Abstract
Introduction
Dysregulation of cerebral glucose consumption, alterations in cerebrospinal fluid (CSF) biomarkers, and cognitive impairment have been reported in patients with obstructive sleep apnoea (OSA). On these bases, OSA has been considered a risk factor for Alzheimer’s disease (AD). This study aimed to measure cognitive performance, CSF biomarkers, and cerebral glucose consumption in OSA patients and to evaluate the effects of continuous positive airway pressure (CPAP) treatment on these biomarkers over a 12-month period.
Methods
Thirty-four OSA patients and 34 controls underwent 18F-fluoro-2-deoxy-d-glucose positron emission tomography (18F-FDG PET), cognitive evaluation, and CSF analysis. A subgroup of 12 OSA patients treated with beneficial CPAP and performing the 12-month follow-up was included in the longitudinal analysis, and cognitive evaluation and 18F-FDG PET were repeated.
Results
Significantly reduced glucose consumption was observed in the bilateral praecuneus, posterior cingulate cortex, and frontal areas in OSA patients than controls. At baseline, OSA patients also showed lower β-amyloid42 and higher phosphorylated-tau CSF levels than controls. Increased total tau and phosphorylated tau levels correlated with a reduction in brain glucose consumption in a cluster of different brain areas. In the longitudinal analysis, OSA patients showed an improvement in cognition and a global increase in cerebral 18F-FDG uptake.
Conclusions
Cognitive impairment, reduced cerebral glucose consumption, and alterations in CSF biomarkers were observed in OSA patients, which may reinforce the hypothesis of AD neurodegenerative processes triggered by OSA. Notably, cognition and brain glucose consumption improved after beneficial CPAP treatment. Further studies are needed to evaluate the long-term effects of CPAP treatment on these AD biomarkers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnoea (OSA) is the most frequent sleep-disordered breathing (SDB) and is highly prevalent in middle-aged and older adults [1]. OSA is characterised by repetitive episodes of upper airway obstruction, leading to apnoea and hypopnoea events, intermittent hypoxia, and sleep fragmentation [2, 3]. OSA is a risk factor for several morbidities [1, 4,5,6,7,8] and is associated with the risk of cognitive impairment [9]. Recent evidence suggests that OSA may also be a risk factor for Alzheimer’s disease (AD) neurodegeneration [10,11,12,13], given that it alters cerebral β-amyloid metabolism and promotes neuroinflammation and oxidative stress [3, 10,11,12,13,14,15,16,17,18,19]. However, in contrast to other proven risk factors for the development of AD, OSA can be treated in clinical practice through the use of continuous positive airway pressure (CPAP), which significantly improves OSA symptoms [20].
Decreased cerebrospinal fluid (CSF) levels or documentation of plaque deposition of β-amyloid42 (Aβ42), reduced cortical temporo-parietal 18F-fluoro-2-deoxy-d-glucose uptake (18F-FDG) on positron emission tomography (PET), and increased CSF levels of total-tau (t-tau) and phosphorylated-tau (p-tau) have been considered biomarkers of AD neuropathology and are currently used to support AD diagnosis in both clinical practice and research [21,22,23,24]. These biomarker alterations appear early during the course of the AD pathology, usually before brain magnetic resonance imaging (MRI) structural changes. Accordingly, they have been validated in providing early high diagnostic accuracy during the work-up for AD [25]. In the recent past, pathological modifications of CSF biomarkers have already been described in patients with OSA, with or without subtle cognitive impairment [10, 14,15,16, 26,27,28,29]. Conversely, fewer 18F-FDG PET brain studies have been performed in patients with OSA and included very small groups of patients. These studies documented a significant modification of 18F-FDG uptake in different brain areas, in particular, glucose hypometabolism in the bilateral prefrontal areas, praecuneus, left hippocampus, and left anterior cingulate cortex, among others [30,31,32]. One of the more recent studies reported that despite the presence of subtle memory impairment, OSA patients displayed a significant reduction in cerebral glucose consumption in the praecuneus, cingulate, parieto-occipital, and prefrontal cortices [33]. However, studies evaluating brain glucose consumption using 18F-FDG PET in OSA patients remain limited, and the possible effects of prolonged CPAP treatment were not evaluated, since the longitudinal studies were set at a 3-month follow-up [30,31,32,33]. Finally, previous studies evaluating patients with OSA analysed CSF and PET data obtained weeks or months after the diagnosis of OSA. Therefore, the present study aimed to comprehensively measure cognition, CSF biomarkers, and cerebral glucose uptake by 18F-FDG PET in middle-aged adult patients with moderate to severe OSA (apnoea–hypopnoea index [AHI] ≥ 15/h) during the diagnostic work-up of the sleep disorder. A subgroup of patients was also followed for a period of 12 months and repeated cognitive testing and brain 18F-FDG PET. Moreover, to better understand the direction of the biomarker changes obtained at baseline, associations between the measured CSF biomarkers of AD pathology (tau proteins and Aβ42), cerebral glucose uptake and cognitive performance were analysed.
Methods
Participants and study procedures
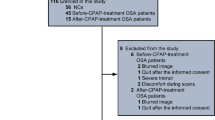
Middle-aged adult patients with OSA who were admitted to the Sleep Medicine Centre at the Neurology Unit of the University Hospital of Rome “Tor Vergata” were recruited between January 2016 and September 2018. All patients underwent a standard sleep medicine visit, polysomnographic recording (PSG), physical and neurological examinations, cognitive evaluation, CSF AD biomarker analysis, and 18F-FDG PET. A subgroup of patients with moderate to severe OSA (AHI ≥ 15/h), compliant with CPAP treatment for a period of 12 months, were included in the longitudinal analysis to evaluate changes in neuropsychological and neuroimaging biomarkers. At follow-up, the patients underwent cognitive evaluation and 18F-FDG PET. Figure 1 shows a flowchart of the recruitment and selection process.
