaction than those caring for people with early or intermediate PD; similar findings were observed for caregivers of people with intermediate versus early PD. Caregivers for people with intermediate PD were also less likely to be employed than those with early PD (25.3% vs 42.4%) and spent more time caring (6.6 vs 3.2 h/day).
Conclusions
This real-world study demonstrates that caregivers of people with APD experience a greater burden than those caring for people with early PD. This highlights the importance of including caregiver-centric measures in future studies, and emphasizes the need for implementing treatments that reduce caregiver burden in APD.
Trial registration: N/A.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Parkinson’s disease (PD) is a progressive neurodegenerative disorder, characterized by tremor, bradykinesia and rigidity, with postural instability commonly seen later in the disease [1]. Considerable evidence demonstrates that PD has a profound impact on the quality of life (QoL) of patients, as well as their ability to carry out activities of daily living (ADLs) [2–12]. The incidence of PD has been estimated to range from five new cases in 100,000 to over 35 in 100,000 per year [13, 14], with both incidence and prevalence of PD increasing with age [15]. The number of cases of PD is predicted to increase from 6 million in 2015 to more than 12 million by 2040 [16].
The increasing prevalence of PD will have substantial implications for healthcare systems and economies worldwide, as people with PD tend to have high medical care needs, reduced productivity, and a dependence on informal and professional caregivers [17]. For example, an analysis based on multiple sources in the United States (US) estimated a total economic PD burden of $51.9 billion in 2017, projected to surpass $79 billion by 2037 [17].
Most people with PD live in the community and are looked after by informal caregivers such as spouses, adult children, friends, or other nonpaid individuals [18, 19]. However, caring for a partner or family member with PD negatively affects the caregiver’s own physical and emotional psychosocial well-being, and worsens QoL [2, 20–30]. Furthermore, the burden on caregivers tends to increase with advancing disease, along with deterioration in the speech and cognitive abilities of the person with PD [31–33].
In addition to the mental impact of caring for someone with PD, caregivers also often experience an economic burden, and sometimes financial hardship [34]. Added to the increased financial cost of caring for someone with PD, a caregiver is also likely to experience reduced productivity at work through absenteeism, disability, or forced retirement. Estimates show that PD caregivers have a higher cumulative income loss over 5 years compared with control subjects ($5967 vs $2634 by year 5; p = 0.03) [34].
The increasing prevalence of PD predicted over the next 20 years will compound each of these issues, placing further burden on healthcare systems, and increasing the financial, mental, and physical strain on caregivers. The problem for caregivers is more acute as pressures on central healthcare resources will increase the reliance on informal care at home [35]. Despite this impending crisis, little research has been undertaken to quantify the increased burden experienced by caregivers with advanced PD (APD), and the impact this has on the caregiver’s ability to care, own well-being, and QoL. The purpose of this study was to examine the impact of APD on caregiver perceived burden, QoL, and health status, using real-world data from the US, Japan and five European countries.
Methods
Data for this study were drawn from the Adelphi Parkinson’s Disease Specific Program (DSP™), a point-in-time survey of physicians and their consulting PD patients presenting in a real-world clinical setting between 2017 and 2019. Dyads of people with PD and their primary caregivers were identified from the Adelphi DSP in five European countries (France, Germany, Italy, Spain, UK), the US, and Japan. The DSP methodology has been previously published and validated [36]. In brief, participating physicians completed a record form for the next 12 consecutively consulting eligible patients, and each person with PD was then invited to complete a patient-reported questionnaire. Caregivers were also asked to complete a form, reporting on their own demographic characteristics and their perceived burden of care. Validated translations of the survey materials were developed for each of the countries where data collection took place.
Respondents provided informed consent for use of their data; all data were aggregated and de-identified before receipt. Surveys were conducted in full accordance with the US Health Insurance Portability and Accountability Act of 1996. The study protocol was approved by the Western Institutional Review Board (Puyallup, Washington, US) on 3 July 2019. All data were collected according to the requirements of ethics committee approval, including obtaining participants’ informed consent.
Participants
Physicians were eligible to participate in the survey if they were a neurologist experienced in the management of people with movement disorders, and were personally responsible for the treatment and management of three or more people with PD per week. Individuals were eligible for inclusion if they were diagnosed with PD on or before the date of their last consultation (i.e., at the time of data collection), were aged ≥ 18 years, were not currently involved in any clinical trials, and had a caregiver who was also willing to participate. Participants were classified by their physician as having early, intermediate, or advanced Parkinson’s disease (APD) based on their best clinical judgment after considering overall patient history including demographics, clinical characteristic, concomitant medications, and other patient-reported outcomes.
