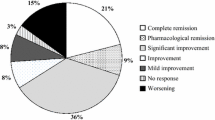
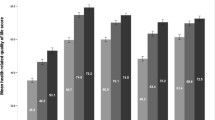
Abstract
A few observational studies and randomized trials suggest that exercise and rehabilitation may improve activity limitation and quality of life (QoL) in patients with chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), but the impact of other modifiable factors on the severity of the disease is not well understood. Using a structured questionnaire, we collected data on lifestyle and dietary habits of the patients included in the Italian CIDP database to investigate the possible influence of modifiable lifestyle factors on disability and QoL. Questionnaire data were available for 323 patients. The effect of lifestyle and dietary exposures on impairment, disability and QoL was evaluated using logistic regression models, adjusting for age, sex, disease duration, physical activity and smoking. Physical activity was associated with lower sensory impairment by the ISS scale, less disability by the INCAT and RODS scale and a better QoL in all the domains of EURO-QoL scale with the exception of anxiety/depression. None of the other parameters had an impact on these scales. This study adds evidence to the possible role of physical activity in improving symptom severity, disability and QoL in patients with CIDP. None of the other environmental factors investigated appeared to have an impact on the severity and health perception of CIDP.
Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, upon request.
References
Nobile-Orazio E (2014) Chronic Inflammatory demyelinating polyradiculoneuropathy. Where we are, where we should go. J Peripher Nerv Syst 19:2–13. https://doi.org/10.1111/jns5.12053
Jelinek GA, De Livera AM, Marck CH, Brown CR, Neate SL, Taylor KL et al (2016) Associations of lifestyle, medication, and socio-demographic factors with disability in people with multiple sclerosis: an international cross-sectional study. PLoS ONE 25(11):e0161701. https://doi.org/10.1371/journal.pone.0161701
Belbasis L, Bellou V, Evangelou E, Ioannidis JPA, Tzoulaki I (2015) Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol 14:263–273. https://doi.org/10.1016/S1474-4422(14)70267-4
Manzel A, Muller DN, Hafler DA, Erdman SE, Linker RA, Kleinewietfeld M (2014) Role of “Western Diet” in inflammatory autoimmune diseases. Curr Allergy Asthma Rep 14:404. https://doi.org/10.1007/s11882-013-0404-6
Fitzgerald KC, Tyry T, Salter A, Cofield SS, Cutter G, Fox R et al (2018) Diet quality is associated with disability and symptom severity in multiple sclerosis. Neurology 90:1–11. https://doi.org/10.1212/WNL.0000000000004768
Harel-Meir M, Sherer Y, Shoenfeld Y (2007) Tobacco smoking and autoimmune rheumatic diseases. Nat Clin Pract Rheumatol 3:707–715. https://doi.org/10.1038/ncprheum0655
Weiland TJ, Hadgkiss EJ, Jelinek GA, Pereira NG, Marck CH, van der Meer DM (2014) The association of alcohol consumption and smoking with quality of life, disability and disease activity in an international sample of people with multiple sclerosis. J Neurol Sci 336:211–219. https://doi.org/10.1016/j.jns.2013.10.046
Bombardier CH, Blake KD, Ehde DM, Gibbons LE, Moore D, Kraft GH (2004) Alcohol and drug abuse among persons with multiple sclerosis. Mult Scler 10:35–40. https://doi.org/10.1191/1352458504ms989oa
Beier M, D'Orio V, Spat J, Shuman M, Foley FW (2014) Alcohol and substance use in multiple sclerosis. J Neurol Sci 338:122–127. https://doi.org/10.1016/j.jns.2013.12.029
Swanson GR, Sedghi S, Farhadi A, Keshavarzian A (2010) Pattern of alcohol consumption and its effect on gastrointestinal symptoms in inflammatory bowel disease. Alcohol 44:223–228. https://doi.org/10.1016/j.alcohol.2009.10.019
Stejskal J, Stejskal VD (1999) The role of metals in autoimmunity and the link to neuroendocrinology. Neuro Endocrinol Lett 20:351–364
Garssen MP, Bussmann JB, Schmitz PI, Zandbergen A, Welter TG, Merkies IS et al (2004) Physical training and fatigue, fitness, and quality of life in Guillain-Barré syndrome and CIDP. Neurology 63:2393–2395. https://doi.org/10.1212/01.WNL.0000148589.87107.9C