The inclusion criteria for OSA patients were as follows: no concomitant neurological or psychiatric diseases, no dementia (Clinical Dementia Rating [CDR] > 1), no concomitant other sleep disorders, and no use of CNS-active drugs. Participants with systemic and/or neurologic infectious, inflammatory, or autoimmune diseases; diabetes; clinical conditions influencing cognitive performance (such as hypothyroidism or B12 vitamin depletion); and history of alcohol or other substance abuse were excluded from the present study. Moreover, brain MRI was used to exclude signs of brain atrophy, particularly in the hippocampus, and white matter abnormalities due to stroke or asymptomatic vessel disease. Inclusion criteria for the longitudinal analysis were as follows: good compliance to CPAP treatment (as previously defined as greater than 4 h of CPAP use per night on a routine basis [> 5 nights per week]) [34,35,36] and a mean residual of AHI, obtained by the ventilator software report, < 10 per hour.
A group of controls was also recruited at the University Hospital of Rome “Tor Vergata”. Specifically, the control group (CG) included inpatients at the same medical centre undergoing clinical neurologic examination and 18F-FDG PET for suspected malignancies, which were ruled out after diagnostic investigations. All the controls underwent physical and neurological examinations, cognitive evaluation, lumbar puncture (LP), and 18F-FDG PET. Moreover, a sleep medicine interview to exclude sleep disorders was performed in the controls. Exclusion criteria were signs or symptoms of central or peripheral nervous system diseases, diabetes, depression or other psychiatric symptoms, cognitive decline, and sleep disorders.
The study was performed according to the STROBE statement, and the study protocol was considered as observational and approved by the Ethical Committee of the University Hospital of Rome “Tor Vergata”. Written informed consent was obtained from all participants in the study, and subjects and procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013 [37].
Polysomnographic recordings
All OSA patients underwent PSG recording to evaluate nocturnal sleep (SOMNOscreen, SOMNOmedics GmbH, Randersacker, Germany). The following standard parameters were computed: sleep onset latency (SL; the time-interval between the lights off and the first sleep epoch), total sleep time (TST; the actual sleep time without SL and awakenings), sleep efficiency (SE; the ratio between TST and time in bed), rapid eye movement (REM) sleep latency (REML; the time interval between the sleep onset and the first epoch of REM sleep), stage 1 of non-REM sleep (N1), stage 2 of non-REM sleep (N2), stage 3 of non-REM sleep (N3), REM sleep (REM), and wakefulness after sleep onset (WASO). Sleep stages were calculated as percentages of the TST. Apnoea was defined as a reduction of > 90% of respiratory airflow for 10 or more seconds, while hypopnoea has been determined as the reduction of > 30% of respiratory airflow for 10 or more seconds associated with an oxygen desaturation of ≥ 3%. The severity of OSA is determined by AHI (the sum of all apnoeas and hypopnoeas per hour of sleep). The following oxygen saturation (SaO2) parameters were evaluated: mean SaO2, nadir SaO2, time spent with SaO2 < 90% (T < 90), and ODI (number of oxygen desaturations ≥ 3% per hour). PSG recordings were scored based on the international standard criteria of the American Academy of Sleep Medicine [38].
Neuropsychological tests
Cognitive evaluation was performed the day after PSG recording, immediately before performing LP. The Mini-Mental State Examination (MMSE) [39] and a brief revised version of the Mental Deterioration Battery [40, 41] were performed in all participants. All scores were corrected for age and education level. Short- and long-term memory, executive function, attention, and general intelligence were evaluated [26].
Short- and long-term memory was evaluated using the Rey Auditory-Verbal Learning Test (RAVLT) [42]. A list of 15 words was read to the patient five times and their ability to recall was measured immediately (the sum of the words recalled in the five trials, RAVLT-I) and after 15 min (the number of words recalled 15 min after the last word presentation, RAVLT-D).
Executive functions were evaluated using the Stroop Colour/Word test in which the participants were required to name the colour ink that a colour-word (e.g. RED) is presented in, both in congruent (e.g. when the word RED is printed in red link) and incongruent conditions (e.g. when the word BLUE is printed in red link). In the latter condition, an increase in the number of errors and the time taken to respond is observed (“Stroop interference effect”) [43]. The test is considered the “paradigmatic measure of selective attention” [44].
General intelligence was tested using Raven Progressive Matrices (36 items), which consists of choosing from a set of distractors the item logically missing in a given visual/spatial set [45]. The items are regrouped in a set of three subtests (labelled A, Ab, and B) and evaluate non-verbal intelligence, visual processing speed, cognitive speed, and flexibility.
CSF biomarker analysis
All CSF samples were obtained the day after the PSG recording by LP performed in the decubitus position between 8:00 and 9:30 AM, within 1–2 h after morning awakening, using an atraumatic needle. CSF samples were collected in polypropylene tubes using standard sterile techniques. The first 4 mL CSF sample was used for routine biochemistry analysis, including the total cell count. A second 4 mL CSF sample was centrifuged to eliminate cells and cellular debris and immediately frozen at − 80 °C until the analysis to assess t-tau, p-tau, and Aβ42 levels could be performed. The CSF Aβ42, t-tau, and p-tau levels were determined according to previously published standard procedures, using commercially available sandwich enzyme-linked immunosorbent assays (Innotest β-Amyloid 1–42, Innotest h-T-tau, Innotest Phospho-T-tau 181; Innogenetics, Ghent, Belgium) [15, 46,47,48]. The cut-off values for the CSF biomarkers positive for AD pathology were set in-house [49, 50], and our laboratory results are in line with those of the external quality control program for the CSF biomarkers, promoted by the Alzheimer’s Association [51]. Specifically, Aβ42, t-tau, and p-tau were dichotomized on the basis of previously established cut-off values: < 500 pg/mL for Aβ42, > 375 pg/mL for t-tau, and > 52 pg/mL for p-tau [46, 48].