Measures
Demographic and disease characteristics of enrolled participants were captured. Measures of clinical burden included the physician’s best clinical judgment of disease severity (early PD, intermediate PD or APD), Charlson Comorbidity Index score (CCI; based on a number of conditions that are each assigned an integer weight from one to six; higher score indicates greater comorbidity burden) [37], time since diagnosis, Hoehn and Yahr scale (HY; measure of progression of symptoms and disability in PD; higher score indicates greater burden) [38], off-time, dyskinesia, axial symptoms (uncontrolled shuffling walk, freezing of gait, and falling/imbalance), Unified Parkinson’s Disease Rating Scale (UPDRS; clinical rating scale for PD encompassing behavior and mood, ADLs, motor symptoms, and complications; higher score indicates greater burden) [39], and Mini-Mental State Examination (MMSE; 11-question instrument testing five areas of cognitive function; lower score indicates worse cognitive function) [40].
Caregiver characteristics captured included demographic details, relationship to participant, duration of caring responsibilities, hours per day spent caring, caregiver medication use to support PD-related caregiving, 3-level version of the EuroQol 5 Dimension (EQ-5D-3L; a generic instrument to assess health-related QoL; scores range from 0 to 1, with a lower score indicating poorer QoL) [41], Zarit Burden Interview (ZBI; caregiver self-report 29-item questionnaire; higher score indicates greater burden) [42]. Categories of ZBI were interpreted as follows: slight burden (ZBI score 0–20), mild burden (ZBI score 21–40), moderate burden (ZBI score 41–60), and severe burden (ZBI score 61+) [43], and satisfaction with PD treatment (linear scale from 1 [very unsatisfied] to 7 [very satisfied]).
Statistical analysis
Demographic data of people with PD and caregivers were analyzed descriptively. Participant clinical characteristics, and measures of caregiver burden were also analyzed descriptively. Group comparisons were conducted using analysis of variance (ANOVA), chi-squared, and Kruskal–Wallis tests. Incremental caregiver burden was evaluated using generalized linear models (Gaussian, with identity link), the partial proportional odds model, and logistic regression models (reference = early PD) adjusting for country, participant factors (age, sex, CCI), and caregiver factors (age, sex, marital status). Pairwise comparisons were made using Sidak’s method to adjust for multiple comparisons. All analyses were conducted in Stata v17.0 (StataCorp LLC, College Station, Texas, USA).
Results
Patient demographics
In total, 222 physicians enlisted 721 patient-caregiver dyads from seven countries, with Germany accounting for the highest percentage of people with PD (23.2%). The mean (SD) age of people with PD at enrollment was 70.7 (9.6) years, and the mean age (SD) at diagnosis was 65.2 (9.7 years). Most (61.9%) people with PD were male, and the mean (SD) CCI score was 0.5 (1.1).
One hundred and twenty-seven participants (17.6%) were classified as having APD, with 258 (35.8%) and 336 (46.6%) recorded as having early and intermediate disease, respectively. The demographic and disease characteristics of enrolled participants are summarized in Table 1.
Caregiver demographics
The majority (70.4%) of caregivers enrolled in this study were the spouse of the person with PD, and most (71.6%) were female. The mean (SD) age of caregivers was 62.6 (12.8) years, and they had been caring for the person with PD for a mean (SD) of 4.6 (4.2) years. The characteristics of the caregivers are summarized in Table 2.
Impact of disease severity on caregiver productivity
A lower percentage of caregivers for people with intermediate PD were currently employed compared with caregivers for people with early PD (25.3% vs 42.4%; p < 0.001). However, the proportion of employed caregivers was numerically higher for people with APD than intermediate PD (31.2% vs 25.3%; p = NS) (Fig. 1a). This mirrors the age profile for caregivers, whereby the care-givers for people with early PD were younger than those for intermediate PD, while caregivers for people with APD were also younger than those for intermediate PD (Table 2).
The number of hours per day spent caring for the person with PD was significantly greater in caregivers of people with intermediate PD (6.6 [6.2] h/day) compared with those caring for individuals with early PD (3.2 [4.7] h/day; p = 0.001) (Fig. 1b). Caregivers of people with APD spent 8.4 (6.5) h caring per day (p = 0.336 vs early PD and p = 0.719 vs intermediate PD).
Impact of disease severity on caregiver burden
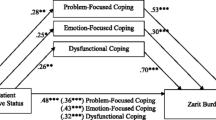
After adjusting for confounders, caregivers of people with intermediate PD had significantly higher self-perceived burden compared with those caring for people with early PD (ZBI = 29.5 vs 24.7; p < 0.001). In addition, caregivers of people with APD had significantly higher self-perceived burden (ZBI = 34.5) than those caring for people with intermediate PD (p = 0.005) or early PD (p < 0.001) (Table 3).
Similarly, caregivers of people with intermediate PD were significantly more likely to take medication due to PD-related caregiving than those caring for people with early PD (25.2% vs 13.8%, respectively, of caregivers took medication; p = 0.037). More than one-third (34.7%) of caregivers of people with APD took medication, which was significantly more than among caregivers of people with intermediate PD (p = 0.017) or early PD (p < 0.001) (Table 3).
