Abstract
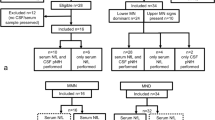
Misdiagnosis is frequent in early motor neuron disease (MND), typically compressive radiculopathy, or in patients with restricted MND phenotype. In this retrospective, single tertiary centre study, we measured levels of neurofilament light (NfL) and phosphorylated neurofilament heavy (p-NfH) chain in cerebrospinal fluid (CSF) and of p-NfH in serum with commercially available ELISA kits and assessed their respective diagnostic performance as a marker of MND. The entire study population (n = 164) comprised 71 MND patients, 30 patients with compressive myelo- or radiculopathy, and 63 disease controls (DC). Among MND patients, we specified subgroups with only lower motoneuron involvement (MND-LMN, n = 15) and with confounding nerve roots or spinal cord compression (MND-C, n = 18), representing clinical diagnostic pitfalls. MND-LMN displayed significantly lower CSF NfL (p = 0.003) and p-NFH (p = 0.017), but not serum p-NfH (p = 0.347) levels compared to other MND patients (n = 56). The discriminative ability (area under the curve—AUC) of both CSF Nfs towards all MND patients was comparable to each other but significantly higher than that of p-NfH in serum (ps < 0.001). AUC of both CSF Nfs between MND-LMN and DC and also between MND-C and myelo-/radiculopathies were reduced, as compared to AUC between other MND and DC or myelo-/radiculopathies, respectively. Our results suggest that both Nfs in CSF represent a reliable diagnostic marker in a general MND population, fulfilling Awaji criteria. As for diagnostic pitfalls, and also for p-NfH in serum, their discriminative ability and, therefore, clinical utility appears to be limited.



Similar content being viewed by others
Data sharing statement
The data used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Chiò A, Calvo A, Moglia C, Mazzini L, Mora G, PARALS study group (2011) Phenotypic heterogeneity of amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry 82(7):740–746. https://doi.org/10.1136/jnnp.2010.235952
Bendotti C, Bonetto V, Pupillo E et al (2020) Focus on the heterogeneity of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 21(7–8):485–495. https://doi.org/10.1080/21678421.2020.1779298
Cellura E, Spataro R, Taiello AC, La Bella V (2012) Factors affecting the diagnostic delay in amyotrophic lateral sclerosis. Clin Neurol Neurosurg 114(6):550–554. https://doi.org/10.1016/j.clineuro.2011.11.026
Srinivasan J, Scala S, Jones HR, Saleh F, Russell JA (2006) Inappropriate surgeries resulting from misdiagnosis of early amyotrophic lateral sclerosis. Muscle Nerve 34(3):359–360. https://doi.org/10.1002/mus.20555
Pinto S, Swash M, de Carvalho M (2014) Does surgery accelerate progression of amyotrophic lateral sclerosis? J Neurol Neurosurg Psychiatry 85(6):643–646. https://doi.org/10.1136/jnnp-2013-305770
Sanderson AB, Arnold WD, Elsheikh B, Kissel JT (2015) The clinical spectrum of isolated peripheral motor dysfunction. Muscle Nerve 51(3):358–362. https://doi.org/10.1002/mus.24326
Simon NG, Ayer G, Lomen-Hoerth C (2013) Is IVIg therapy warranted in progressive lower motor neuron syndromes without conduction block? Neurology 81(24):2116–2120. https://doi.org/10.1212/01.wnl.0000437301.28441.7e
Poesen K, Van Damme P (2019) Diagnostic and prognostic performance of neurofilaments in ALS. Front Neurol 18(9):1167. https://doi.org/10.3389/fneur.2018.01167
Yuan A, Rao MV, Veeranna, Nixon RA (2017) Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol 9(4):a018309. https://doi.org/10.1101/cshperspect.a018309 (Published 2017 Apr 3)
Lobsiger CS, Cleveland DW (2009) Neurofilaments: organization and function in neurons. In: Squire LR (ed) Encyclopedia of neuroscience. Elsevier, Amsterdam, pp 433–436. https://doi.org/10.1016/B978-008045046-9.00728-2
Gaiottino J, Norgren N, Dobson R et al (2013) Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS ONE 8(9):e75091. https://doi.org/10.1371/journal.pone.0075091
Bridel C, van Wieringen WN, Zetterberg H et al (2019) Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol 76(9):1035–1048. https://doi.org/10.1001/jamaneurol.2019.1534
Kuhle J, Regeniter A, Leppert D, Mehling M, Kappos L, Lindberg RL, Petzold A (2010) A highly sensitive electrochemiluminescence immunoassay for the neurofilament heavy chain protein. J Neuroimmunol 220(1–2):114–119. https://doi.org/10.1016/j.jneuroim.2010.01.004
Kušnierová P, Zeman D, Hradílek P, Čábal M, Zapletalová O (2019) Neurofilament levels in patients with neurological diseases: a comparison of neurofilament light and heavy chain levels. J Clin Lab Anal 33(7):e22948. https://doi.org/10.1002/jcla.22948
Rossi D, Volanti P, Brambilla L, Colletti T, Spataro R, La Bella V (2018) CSF neurofilament proteins as diagnostic and prognostic biomarkers for amyotrophic lateral sclerosis. J Neurol 265(3):510–521. https://doi.org/10.1007/s00415-017-8730-6
Ohya J, Chikuda H, Kato S, Hayakawa K, Oka H, Takeshita K, Tanaka S, Ogata T (2015) Elevated levels of phosphorylated neurofilament heavy subunit in the cerebrospinal fluid of patients with lumbar spinal stenosis: preliminary findings. Spine J 15(7):1587–1592. https://doi.org/10.1016/j.spinee.2015.03.013