PET/CT scanning protocol
The PET/CT protocol study was conducted at the Nuclear Medicine facility of the University Hospital of Rome “Tor Vergata”. All subjects, the day before performing PSG, were intravenously injected with 18F-FDG (dose range 185–295 megabequerels) and hydrated with 500 mL of saline (0.9% sodium chloride). PET/CT acquisition, using a General Electric VCT PET/CT scanner, started 30 ± 5 min after 18F-FDG injection and lasted 10 min for all participants. The reconstruction parameters were as follows: ordered subset expectation maximisation, 4 subsets and 14 iterations; matrix 256 × 256; full width at half maximum (FWHM): 5 mm [52].
Data and statistical analysis
PSG, neuropsychological, and CSF data analysis
A commercial statistical software was used for statistical analysis (SPSS version 25, IBM Corporation, Armonk, NY, USA) [53]. The Kolmogorov–Smirnov test was used to check for normal distribution of PSG, neuropsychological, and CSF data. All demographic, clinical, PSG, neuropsychological, and CSF data were then compared between the two groups (OSA vs. CG) using the Student’s t-test. For the subgroup analyses, considering the small sample size, differences between clinical and cognitive functioning of patients at baseline and at the 12-month follow-up were tested using the Wilcoxon signed rank test.
Correlations between the CSF biomarker levels, neuropsychological, 18F-FDG PET, and PSG data in OSA patients were performed using Pearson’s correlation test. Only the statistically different 18F-FDG PET areas between the OSA and CG were included in the Pearson correlations. To reduce the chances of obtaining false-positive results, due to the multiple comparisons among the demographic, clinical, sleep parameters and neuropsychological scores, a Bonferroni correction was used. The Bonferroni correction divides the unadjusted p values by the total number of tests, which corresponds to an alpha level of 0.003 (0.05/20). All p-values lower or equal to 0.003 were considered as statistically significant. Effect sizes were quantified by Cohen’s d.
18 F-FDG PET analysis
Statistical parametric mapping 12 (SPM12) implemented in MATLAB 2018a was used to analyse PET scans in this study (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/), as previously reported [54]. PET data were converted from DICOM to NIfTI format using MRIcro software available at https://www.nitrc.org/projects/mricron and then subjected to a normalisation process. A bias regularisation was applied (0.0001) to limit biases due to smooth, spatially varying artefacts that modulate the image’s intensity and impede the automated processing of the images. To prevent the algorithm from trying to model the intensity variation due to different tissue types, the FWHM of the Gaussian smoothness of bias was set at a 60-mm cut-off. A tissue probability map implemented in SPM12 was used (TPM.nii). To achieve approximate alignment to the ICBM space template—European brains [55, 56], a mutual information affine registration was used with the tissue probability maps [57].
Warping regularisation was set with the following 1 × 5 arrays (0, 0.001, 0.5, 0.05, 0.2). To cope with functional anatomical variability that is not compensated by spatial normalisation and improves the signal-to-noise ratio, smoothness was set at 5 mm, and the sampling distance, which encodes the approximate distance between sampled points when estimating the model parameters, was set at 3.
To blur the individual variations (especially gyral variations) and to increase the signal-to-noise ratio, an 8-mm isotropic Gaussian filter was applied. Before regression analysis was applied, it was necessary to define the parameters and post-processing tools; global normalisation (which escalates images to a global value) = 50 (using proportional scaling); the masking threshold (to help identify voxels with an acceptable signal in them) was set to 0.8; a transformation tool of statistical parametric maps into normal distribution was used; the correction of SPM coordinates to match the Talairach coordinates, subroutine implemented by Matthew Brett (http://www.mrc-cbu.cam.ac.uk/Imaging) was made. Brodmann areas (BA) were identified at a range from 0 to 3 mm from the corrected Talairach coordinates of the SPM output isocentre using a Talairach client available at http://www.talairach.org/index.html. As proposed by Bennett et al. [58], SPM t-maps were corrected for multiple comparisons using the false discovery rate (P ≤ 0.05) and corrected for multiple comparisons at the cluster level (P ≤ 0.001). The level of significance was set at 100 (5 × 5 × 5 voxels, i.e. 11 × 11 × 11 mm) contiguous voxels [58]. The following voxel-based comparisons were assessed: (1) CG versus OSA patients at baseline and vice versa; (2) OSA patients at baseline vs. OSA patients after CPAP therapy and vice versa. In order to exclude possible abnormalities or biases due to the presence of a very early AD stage in the subgroup of OSA patients, a separate analysis was performed, comparing patients with normal CSF biomarkers to patients showing the CSF AD biomarkers consistent with possible AD pathology (low Aβ42 and/or high t-tau or p-tau). Moreover, a single subject analysis was performed to identify the presence of typical AD 18F-FDG PET patterns at baseline [59]. All comparisons were performed using the “two-sample t-test” design model available in SPM12 [52]. Sex and MMSE were used as covariates in the analyses of the CG and OSA patients.
To investigate the relationship between 18F-FDG brain uptake and neuropsychological testing in OSA subjects, a selected ROI was placed on the cortical grey matter of Bas found to be affected by a relative hypometabolism in OSA patient vs. control comparisons using the WFU Pickatlas tool (https://www.nitrc.org/projects/wfu_pickatlas/) implemented in SPM 12 and further analysed after a normalisation process [60]. The mean signal intensities computed for the whole cluster were normalised within each subject to the average intensities of the cerebellar volume of interest, as defined by other reports published previously [60].