A similar pattern was found for caregiver treatment satisfaction, which was significantly lower among caregivers of people with intermediate PD compared with those caring for an individual with early PD (4.9 vs 5.3 on a linear scale where 1 = very unsatisfied and 7 = very satisfied; p = 0.003), and for APD compared with intermediate PD (4.4 vs 4.9; p < 0.001). Caregivers of people with APD also had a significantly lower caregiver treatment satisfaction than those of people with early PD (p < 0.001) (Table 3).
In terms of caregiver QoL as assessed by the EQ-5D-3L score, no pairwise significant differences were found between caregivers of people with APD, intermediate PD, or early PD (Table 3).
As assessed by the ZBI, the proportion of caregivers experiencing moderate burden increased significantly with severity of PD (16.7%, 23.9%, and 36.0%, respectively; Sidak adjusted p = 0.001 for early vs intermediate PD; p = 0.007 for intermediate vs APD; and p < 0.001 for early vs APD). A similar increase with PD severity was observed among caregivers experiencing severe burden (2.0%, 5.1%, 6.4%, respectively; p = 0.006 for early vs intermediate PD; p = 0.045 for intermediate vs APD; and p = 0.002 for early vs APD) (Fig. 2).
Discussion
As pressure on healthcare resources increases, more individuals with chronic diseases are being cared for in the home, with the burden of care falling on family members (particularly a spouse) [35, 44, 45]. As PD is largely a disease of older age, it is likely that a person with PD will be cared for by a spouse who is him or herself elderly, and may have other comorbid conditions [46, 47]. Thus, national healthcare systems are being protected from a substantial amount of time and resource that is currently being provided by informal caregivers [48].
This study uses real-world data from 721 patient–caregiver dyads in seven countries to quantify the burden on caregivers, and particularly to evaluate the burden that falls on caregivers of people with APD. With a mean age 62.6 years, and a greater likelihood of being female (71.6%) and a spouse (70.4%), the profile of caregivers in this study is consistent with that reported in previous studies [35, 44–47].
One key finding of this study is that someone looking after a person with APD had significantly higher perceived burden, was significantly more likely to start medications due to PD-related caregiving, and was significantly less likely to be satisfied with treatment compared with the caregiver of someone with early or intermediate PD. Similar findings were also found for caregivers of people with intermediate PD compared with those looking after someone with early PD. Interestingly, no significant pairwise differences were observed for caregiver generic QoL according to severity of PD, as measured by the EQ-5D-3L score.
The range of ZBI scores observed in this study (24.7–34.5, depending on PD severity) are largely consistent with previous reports, which have also reported a greater burden with more severe PD [24, 46, 49–51]. The mean EQ-5D-3L score of 0.9 reported here irrespective of severity also suggests a slightly greater effect on caregiver QoL in the current study compared with previously reported studies reporting an EQ-5D-3L score of 0.7 [51, 52].
The amount of time required for caring also increased between intermediate and early PD (3.2 vs 6.6 h/day; p = 0.001); consistent with previous studies [47, 53]. However, while caregivers of people with intermediate PD were significantly less likely to be employed than caregivers of people with early PD, a numerically greater (but statistically non-significant) proportion of caregivers of people with advanced than intermediate PD were employed. This is initially surprising, as people with APD tended to be older than those with intermediate or early PD, suggesting that their spouses may also be older. However, people with more advanced disease are more likely to rely on professional care, including residential care, potentially allowing more time for a caregiver to work outside the home. In addition, there was a greater percentage of children caring for people with APD (35.4%) compared with intermediate (22.3%) and early PD (19.1%), which may reflect the greater age of the patients themselves.
The burden on caregivers also depends on the specific characteristics of people with PD, with improved spiritual well-being [46], HRQoL [46], and mood [54] reducing caregiver burden, and prolonged disease duration [53] increasing the burden. Increasing motor symptoms [53, 54], overall disability, and greater HY score have also been shown to be correlated with caregiver burden [47]. Interestingly, non-motor symptoms appear to have a greater effect on caregiver burden than motor symptoms [47, 54–57], as they disproportionately increase the need for supervision, and affect the emotional relationship between person with PD and the caregiver [47].
A failure to intervene to support families caring for an individual with APD is likely to result in a greater healthcare burden, as the caregivers themselves become ill, unable to offer support, and require care of their own. The development of strategies to reduce the burden of caregivers of people with PD are thus urgently required. Suggestions under consideration include more home-based community-centered integrated care [58], home adaptation [59], financial support for caregivers [59], and targeted interventions and counseling to help caregivers manage stress [59, 60]. In addition, health and wellness checks for caregivers may be effective in mitigating risk of future decline in caregiver QoL [60]. Offering caregiver interventions relatively early in the course of PD may mitigate decline in QoL as well as alter the trajectory of caregiver functioning [60]. Furthermore, experience during the coronavirus disease 2019 (COVID-19) pandemic has demonstrated that the use of remote consultations and the availability of digital communications can also offer access to care in difficult circumstances [61–63]. Use of this technology may allow caregivers to receive ongoing support and medical intervention, even if they are not easily able to leave the house.