Takahashi H, Aoki Y, Nakajima A et al (2018) Axonal damage is remarkable in patients with acutely worsening symptoms of compression myelopathy: biomarkers in cerebrospinal fluid samples. Eur Spine J 27(8):1824–1830. https://doi.org/10.1007/s00586-018-5549-5
Poesen K, De Schaepdryver M, Stubendorff B et al (2017) Neurofilament markers for ALS correlate with extent of upper and lower motor neuron disease. Neurology 88(24):2302–2309. https://doi.org/10.1212/WNL.0000000000004029
De Schaepdryver M, Jeromin A, Gille B, Claeys KG, Herbst V, Brix B, Van Damme P, Poesen K (2018) Comparison of elevated phosphorylated neurofilament heavy chains in serum and cerebrospinal fluid of patients with amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 89(4):367–373. https://doi.org/10.1136/jnnp-2017-316605
Steinacker P, Feneberg E, Weishaupt J et al (2016) Neurofilaments in the diagnosis of motoneuron diseases: a prospective study on 455 patients. J Neurol Neurosurg Psychiatry 87(1):12–20. https://doi.org/10.1136/jnnp-2015-311387
Gaiani A, Martinelli I, Bello L et al (2017) Diagnostic and prognostic biomarkers in amyotrophic lateral sclerosis: neurofilament light chain levels in definite subtypes of disease. JAMA Neurol 74(5):525–532. https://doi.org/10.1001/jamaneurol.2016.5398
Falzone YM, Domi T, Agosta F et al (2020) Serum phosphorylated neurofilament heavy-chain levels reflect phenotypic heterogeneity and are an independent predictor of survival in motor neuron disease. J Neurol 267(8):2272–2280. https://doi.org/10.1007/s00415-020-09838-9
Ludolph A, Drory V, Hardiman O, Nakano I, Ravits J, Robberecht W, Shefner J, WFN Research Group On ALS/MND (2015) A revision of the El Escorial criteria 2015. Amyotroph Lateral Scler Frontotemporal Degener. 16(56):291–292. https://doi.org/10.3109/21678421.2015.1049183
Kimura F, Fujimura C, Ishida S, Nakajima H, Furutama D, Uehara H, Shinoda K, Sugino M, Hanafusa T (2006) Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology 66(2):265–267. https://doi.org/10.1212/01.wnl.0000194316.91908.8a
Lee SH, Kim KT, Suk KS, Lee JH, Shin JH, So DH, Kwack YH (2010) Asymptomatic cervical cord compression in lumbar spinal stenosis patients: a whole spine magnetic resonance imaging study. Spine (Phila Pa). 35(23):2057–2063. https://doi.org/10.1097/BRS.0b013e3181f4588a
Schönström N, Willén J (2001) Imaging lumbar spinal stenosis. Radiol Clin N Am 39(1):31–53. https://doi.org/10.1016/s0033-8389(05)70262-1
Halbgebauer S, Steinacker P, Verde F, Weishaupt J, Oeckl P, von Arnim C, Dorst J, Feneberg E, Mayer B, Rosenbohm A, Silani V, Ludolph AC, Otto M (2022) Comparison of CSF and serum neurofilament light and heavy chain as differential diagnostic biomarkers for ALS. J Neurol Neurosurg Psychiatry 93(1):68–74. https://doi.org/10.1136/jnnp-2021-327129
Lu CH, Petzold A, Topping J et al (2015) Plasma neurofilament heavy chain levels and disease progression in amyotrophic lateral sclerosis: insights from a longitudinal study. J Neurol Neurosurg Psychiatry 86(5):565–573. https://doi.org/10.1136/jnnp-2014-307672
Wilke C, Pujol-Calderón F, Barro C et al (2019) Correlations between serum and CSF pNfH levels in ALS, FTD and controls: a comparison of three analytical approaches. Clin Chem Lab Med 57(10):1556–1564. https://doi.org/10.1515/cclm-2019-0015
Schreiber S, Spotorno N, Schreiber F et al (2018) Significance of CSF NfL and tau in ALS. J Neurol 265(11):2633–2645. https://doi.org/10.1007/s00415-018-9043-0
Menke RA, Gray E, Lu CH, Kuhle J, Talbot K, Malaspina A, Turner MR (2015) CSF neurofilament light chain reflects corticospinal tract degeneration in ALS. Ann Clin Transl Neurol 2(7):748–755. https://doi.org/10.1002/acn3.212
Funding
This study was supported by the Ministry of Health, Czech Republic—conceptual development of research organization, University Hospital Motol, Prague, Czech Republic grant no. 00064203.
Author information
Authors and Affiliations
Contributions
JH performed the experiments; JH and DB contributed to analysis and interpretation of the data. DB and RM recruited the patients, performed the clinical evaluation, and acquired clinical data. DB and RM drafted the manuscript. DB and RM conceptualized and designed the study. All authors had full access to the data in the study, critically revised, and approved the final version of the manuscript. The two (DB, RM) of the three authors of this publication are members of the European Reference Network for Neuromuscular Diseases—Project ID No. 870177.
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Patient consent
Informed consent was obtained from the patients who participated in the study.
Ethical approval
The study was approved by the Ethics Committee of the University Hospital Motol, Prague and has, therefore, been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baumgartner, D., Mazanec, R. & Hanzalová, J. Diagnostic utility of neurofilament markers for MND is limited in restricted disease phenotype and for differentiation from compressive myeloradiculopathies. J Neurol 270, 1600–1614 (2023). https://doi.org/10.1007/s00415-022-11504-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11504-1