Results
Demographic and clinical data
Thirty-four patients affected by moderate to severe OSA were recruited for this study, and the same number of subjects was included in the CG. Demographic and clinical data of the study groups are reported in Table 1, and the PSG results and neuropsychological data of OSA patients are presented in Table 2. According to the neuropsychological data, OSA patients showed lower MMSE scores (25.87 ± 2.63) than controls (29.08 ± 0.90) (p = 0.001, d = 1.63).
CSF data
OSA patients showed lower CSF Aβ42 levels than controls (p = 0.002, d = 1.90), with 7 OSA patients (20.5% of the sample) presenting pathologic Aβ42 values (below cut-off < 500 pg/mL). OSA patients also showed higher CSF levels of p-tau (p = 0.001, d = 0.84) when compared to controls. Moreover, six OSA patients (17.6% of the sample) showed pathological t-tau CSF levels (above cut-off > 375 pg/mL) and 9 patients (26.4%) showed pathological p-tau CSF levels (above cut-off > 52 pg/mL). No AD biomarker CSF values were pathological in the CG. All CSF data for patients with OSA and controls are reported in Table 1.
18F-FDG PET analysis
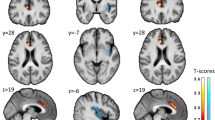
Considering 18F-FDG PET analysis, a reduction in 18F-FDG PET uptake was found in patients with OSA in the bilateral praecuneus (BA 7), posterior cingulate cortex (BA 23), and frontal areas (BA8-9-10) when compared to the CG (Table 3 and Fig. 2). No significant differences were found when comparing patients with normal CSF AD biomarkers to patients with CSF AD biomarkers alterations. Moreover, no significant results were obtained in the single-subject analyses.
Frontal (a), upper (b) and lateral (c) view of the three-dimensional (3D) rendering showing the results of SPM comparisons between 18 F-FDG uptake in OSA patients as compared to the control group. The significance values above a chosen threshold and the “T value” in this voxel for a given contrast is represented by use of a colour intensity code. OSA patients show a significant reduction of brain glucose consumption in left and right frontal, parietal and in right limbic cortex. Details are provided in Table 3. R: right; L: left
Correlations analysis
In the OSA patient group, a significant correlation was found between 18F-FDG PET and CSF biomarkers, indicating that a reduction in 18F-FDG PET cerebral uptake was associated with higher t-tau and p-tau CSF concentrations. No significant correlations were observed between the CSF levels and cognitive performance. Table 4 shows the significant correlations among 18F-FDG PET, demographic characteristics, CSF biomarkers, and cognitive data.
Longitudinal analysis
A subgroup of 12 OSA patients treated with beneficial CPAP therapy for a period of 12 months was included in the longitudinal analysis. In this subgroup, there were 10 men and 2 women, with a mean age of 68.64 ± 10.5. Patients showed an mean AHI of 42.00 ± 23.20 at baseline and of 4.48 ± 2.84 after 12 months of CPAP therapy (p = 0.003, d = 3.19). Baseline and longitudinal data of the OSA patient subgroup at the 12-month follow-up are presented in Table 5. No significant differences were found between the sample of patients undergoing follow-up analysis and the whole group of OSA patients evaluated at baseline in terms of demographic and clinical characteristics. Specifically, no differences were found for gender (p = 0.68), age (p = 0.66), CSF biomarkers (Aβ42, p = 0.10; t-tau, p = 0.14; p-tau, p = 0.72), and MMSE (p = 0.96). In particular, considering CSF biomarkers analysis, the percentage of patients with pathological values was comparable between the two groups.
Therefore, considering the group of patients undergoing the longitudinal analysis, the patients showed an improvement in the attention domain (Stroop [T]) (p = 0.002, d = 2.07) after 12 months of CPAP therapy. Moreover, a significant increase in 18F-FDG uptake in a wide cluster that involved the right and left frontal areas and left parietal lobe (BA 4, BA 6, BA 10) was also evident at the comparison with the baseline 18F-FDG PET data (see Table 6 and Fig. 3). No significant correlation was found between the AHI reduction between baseline and follow-up, cognitive performance, and the 18F-FDG uptake variation in the different BAs.
3D rendering showing the results of SPM comparisons between 18 F-FDG uptake in patients OSA patients undergoing the longitudinal evaluation. At the 12-month follow-up, a significant increase of cortical glucose consumption is detectable in right and left frontal and parietal cortex and in in OSA patients. R: right; L: left
Discussion
Several efforts have been made in the recent years to better understand the risk of sleep disorders, particularly OSA, as a potential contributor of neurodegenerative processes [61]. Although it has been documented that OSA can cause cognitive impairment, alteration in CSF AD biomarkers and reduction in cerebral 18F-FDG uptake [21,22,23,24], few studies have comprehensively evaluated all these biomarkers in middle-aged adult patients with OSA. Moreover, these studies investigated all the biomarkers at different times (from weeks to months) during the research work-up. Considering the importance of recognizing the increased risk of AD pathology in patients with OSA, the present study design was built to evaluate all these AD biomarkers in a very short period in order to check the relation among brain neurodegenerative processes, sleep quality and continuity, and cognitive performance in OSA patients. Finally, the longitudinal analysis represented a pilot evaluation to better understand the potential beneficial effects of a long period of CPAP treatment on AD biomarkers and cognition in patients with OSA (12 months—with respect to shorter follow-ups reported in previous investigations) [30,31,32,33]. Therefore, the present study focused on two aspects: on the one hand, to better define the effect of OSA on these biomarkers, it evaluated changes in different CSF, cognitive and 18F-FDG PET biomarkers in a large group of middle-aged adult patients with moderate to severe OSA compared to controls; on the other hand, to evaluate the possibility of improving cognitive performance and increasing brain function (measured by glucose consumption) in these patients, it explored the longitudinal effects of CPAP treatment on cognition and cerebral glucose consumption.