From the side of the person with PD, new treatments are urgently required to optimize symptom control, allowing people with PD to function to a higher level without the need for substantial caregiver support [64]. Development of new treatments for PD has been slow over recent years, and available therapies have focused on slowing symptomatic decline rather than addressing the underlying pathophysiology of PD [65]. However, interest in disease-modifying therapies (DMTs) is currently flourishing [65], with exciting therapeutic approaches including cellular therapies, immunotherapies, and vaccines, as well as repurposed drugs [66]. Given the number of different approaches currently in the treatment pipeline, there may be grounds for cautious optimism that more effective treatments will start to become available in the near future. Indeed, evidence already suggests that the availability of advanced therapies allows caregivers more time for themselves, improves QoL, and tends to improve mood compared with standard PD therapies [24, 67]. In particular, an observational prospective study demonstrated that 6 months’ use of levodopa/carbidopa intestinal gel among people with APD significantly improved the QoL and SF-36 health status of caregivers [68]. Another study demonstrated that 12 months of use significantly improved MCSI outcomes among caregivers [69]. A systematic review of studies investigating the effects of deep brain stimulation revealed some variation in studies regarding the impact of this intervention on caregiver burden [70].
This large, international dataset is derived from the Parkinson’s DSP, which is validated for capturing large, statistically robust samples of global real-world evidence. The strengths of this study lie in its basis in real-world clinical practice, while the pairing of caregivers and people with PD allows us to investigate the impact of patient characteristics on the caregiver burden. In particular, this study demonstrates that in the real world, caregivers of people with APD are more likely to be female, spend more hours caring per day, have a higher self-perceived burden, worse QoL and a greater reliance on medication compared with those caring for people with early PD. Although the lack of restrictions and control provide us with true real-world insights, they may also be considered limitations of the study. For example, the risk of bias and confounding factors due to the absence of randomization, risk of missing or misclassified data, and the self-reported nature of the caregiver data are potential deficiencies of the study. As the DSP methodology required caregivers to be present at physician consultation, there is a risk of bias towards those most actively involved in caring for the patient. Situations where caregivers avoid caring for the individual with PD, or other complex interactions of this nature, may be unaccounted for. In addition, there was an imbalance in the geographic distribution of participants included in this study, while the caring setting was not captured in the US and Japan. There is also the risk of recall bias when caregivers were asked about their recent experience of caring for the person with PD. One further limitation of the study was the use of physician’s opinion of disease severity. However, as this judgement was based on the overall condition of the patient, this was considered the best reflection of management in clinical practice. Based on the inclusion criteria for physicians it was felt that all were highly experienced and could reasonably be expected to reliably distinguish degree of PD severity.
In the future, additional data should be collected on the value of educational programs for caregivers of people with PD, in helping them understand the nature of the disease and its progressive nature. In addition, it would be useful to evaluate the potential role of online technology in supporting the caregivers of people with APD, to assess whether this approach can provide meaningful access to healthcare for caregivers unable to travel or leave their family member unattended. Finally, future studies should evaluate the impact of optimal PD symptom control on alleviating the burden of caregiving.
Conclusions
This real-world study demonstrated that caregivers of people with advancing PD are more likely to be female, spend more hours caring per day, have a higher self-perceived burden, and a greater reliance on medication compared with those caring for people with early PD. These findings emphasize the importance of including caregiver-centric measures in future clinical study designs, and highlight the urgent need for new treatment options that lower burden among caregivers of people with advanced PD.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CCI:
-
Charlson comorbidity index
- DMT:
-
Disease-modifying therapies
- DSP:
-
Disease specific program
- HY:
-
Hoehn and Yahr
- MDS:
-
Movement disorder society
- MIUR:
-
Ministry of Education University and Research
- PD:
-
Parkinson’s disease
- QoL:
-
Quality of life
- UPDRS:
-
Unified Parkinson’s disease rating scale
- US:
-
United States
- ZBI:
-
Zarit Burden interview
References
Zafar S, Yaddanapudi SS (2021) Parkinson disease. StatPearls. StatPearls Publishing Copyright © 2021. StatPearls Publishing LLC., Treasure Island, FL
Rajiah K, Maharajan MK, Yeen SJ, Lew S (2017) Quality of Life and caregivers’ burden of Parkinson’s disease. Neuroepidemiology 48(3–4):131–137. https://doi.org/10.1159/000479031
Balestrino R, Martinez-Martin P (2017) Neuropsychiatric symptoms, behavioural disorders, and quality of life in Parkinson’s disease. J Neurol Sci 373:173–178. https://doi.org/10.1016/j.jns.2016.12.060
Kuhlman GD, Flanigan JL, Sperling SA, Barrett MJ (2019) Predictors of health-related quality of life in Parkinson’s disease. Parkinsonism Relat Disord 65:86–90. https://doi.org/10.1016/j.parkreldis.2019.05.009
Crispino P, Gino M, Barbagelata E, Ciarambino T, Politi C, Ambrosino I et al (2020) Gender differences and quality of life in Parkinson’s disease. Int J Environ Res Public Health 18(1):198. https://doi.org/10.3390/ijerph18010198
Balash Y, Korczyn AD, Migirov AA, Gurevich T (2019) Quality of life in Parkinson’s disease: a gender-specific perspective. Acta Neurol Scand 140(1):17–22. https://doi.org/10.1111/ane.13095
Limongi JCP (2017) Quality of life in Parkinson’s disease. Arq Neuropsiquiatr 75(8):493–494. https://doi.org/10.1590/0004-282x20170114
Palmeri R, Lo Buono V, Bonanno L, Sorbera C, Cimino V, Bramanti P et al (2019) Potential predictors of quality of life in Parkinson’s disease: sleep and mood disorders. J Clin Neurosci 70:113–117. https://doi.org/10.1016/j.jocn.2019.08.058
Kwok JYY, Auyeung M, Chan HYL (2020) Examining factors related to health-related quality of life in people with Parkinson’s disease. Rehabil Nurs. 45(3):122–130. https://doi.org/10.1097/rnj.0000000000000179
Lubomski M, Davis RL, Sue CM (2021) Health-Related Quality of life for Parkinson’s disease patients and their caregivers. J Mov Disord 14(1):42–52. https://doi.org/10.14802/jmd.20079
Sjödahl Hammarlund C, Westergren A, Åström I, Edberg A-K, Hagell P (2018) The impact of living with Parkinson’s disease: balancing within a web of needs and demands. Parkinson’s Dis 2018:4598651. https://doi.org/10.1155/2018/4598651
Zhao N, Yang Y, Zhang L, Zhang Q, Balbuena L, Ungvari GS et al (2021) Quality of life in Parkinson’s disease: a systematic review and meta-analysis of comparative studies. CNS Neurosci Ther 27(3):270–279. https://doi.org/10.1111/cns.13549
Simon DK, Tanner CM, Brundin P (2020) Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clin Geriatr Med. 36(1):1–12. https://doi.org/10.1016/j.cger.2019.08.002
Twelves D, Perkins KS, Counsell C (2003) Systematic review of incidence studies of Parkinson’s disease. Mov Disord 18(1):19–31. https://doi.org/10.1002/mds.10305
Marras C, Beck JC, Bower JH, Roberts E, Ritz B, Ross GW et al (2018) Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis 4:21. https://doi.org/10.1038/s41531-018-0058-0
Dorsey ER, Sherer T, Okun MS, Bloem BR (2018) The emerging evidence of the Parkinson pandemic. J Parkinsons Dis 8(s1):S3–S8. https://doi.org/10.3233/JPD-181474
Yang W, Hamilton JL, Kopil C, Beck JC, Tanner CM, Albin RL et al (2020) Current and projected future economic burden of Parkinson’s disease in the US. NPJ Parkinsons Dis 6:15. https://doi.org/10.1038/s41531-020-0117-1
Martinez-Martin P, Rodriguez-Blazquez C, Forjaz MJ (2012) Quality of life and burden in caregivers for patients with Parkinson’s disease: concepts, assessment and related factors. Expert Rev Pharmacoecon Outcomes Res 12(2):221–230. https://doi.org/10.1586/erp.11.106
Boersma I, Jones J, Coughlan C, Carter J, Bekelman D, Miyasaki J et al (2017) Palliative care and Parkinson’s disease: caregiver perspectives. J Palliat Med 20(9):930–938. https://doi.org/10.1089/jpm.2016.0325
Lee J, Kim SH, Kim Y, Kim YL, Sohn Y (2019) Quality of life of caregivers of individuals with Parkinson’s disease. Rehabil Nurs 44(6):338–348. https://doi.org/10.1097/rnj.0000000000000158
Yuksel B, Ak PD, Sen A, Sariahmetoglu H, Uslu SC, Atakli D (2018) Caregiver burden and quality of life in early stages of idiopathic Parkinson’s disease. Ideggyogy Sz 71(9–10):343–350
Tan SB, Williams AF, Tan EK, Clark RB, Morris ME (2020) Parkinson’s disease caregiver strain in Singapore. Front Neurol 11:455. https://doi.org/10.3389/fneur.2020.00455