The main finding of the present study is the documentation of 18F-FDG PET uptake reduction in eloquent brain areas for AD pathology (also owing to the default mode network), such as the bilateral praecuneus, posterior cingulate cortex, and frontal areas, in OSA patients when compared to the CG. This finding concords with the very few previous PET studies including smaller groups of patients, which found a significantly reduced glucose consumption in the bilateral prefrontal areas, praecuneus, and left anterior cingulate cortex in OSA patients compared to controls [30,31,32]. Although the present study confirmed the impaired function of several brain areas in patients with OSA, the mechanisms at the basis of this malfunction have not been completely understood. For this reason, we also evaluated CSF AD biomarkers to test if this brain functional impairment can be due to pathological processes owing to AD pathology. Consistently, patients with OSA showed lower Aβ42 and higher p-tau proteins CSF levels compared to controls, and higher CSF tau proteins levels correlated with the reduction of brain glucose metabolism in a cluster of different brain frontal and parietal regions. Moreover, cognition was impaired in patients with OSA, in particular in the attention domain, which may be related to the observed pathological uptake of glucose in the frontal and temporal brain areas.
The main triggering events in OSA patients that may be responsible for these pathological modifications are nocturnal hypoxia and sleep fragmentation. Hypoxia is thought to be an important trigger in affecting brain glucose consumption in eloquent brain areas usually impaired at the beginning of AD pathology and associated with future cognitive impairment [62, 63]. Moreover, it has been suggested that sleep impairment due to OSA may induce preclinical AD biomarker changes, such as a reduction in CSF Aβ42 levels and an increase in brain amyloid plaque deposition [14,15,16]. Therefore, the CSF biomarker alterations in OSA patients may be explained by these two mechanisms: intermittent hypoxia, which has been hypothesized to alter brain amyloid metabolism inducing the activity of β-secretases, and sleep fragmentation, which may affect the glymphatic system [64,65,66].
Considering the CSF results, although the reduction of CSF Aβ42 levels in OSA patients has been already reported, the documentation of a significant increase in p-tau CSF levels is a novel finding with respect to the previous literature [15, 20]. The present finding may be due to the larger group of patients with OSA included in this study, compared to the past literature [15, 20]. Hence, these findings extend the previous literature on CSF AD biomarker changes in OSA patients and add evidence that p-tau protein alteration may reflect the presence of synaptic damage, neurodegeneration, and neuronal dysfunction, and the modification of brain glucose uptake documented by 18F-FDG PET as a result [67].
The second part of the present study describes the longitudinal analysis performed in a subgroup of OSA patients who were compliant to CPAP treatment. The positive effects of CPAP on cognitive performance and CSF AD biomarkers have been previously described [68, 69], and in this study, we confirmed not only the improvement in cognitive performance after long-term CPAP treatment, but also significantly documented the increase in cerebral glucose consumption in a wide cluster of areas, including the right and left frontal areas and the left parietal lobe. Notably, several areas improving glucose uptake take part to the default mode network, also with a central role. This finding reinforces the previously documented improvement in brain glucose metabolism in the bilateral precentral gyrus and left anterior cingulate cortex after a shorter period of CPAP treatment [30]. Consistently, beneficial CPAP treatment induces neural compensation and reduces cerebral dysfunction [30, 63, 70], thus improving brain glucose consumption and cognitive performance. Our findings, besides supporting previous longitudinal studies with a 3-month follow-up [30,31,32,33], broaden the previous knowledge through the documentation of the positive effects of long-term longitudinal CPAP treatment on both brain 18F-FDG PET and cognitive functioning, highlighting the clinical relevance of treating OSA by CPAP. Accordingly, recent evidence documented that the use of CPAP is associated with a lower risk of developing AD, dementia and mild cognitive impairment in older adults [71]. Although the focus of the current study was the long-term effects of CPAP treatment, future studies should also consider the effect of behavioural interventions that an be concomitantly used in these patients, such as increasing physical activity, reducing alcohol consumption, or improving sleep hygiene.
The present study presents some limitations. The small sample size of patients recruited for the longitudinal analysis might have affected the statistical power of the results and prevented the generalisation of the longitudinal findings. In particular, the sample size of the subgroup of patients included in the longitudinal analysis was too small to perform further analysis in patients showing AD-typical biomarker changes at baseline. Another potential design limitation is that only patients compliant to CPAP treatment were included in the longitudinal analysis, which can reflect a selection bias of the study since those patients may be more likely those who experienced cognitive benefit. However, the novelty of these results invites future studies with a larger sample to further explore this aspect, possibly focusing on CSF biomarker values and on the cognitive trajectories of OSA patients treated by CPAP. Future studies may also explore if there is a dose-related response to CPAP therapy in terms of reduction of AHI or greater usage (e.g. 4.1 h vs 8 h of CPAP use a night) associated with greater improvement in cognitive performance. Another limitation is that sleep assessment through PSG was not repeated at follow-up in OSA patients; therefore, it is difficult to determine whether the changes in cognition and brain glucose consumption may be due to slow-wave sleep and REM sleep improvement and/or less sleep fragmentation. Further, sleepiness and fatigue were not assessed through self-reported scales, which raises the possibility that these factors may also account for the improvement of cognitive performance at follow-up. Although brain MRI was performed to exclude signs of brain atrophy and white matter abnormalities, a quantitative measure of brain atrophy was missing. Future studies should also consider measuring brain atrophy or white matter damage to assess whether OSA patients already demonstrate early signs of neurodegeneration, especially considering that both OSA and AD could have a bidirectional and cyclic potentiating effect on each other’s pathogenesis. For instance the progressive brain accumulation of amyloid plaques and intraneuronal neurofibrillary tangles of tau proteins in AD may determine changes in sleep patterns, even before dementia is recognized, thus potentially increasing the risk for OSA in AD. Finally, the CG did not constitute healthy volunteers and comprised of subjects with suspected malignancies undergoing 18F-FDG PET/CT and was found to be completely negative for various diseases. The lack of a PSG evaluation and a broad cognitive evaluation, as well as long-term follow-up in the CG is another limitation of the present study.
Conclusion
Although increasing evidence shows pathological modifications of cognitive, CSF, and nuclear medicine biomarkers in OSA patients that may promote AD neurodegeneration [21,22,23,24], few studies have comprehensively assessed all these aspects. The present findings documented that patients with OSA show cerebral glucose consumption dysregulation, CSF AD biomarker alterations (both p-tau proteins and Aβ42) and cognitive impairment, thus highlighting the importance of increasing OSA screening and diagnosis in the middle-aged adult and elderly population, since CPAP treatment may improve cognitive performance and possibly restore brain functioning. Further studies are needed to evaluate the long-term effects of CPAP treatment to reinforce the hypothesis that AD biomarker changes in OSA conditions induced by hypoxia and sleep fragmentation can be considered reversible by CPAP, thus allowing the planning of preventive strategies against AD through CPAP treatment of OSA in middle-aged subjects presenting with this sleep disorder.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Change history
26 August 2022
Missing Open Access funding information has been added in the Funding Note.
References
Heinzer R, Vat S, Marques-Vidal P et al (2015) Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 3:310–318. https://doi.org/10.1016/S2213-2600(15)00043-0
Ryan CM, Bradley TD (2005) Pathogenesis of obstructive sleep apnea. J Appl Physiol 99:2440–2450. https://doi.org/10.1152/japplphysiol.00772.2005
Daulatzai MA (2015) Evidence of neurodegeneration in obstructive sleep apnea: Relationship between obstructive sleep apnea and cognitive dysfunction in the elderly. J Neurosci Res 93:1778–1794. https://doi.org/10.1002/jnr.23634
Khot SP, Morgenstern LB (2019) Sleep and stroke. Stroke 50:1612–1617. https://doi.org/10.1161/STROKEAHA.118.023553
Neighbors CLP, Noller MW, Song SA et al (2018) Vitamin D and obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med 43:100–108. https://doi.org/10.1016/j.sleep.2017.10.016
Mentek M, Aptel F, Godin-Ribuot D et al (2018) Diseases of the retina and the optic nerve associated with obstructive sleep apnea. Sleep Med Rev 38:113–130. https://doi.org/10.1016/j.smrv.2017.05.003
Upala S, Sanguankeo A, Congrete S (2016) Association between obstructive sleep apnea and osteoporosis: a systematic review and meta-analysis. Int J Endocrinol Metab 14:129–140. https://doi.org/10.5812/ijem.36317
Liguori C, Mercuri NB, Izzi F et al (2016) Obstructive sleep apnoea as a risk factor for osteopenia and osteoporosis in the male population. Eur Respir J 47:987–990. https://doi.org/10.1183/13993003.01830-2015
Vaessen TJA, Overeem S, Sitskoorn MM (2015) Cognitive complaints in obstructive sleep apnea. Sleep Med Rev 19:51–58. https://doi.org/10.1016/j.smrv.2014.03.008
Osorio RS, Ayappa I, Mantua J et al (2014) The interaction between sleep-disordered breathing and apolipoprotein E genotype on cerebrospinal fluid biomarkers for Alzheimer’s disease in cognitively normal elderly individuals. Neurobiol Aging 35:1318–1324. https://doi.org/10.1016/j.neurobiolaging.2013.12.030
Andrade AG, Bubu OM, Varga AW, Osorio RS (2018) The relationship between obstructive sleep apnea and Alzheimer’s Disease. J Alzheimer’s Dis 64:S255–S270. https://doi.org/10.3233/JAD-179936
Bubu OM, Andrade AG, Umasabor-Bubu OQ et al (2020) Obstructive sleep apnea, cognition and Alzheimer’s disease: a systematic review integrating three decades of multidisciplinary research. Sleep Med Rev 50:101250. https://doi.org/10.1016/j.smrv.2019.101250
Ju Y-ES, Lucey BP, Holtzman DM (2014) Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat Rev Neurol 10:115–119. https://doi.org/10.1038/nrneurol.2013.269
Sharma RA, Varga AW, Bubu OM et al (2018) Obstructive sleep apnea severity affects amyloid burden in cognitively normal elderly: a longitudinal study. Am J Respir Crit Care Med 197:933–943. https://doi.org/10.1164/rccm.201704-0704OC
Liguori C, Mercuri NB, Nuccetelli M et al (2019) Obstructive sleep apnea may induce orexinergic system and cerebral β-amyloid metabolism dysregulation: is it a further proof for Alzheimer’s disease risk? Sleep Med 56:171–176. https://doi.org/10.1016/j.sleep.2019.01.003
Ju YS, Finn MB, Sutphen CL et al (2016) Obstructive sleep apnea decreases central nervous system-derived proteins in the cerebrospinal fluid. Ann Neurol 80:154–159. https://doi.org/10.1002/ana.24672
Lavie L (2012) Oxidative stress inflammation and endothelial dysfunction in obstructive sleep apnea. Front Biosci E4:1391. https://doi.org/10.2741/469
Yamauchi M, Nakano H, Maekawa J et al (2005) Oxidative stress in obstructive sleep apnea. Chest 127:1674–1679. https://doi.org/10.1378/chest.127.5.1674
Ryan S (2017) Adipose tissue inflammation by intermittent hypoxia: mechanistic link between obstructive sleep apnoea and metabolic dysfunction. J Physiol 595:2423–2430. https://doi.org/10.1113/JP273312
Liguori C, Mercuri NB, Izzi F et al (2017) Obstructive sleep apnea is associated with early but possibly modifiable Alzheimer’s disease biomarkers changes. Sleep 40:1–10. https://doi.org/10.1093/sleep/zsx011