Perry SE, Borders JC, Dakin AE, Troche MS (2021) Characterizing quality of life in caregivers of people with Parkinson’s disease and dysphagia. Dysphagia. https://doi.org/10.1007/s00455-021-10299-z
Modugno N, Antonini A, Tessitore A, Marano P, Pontieri FE, Tambasco N et al (2020) Impact of supporting people with advanced Parkinson’s disease on career’s quality of life and burden. Neuropsychiatr Dis Treat 16:2899–2912. https://doi.org/10.2147/ndt.S256217
Tessitore A, Marano P, Modugno N, Pontieri FE, Tambasco N, Canesi M et al (2018) Caregiver burden and its related factors in advanced Parkinson’s disease: data from the PREDICT study. J Neurol 265(5):1124–1137. https://doi.org/10.1007/s00415-018-8816-9
Caap-Ahlgren M, Dehlin O (2002) Factors of importance to the caregiver burden experienced by family caregivers of Parkinson’s disease patients. Aging Clin Exp Res 14(5):371–377. https://doi.org/10.1007/bf03324464
Henry RS, Lageman SK, Perrin PB (2020) The relationship between Parkinson’s disease symptoms and caregiver quality of life. Rehabil Psychol. 65(2):137–144. https://doi.org/10.1037/rep0000313
Gultekin M, Ekinci A, Erturk G, Mirza M (2017) Female Parkinson’s disease caregivers have much anxiety and depressive symptom. Brain Behav 7(9):e00787. https://doi.org/10.1002/brb3.787
Saadat P, Faramarzi M, Salimkhani F, Khafri S (2020) Psychiatric symptoms in patients and caregivers with Parkinson’s disease. Oman Med J 35(6):e205. https://doi.org/10.5001/omj.2020.91
Martínez-Martín P, Forjaz MJ, Frades-Payo B, Rusiñol AB, Fernández-García JM, Benito-León J et al (2007) Caregiver burden in Parkinson’s disease. Mov Disord 22(7):924–931. https://doi.org/10.1002/mds.21355
Schrag A, Hovris A, Morley D, Quinn N, Jahanshahi M (2006) Caregiver-burden in Parkinson’s disease is closely associated with psychiatric symptoms, falls, and disability. Parkinsonism Relat Disord 12(1):35–41. https://doi.org/10.1016/j.parkreldis.2005.06.011
Oh YS, Lee JE, Lee PH, Kim JS (2015) Neuropsychiatric symptoms in Parkinson’s disease dementia are associated with increased caregiver burden. J Mov Disord 8(1):26–32. https://doi.org/10.14802/jmd.14019
Sensi M, Cossu G, Mancini F, Pilleri M, Zibetti M, Modugno N et al (2017) Which patients discontinue? Issues on Levodopa/carbidopa intestinal gel treatment: Italian multicentre survey of 905 patients with long-term follow-up. Parkinsonism Relat Disord 38:90–92. https://doi.org/10.1016/j.parkreldis.2017.02.020
Martinez-Martin P, Macaulay D, Jalundhwala YJ, Mu F, Ohashi E, Marshall T et al (2019) The long-term direct and indirect economic burden among Parkinson’s disease caregivers in the United States. Mov Disord 34(2):236–245. https://doi.org/10.1002/mds.27579
Petrini M, Cirulli F, D’Amore A, Masella R, Venerosi A, Carè A (2019) Health issues and informal caregiving in Europe and Italy. Ann Ist Super Sanita 55(1):41–50. https://doi.org/10.4415/ann_19_01_08
Anderson P, Benford M, Harris N, Karavali M, Piercy J (2008) Real-world physician and patient behaviour across countries: disease-specific programmes—a means to understand. Curr Med Res Opin 24(11):3063–3072. https://doi.org/10.1185/03007990802457040
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383. https://doi.org/10.1016/0021-9681(87)90171-8
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17(5):427–442. https://doi.org/10.1212/wnl.17.5.427
Fahn S, Elton RL, UPDRS program members (1987) Unified Parkinsons Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB (Eds) Recent developments in Parkinsons disease, vol 2. Macmillan Healthcare Information, Florham Park, NJ, pp 153–163
Folstein MF, Folstein SE, McHugh PR (1975) "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198. https://doi.org/10.1016/0022-3956(75)90026-6
Devlin NJ, Brooks R (2017) EQ-5D and the EuroQol group: past, present and future. Appl Health Econ Health Policy 15(2):127–137. https://doi.org/10.1007/s40258-017-0310-5
Zarit SH, Reever KE, Bach-Peterson J (1980) Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist 20(6):649–655. https://doi.org/10.1093/geront/20.6.649
Hébert R, Bravo G, Préville M (2000) Reliability, validity and reference values of the Zarit Burden interview for assessing informal caregivers of community-dwelling older persons with dementia. Can J Aging La Rev Can Vieilliss 19(4):494–507. https://doi.org/10.1017/S0714980800012484
Tur-Sinai A, Teti A, Rommel A, Hlebec V, Lamura G (2020) How many older informal caregivers are there in Europe? Comparison of estimates of their prevalence from three European surveys. Int J Environ Res Public Health 17(24):9531. https://doi.org/10.3390/ijerph17249531
Verbakel E (2018) How to understand informal caregiving patterns in Europe? The role of formal long-term care provisions and family care norms. Scand J Public Health. 46(4):436–447. https://doi.org/10.1177/1403494817726197