Martin P, Anders W, Maëlenn G et al (2015) World Alzheimer Report 2015: the global impact of dementia an analysis of prevalence, incidence, cost and trends. London
Simrén J, Ashton NJ, Blennow K, Zetterberg H (2020) An update on fluid biomarkers for neurodegenerative diseases: recent success and challenges ahead. Curr Opin Neurobiol 61:29–39. https://doi.org/10.1016/j.conb.2019.11.019
Fossati S, Ramos Cejudo J, Debure L et al (2019) Plasma tau complements CSF tau and P-tau in the diagnosis of Alzheimer’s disease. Alzheimer’s Dement Diagnosis, Assess Dis Monit 11:483–492. https://doi.org/10.1016/j.dadm.2019.05.001
McKhann GM, Knopman DS, Chertkow H et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement 7:263–269. https://doi.org/10.1016/j.jalz.2011.03.005
Blennow K (2017) A review of fluid biomarkers for Alzheimer’s disease: moving from CSF to blood. Neurol Ther 6:15–24. https://doi.org/10.1007/s40120-017-0073-9
Torelli F, Moscufo N, Garreffa G et al (2011) Cognitive profile and brain morphological changes in obstructive sleep apnea. Neuroimage 54:787–793. https://doi.org/10.1016/j.neuroimage.2010.09.065
Huang X, Tang S, Lyu X et al (2019) Structural and functional brain alterations in obstructive sleep apnea: a multimodal meta-analysis. Sleep Med 54:195–204. https://doi.org/10.1016/j.sleep.2018.09.025
Shi Y, Chen L, Chen T et al (2017) A meta-analysis of Voxel-based brain morphometry studies in obstructive sleep apnea. Sci Rep 7:1–13. https://doi.org/10.1038/s41598-017-09319-6
Bubu OM, Pirraglia E, Andrade AG et al (2019) Obstructive sleep apnea and longitudinal Alzheimer’s disease biomarker changes. Sleep 42:1–13. https://doi.org/10.1093/sleep/zsz048
Ju G, Yoon IY, Lee SD et al (2012) Modest changes in cerebral glucose metabolism in patients with sleep apnea syndrome after continuous positive airway pressure treatment. Respiration 84:212–218. https://doi.org/10.1159/000338117
Pietrini P, Dani A, Raphaelson M et al (1998) Cerebral glucose metabolic and neuropsychological dysfunction in patients with untreated sleep apnea syndrome (SAS). Sleep 21:82
Dani A, Pietrini P, Furey M et al (1996) Patients with sleep apnea syndrome (SAS) show neuropsychological impairment and regional cerebral glucose metabolic deficits: a pre-treatment positron emission tomography (PET) study. J Sleep Res Res 5:43
Yaouhi K, Bertran F, Clochon P et al (2009) A combined neuropsychological and brain imaging study of obstructive sleep apnea. J Sleep Res 18:36–48. https://doi.org/10.1111/j.1365-2869.2008.00705.x
Reeves-Hoche MK, Meck R, Zwillich CW (1994) Nasal CPAP: an objective evaluation of patient compliance. Am J Respir Crit Care Med 149:149–154. https://doi.org/10.1164/ajrccm.149.1.8111574
Parish JM, Lyng PJ, Wisbey J (2000) Compliance with CPAP in elderly patients with OSA. Sleep Med 1:209–214. https://doi.org/10.1016/S1389-9457(00)00011-3
Ayalon L, Ancoli-Israel S, Stepnowsky C et al (2006) Adherence to continuous positive airway pressure treatment in patients with Alzheimer disease and obstructive sleep apnea. Am J Geriatr Psychiatry 14:176–180. https://doi.org/10.1097/01.JGP.0000192484.12684.cd
World Medical Association (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310:2191–2194. https://doi.org/10.1001/jama.2013.281053
Iber C, Ancoli-Israel S, Chesson AL, Quan SF (2007) The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. American academy of sleep medicine, Westchester
Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state (MMSE). J Psychiatr Res 12:189–198
Carlesimo GA, Caltagirone C, Gainotti G et al (1996) The mental deterioration battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. Eur Neurol 36:378–384
Caltagirone C, Gainotti G, Masullo C, Miceli G (1979) Validity of some neuropsychological tests in the assessment of mental deterioration. Acta Psychiatr Scand 60:50–56
Rey A (1964) L’Examin clinique en psychologie. Universitaire de France, Paris
Stroop JR (1935) Studies of interference in serial verbal reactions. J Exp Psychol 18:643–662. https://doi.org/10.1037/h0054651
Carter CS, Mintun M, Cohen JD (1995) Interference and facilitation effects during selective attention: an H215O PET study of Stroop task performance. Neuroimage 2(4):264–272
Raven JC (1947) Guide to using coloured progressive matrices: Set A, Ab, B. Board and Book Forms, London
Duits FH, Teunissen CE, Bouwman FH et al (2014) The cerebrospinal fluid “alzheimer profile”: Easily said, but what does it mean? Alzheimer’s Dement 10:713-723.e2. https://doi.org/10.1016/j.jalz.2013.12.023
Fagan AM, Mintun MA, Mach RH et al (2006) Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta; 42 in humans. Ann Neurol 59:512–519. https://doi.org/10.1002/ana.20730
Mulder C, Verwey NA, Van Der Flier WM et al (2010) Amyloid-β(1–42), total tau, and phosphorylated tau as cerebrospinal fluid biomarkers for the diagnosis of Alzheimer disease. Clin Chem 56:248–253. https://doi.org/10.1373/clinchem.2009.130518
Liguori C, Spanetta M, Izzi F et al (2020) Sleep-wake cycle in Alzheimer’s disease is associated with tau pathology and orexin dysregulation. J Alzheimer’s Dis 74:501–508. https://doi.org/10.3233/JAD-191124
Sancesario GM, Esposito Z, Nuccetelli M et al (2010) Aβ1-42 Detection in CSF of Alzheimer’s disease is influenced by temperature: indication of reversible Aβ1-42 aggregation? Exp Neurol 223:371–376. https://doi.org/10.1016/j.expneurol.2009.07.028
Mattsson N, Andreasson U, Persson S et al (2011) The Alzheimer’s Association external quality control program for cerebrospinal fluid biomarkers. Alzheimer’s Dement 7:386-395.e6. https://doi.org/10.1016/j.jalz.2011.05.2243
Lancaster JL, Rainey LH, Summerlin JL et al (1997) Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp 5:238–242. https://doi.org/10.1002/(SICI)1097-0193(1997)5:4%3c238::AID-HBM6%3e3.0.CO;2-4
IBM (2020) SPSS - Statistical Package for Social Sciences. IBM, New York
Liguori C, Chiaravalloti A, Sancesario G et al (2016) Cerebrospinal fluid lactate levels and brain [18F]FDG PET hypometabolism within the default mode network in Alzheimer’s disease. Eur J Nucl Med Mol Imaging 43:2040–2049. https://doi.org/10.1007/s00259-016-3417-2
Mazziotta JC, Toga AW, Evans A et al (1995) A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM). Neuroimage 2:89–101. https://doi.org/10.1006/nimg.1995.1012
Mazziotta J, Toga A, Evans A et al (2001) A four-dimensional probabilistic Atlas of the human brain. J Am Med Informatics Assoc 8:401–430. https://doi.org/10.1136/jamia.2001.0080401
D’Agostino E, Maes F, Vandermeulen D, Suetens P (2007) Atlas-to-image non-rigid registration by minimization of conditional local entropy. Inf Process Med Imaging 20:320–332. https://doi.org/10.1007/978-3-540-73273-0_27
Bennett CM, Wolford GL, Miller MB (2009) The principled control of false positives in neuroimaging. Soc Cogn Affect Neurosci 4:417–422. https://doi.org/10.1093/scan/nsp053
Presotto L, Ballarini T, Caminiti SP et al (2017) Validation of 18F–FDG-PET single-subject optimized SPM procedure with different PET scanners. Neuroinformatics 15:151–163. https://doi.org/10.1007/s12021-016-9322-9
Chiaravalloti A, Barbagallo G, Martorana A et al (2019) Brain metabolic patterns in patients with suspected non-Alzheimer’s pathophysiology (SNAP) and Alzheimer’s disease (AD): is [18F] FDG a specific biomarker in these patients? Eur J Nucl Med Mol Imaging 46:1796–1805. https://doi.org/10.1007/s00259-019-04379-4
Liguori C, Spanetta M, Romoli M et al (2021) Sleep disorders and late-onset epilepsy of unknown origin: understanding new trajectories to brain amyloidopathy. Mech Ageing Dev 194:111434. https://doi.org/10.1016/j.mad.2021.111434
Lau EYY, Eskes GA, Morrison DL et al (2010) Executive function in patients with obstructive sleep apnea treated with continuous positive airway pressure. J Int Neuropsychol Soc 16:1077–1088. https://doi.org/10.1017/S1355617710000901
Castronovo V, Canessa N, Strambi LF et al (2009) Brain activation changes before and after PAP treatment in obstructive sleep apnea. Sleep 32:1161–1172. https://doi.org/10.1093/sleep/32.9.1161
Shiota S, Takekawa H, Matsumoto SE et al (2013) Chronic intermittent hypoxia/reoxygenation facilitate amyloid-β generation in mice. J Alzheimer’s Dis 37:325–333. https://doi.org/10.3233/JAD-130419
Ooms S, Overeem S, Besse K et al (2014) Effect of 1 night of total sleep deprivation on cerebrospinal fluid β-amyloid 42 in healthy middle-aged men a randomized clinical trial. JAMA Neurol 71:971–977. https://doi.org/10.1001/jamaneurol.2014.1173
Lulu X, Hongyi K, Qiwu X et al (2013) Sleep drives metabolite clearance from the adult brain. Science (80-) 342:373–377. https://doi.org/10.1126/science.1241224
Liguori C, Maestri M, Spanetta M et al (2021) Sleep-disordered breathing and the risk of Alzheimer’s disease. Sleep Med Rev 55:1–16. https://doi.org/10.1016/j.smrv.2020.101375
Liguori C, Chiaravalloti A, Izzi F et al (2017) Sleep apnoeas may represent a reversible risk factor for amyloid-β pathology. Brain 140:e75. https://doi.org/10.1093/brain/awx281
Maestri M, Liguori C, Bonanni E, Guarnieri B (2020) PAP use in mild cognitive impairment to delay progression to dementia. J Clin Sleep Med 16:1397. https://doi.org/10.5664/jcsm.8536
Canessa N, Castronovo V, Cappa SF et al (2011) Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med 183:1419–1426
Dunietz GL, Chervin RD, Burke JF et al (2021) Obstructive sleep apnea treatment and dementia risk in older adults. Sleep. https://doi.org/10.1093/sleep/zsab076
Funding
Open access funding provided by Università degli Studi di Roma Tor Vergata within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interests or financial or non-financial disclosures that might have influenced the performance or presentation of the work described in this manuscript.
Ethical approval
The authors declare that they complied with ethical standards and that the patients gave written informed consent to participate in the study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fernandes, M., Mari, L., Chiaravalloti, A. et al. 18F-FDG PET, cognitive functioning, and CSF biomarkers in patients with obstructive sleep apnoea before and after continuous positive airway pressure treatment. J Neurol 269, 5356–5367 (2022). https://doi.org/10.1007/s00415-022-11182-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11182-z