Macchi ZA, Koljack CE, Miyasaki JM, Katz M, Galifianakis N, Prizer LP et al (2020) Patient and caregiver characteristics associated with caregiver burden in Parkinson’s disease: a palliative care approach. Ann Palliat Med 9(Suppl 1):S24–S33
Mosley PE, Moodie R, Dissanayaka N (2017) Caregiver burden in parkinson disease: a critical review of recent literature. J Geriatr Psychiatry Neurol 30(5):235–252. https://doi.org/10.1177/0891988717720302
European Observatory on Health Systems and Policies (2021) Caring for people with chronic conditions. A health system perspective 2008. Available from https://www.euro.who.int/__data/assets/pdf_file/0006/96468/E91878.pdf. Accessed May 2021
Jones AJ, Kuijer RG, Livingston L, Myall D, Horne K, MacAskill M et al (2017) Caregiver burden is increased in Parkinson’s disease with mild cognitive impairment (PD-MCI). Transl Neurodegener 6:17. https://doi.org/10.1186/s40035-017-0085-5
Tolosa E, Ebersbach G, Ferreira JJ, Rascol O, Antonini A, Foltynie T et al (2021) The Parkinson’s Real-World Impact Assessment (PRISM) Study: a European survey of the burden of Parkinson’s disease in patients and their careers. J Parkinsons Dis. https://doi.org/10.3233/jpd-212611
Carod-Artal FJ, Mesquita HM, Ziomkowski S, Martinez-Martin P (2013) Burden and health-related quality of life among caregivers of Brazilian Parkinson’s disease patients. Parkinsonism Relat Disord 19(11):943–948. https://doi.org/10.1016/j.parkreldis.2013.06.005
Grün D, Pieri V, Vaillant M, Diederich NJ (2016) Contributory factors to caregiver burden in Parkinson disease. J Am Med Dir Assoc 17(7):626–632. https://doi.org/10.1016/j.jamda.2016.03.004
Tan MMJ, Lim EC, Nadkarni NV, Lye WK, Tan EK, Prakash KM (2019) The characteristics of patients associated with high caregiver burden in Parkinson’s disease in Singapore. Front Neurol 10:561. https://doi.org/10.3389/fneur.2019.00561
Torny F, Videaud H, Chatainier P, Tarrade C, Meissner WG, Couratier P (2018) Factors associated with spousal burden in Parkinson’s disease. Rev Neurol (Paris) 174(10):711–715. https://doi.org/10.1016/j.neurol.2018.01.372
Hiseman JP, Fackrell R (2017) Caregiver burden and the nonmotor symptoms of Parkinson’s Disease. Int Rev Neurobiol 133:479–497. https://doi.org/10.1016/bs.irn.2017.05.035
Carter JH, Stewart BJ, Lyons KS, Archbold PG (2008) Do motor and nonmotor symptoms in PD patients predict caregiver strain and depression? Mov Disord 23(9):1211–1216. https://doi.org/10.1002/mds.21686
Smith ER, Perrin PB, Tyler CM, Lageman SK, Villaseñor T (2019) Parkinson’s symptoms and caregiver burden and mental health: a cross-cultural mediational model. Behav Neurol 2019:1396572. https://doi.org/10.1155/2019/1396572
Fabbri M, Caldas AC, Ramos JB, Sanchez-Ferro Á, Antonini A, Růžička E et al (2020) Moving towards home-based community-centred integrated care in Parkinson’s disease. Parkinsonism Relat Disord 78:21–26. https://doi.org/10.1016/j.parkreldis.2020.07.001
Padovani C, Lopes MCL, Higahashi IH, Pelloso SM, Paiano M, Christophoro R (2018) Being caregiver of people with Parkinson’s disease: experienced situations. Rev Bras Enferm 71(suppl 6):2628–2634. https://doi.org/10.1590/0034-7167-2017-0008
Lageman SK, Mickens MN, Cash TV (2015) Caregiver-identified needs and barriers to care in Parkinson’s disease. Geriatr Nurs 36(3):197–201. https://doi.org/10.1016/j.gerinurse.2015.01.002
Wijesooriya NR, Mishra V, Brand PLP, Rubin BK (2020) COVID-19 and telehealth, education, and research adaptations. Paediatr Respir Rev. 35:38–42. https://doi.org/10.1016/j.prrv.2020.06.009
Wosik J, Fudim M, Cameron B, Gellad ZF, Cho A, Phinney D et al (2020) Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inform Assoc 27(6):957–962. https://doi.org/10.1093/jamia/ocaa067
Temesgen ZM, DeSimone DC, Mahmood M, Libertin CR, VaratharajPalraj BR, Berbari EF (2020) Health care after the COVID-19 pandemic and the influence of telemedicine. Mayo Clin Proc 95(9s):S66–S68. https://doi.org/10.1016/j.mayocp.2020.06.052
LeWitt PA, Chaudhuri KR (2020) Unmet needs in Parkinson disease: motor and non-motor. Parkinsonism Relat Disord 80(Suppl 1):S7–S12. https://doi.org/10.1016/j.parkreldis.2020.09.024
McFarthing K, Rafaloff G, Baptista M, Wyse RK, Stott SRW (2021) Clinical Trial Highlights—Parkinson’s disease drug therapies in the clinical trial pipeline: 2021 update. J Parkinsons Dis. https://doi.org/10.3233/jpd-219006
Elkouzi A, Vedam-Mai V, Eisinger RS, Okun MS (2019) Emerging therapies in Parkinson disease—repurposed drugs and new approaches. Nat Rev Neurol 15(4):204–223. https://doi.org/10.1038/s41582-019-0155-7
Santos-García D, Añón MJ, Fuster-Sanjurjo L, de la Fuente-Fernández R (2012) Duodenal levodopa/carbidopa infusion therapy in patients with advanced Parkinson’s disease leads to improvement in caregivers’ stress and burden. Eur J Neurol 19(9):1261–1265. https://doi.org/10.1111/j.1468-1331.2011.03630.x
Ciurleo R, Corallo F, Bonanno L, Lo Buono V, Di Lorenzo G, Versaci R et al (2018) Assessment of Duodopa(®) effects on quality of life of patients with advanced Parkinson’s disease and their caregivers. J Neurol 265(9):2005–2014. https://doi.org/10.1007/s00415-018-8951-3
Standaert DG, Aldred J, Anca-Herschkovitsch M, Bourgeois P, Cubo E, Davis TL et al (2021) DUOGLOBE: one-year outcomes in a real-world study of levodopa carbidopa intestinal gel for Parkinson’s disease. Mov Disord Clin Pract 8:1061–1074. https://doi.org/10.1002/mdc3.13239
van Hienen MM, Contarino MF, Middelkoop HAM, van Hilten JJ, Geraedts VJ (2020) Effect of deep brain stimulation on caregivers of patients with Parkinson’s disease: a systematic review. Parkinsonism Relat Disord. 81:20–27. https://doi.org/10.1016/j.parkreldis.2020.09.038
Acknowledgements
We acknowledge Alex Gillespie, Jack Wright, and Eddie Jones for their support on conducting the data analysis. We would also like to thank Yash J. Jalundhwala and Niodita Gupta for contributing to the study design and interpretation of the results, and Rachel Danks for assistance with the preparation of this manuscript.
Funding
Financial support for the study was provided by AbbVie. AbbVie participated in the interpretation of the data, review, and approval of the manuscript. All authors contributed to the development of the manuscript and maintained control over the final content.
Author information
Authors and Affiliations
Contributions
All authors read, contributed to, and approved all the drafts and the final manuscript. AA and VSC also contributed to the study design, analysis and interpretation of results. JP performed analysis and interpretation of data.
Corresponding author
Ethics declarations
Conflict of interest
PMM has received honoraria from National School of Public Health (ISCIII), and Editorial Viguera for lecturing in courses; from Bial for advice in a clinical-epidemiological study; and from the International Parkinson and Movement Disorder Society (IPMDS) for management of the Program on Rating Scales. He received grant support from International Parkinson and Movement Disorder Society, for development and validation of the MDS-NMS. MS received speakers’ honoraria and/or consultation fees from AbbVie, Biogen, Boston Scientific, Ever Pharma, Krka, Medtronic and Sandoz, he received grant support from the IBM, from Slovak grant agencies under contracts APVV-18-0547 and VEGA 1/0596/19 and the Operational Program Integrated Infrastructure, funded by the ERDF under No. ITMS2014 + :313011V455. TH has received honorary for speaker related activities for Lundbeck, Lobsor, Zambon, Britannia, EVER Pharma, and AbbVie. AA has received compensation for consultancy and speaker related activities from UCB, Boehringer–Ingelheim, Britannia, AbbVie, Kyowa Kirin, Zambon, Bial, Neuroderm, Theravance Biopharma, Roche; he receives research support from Chiesi Pharmaceuticals, Lundbeck, Horizon 2020—Grant 825785, Horizon2020 Grant 101016902, Ministry of Education University and Research (MIUR) Grant ARS01_01081, Cariparo Foundation. He serves as consultant for Boehringer–Ingelheim for legal cases on pathological gambling. AA, PLK, ABP, and JCP are employees of AbbVie and may own stocks/shares in the company. VSC is a former employee of AbbVie Inc., currently employed by EMD Serono, Inc. and may hold AbbVie stock. JP is an employee of Adelphi Real World, a consulting company that was hired by AbbVie to perform analyses on the Adelphi Disease Specific Program database. SL and JD declare that they have no relevant or material financial interests that relate to the research described in this paper.
Ethics approval and consent to participate
Respondents provided informed consent for use of their data; all data were aggregated and de-identified before receipt. Surveys were conducted in full accordance with the US Health Insurance Portability and Accountability Act of 1996. The study protocol was approved by the Western Institutional Review Board (Puyallup, Washington, US) on 3 July 2019. All data were collected according to the requirements of ethics committee approval, including obtaining participants’ informed consent.
Consent for publication
Consent to publish was provided by all authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martinez-Martin, P., Skorvanek, M., Henriksen, T. et al. Impact of advanced Parkinson’s disease on caregivers: an international real-world study. J Neurol 270, 2162–2173 (2023). https://doi.org/10.1007/s00415-022-11546-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11546-5