Graham RC, Hughes RA, White CM (2007) A prospective study of physiotherapist prescribed community based exercise in inflammatory peripheral neuropathy. J Neurol 254:228–235. https://doi.org/10.1007/s00415-006-0335-4
Khan F, Pallant JF, Amatya B, Ng L, Gorelik A, Brand C (2011) Outcomes of high- and low-intensity rehabilitation programme for persons in chronic phase after Guillain-Barré syndrome: a randomized controlled trial. J Rehabil Med 43:638–646
Bussmann JB, Garssen MP, van Doorn PA, Stam HJ (2007) Analysing the favourable effects of physical exercise: relationships between physical fitness, fatigue and functioning in Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathy. J Rehabil Med 39:121–125
Andrews AW, Middleton A (2018) Improvement during inpatient rehabilitation among older adults with Guillain-Barré syndrome, multiple sclerosis, Parkinson disease, and stroke. Am J Phys Med Rehabil 97:879–884
Markvardsen LH, Overgaard K, Heje K, Sindrup SH, Christiansen I, Vissing J et al (2018) Resistance training and aerobic training improve muscle strength and aerobic capacity in chronic inflammatory demyelinating polyneuropathy. Muscle Nerve 57:70–76. https://doi.org/10.1002/mus.25652
Markvardsen LK, Carstens AR, Knak KL, Overgaard K, Vissing J, Andersen H (2019) Muscle strength and aerobic capacity in patients with CIDP one year after participation in an exercise trial. J Neuromuscul Dis 6:93–97
Novak P, Šmid S, Vidmar G (2017) Rehabilitation of Guillain-Barré syndrome patients: an observational study. Int J Rehabil Res 40:158–163
Ruhland JL, Shields RK (1997) The effects of a home exercise program on impairment and health-related quality of life in persons with chronic peripheral neuropathies. Phys Ther 77:1026–1039
Prada V, Massa F, Salerno A, Fregosi D, Beronio A, Serrati C et al (2020) Importance of intensive and prolonged rehabilitative treatment on the Guillain-Barrè syndrome long-term outcome: a retrospective study. Neurol Sci 41:321–327
Doneddu PE, Bianchi E, Cocito D, Manganelli F, Fazio R, Filosto M et al (2020) Risk factors for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): antecedent events, lifestyle and dietary habits. Data from the Italian CIDP Database. Eur J Neurol 27:136–143. https://doi.org/10.1111/ene.14044
Doneddu PE, Cocito D, Manganelli F, Fazio R, Briani C, Filosto M et al (2019) Atypical CIDP: diagnostic criteria, progression and treatment response. Data from the Italian CIDP Database. J Neurol Neurosurg Psychiatry 90:125–132. https://doi.org/10.1136/jnnp-2018-318714
Joint Task Force of the EFNS and the PNS (2010) European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society First Revision. J Peripher Nerv Syst 15:1–9. https://doi.org/10.1111/j.1529-8027.2010.00245.x
Zimmer P, Trebing S, Timmers-Trebing U, Schenk A, Paust R, Bloch W et al (2018) Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: a randomized controlled trial. Support Care Cancer 26:615–624. https://doi.org/10.1007/s00520-017-3875-5
Streckmann F, Lehmann HC, Balke M, Schenk A, Oberste M, Heller A et al (2018) Sensorimotor training and whole-body vibration training have the potential to reduce motor and sensory symptoms of chemotherapy-induced peripheral neuropathy-a randomized controlled pilot trial. Support Care Cancer 27:2471–2478. https://doi.org/10.1007/s00520-018-4531-4
Gu Y, Dennis SM, Kiernan MC, Harmer AR (2018) Aerobic exercise training may improve nerve function in type 2 diabetes and pre-diabetes: a systematic review. Diabetes Metab Res Rev 35:e3099. https://doi.org/10.1002/dmrr.3099
Cooper MA, Kluding PM, Wright DE (2016) Emerging relationships between exercise, sensory nerves, and neuropathic pain. Front Neurosci 10:372. https://doi.org/10.3389/fnins.2016.00372
Kluding PM, Pasnoor M, Singh R, Jernigan S, Farmer K, Rucker J et al (2012) The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabet Complicat 26:424–429. https://doi.org/10.1016/j.jdiacomp.2012.05.007
Lange-Maia BS, Cauley JA, Newman AB, Boudreau RM, Jakicic JM, Glynn NW et al (2016) Sensorimotor peripheral nerve function and physical activity in older men. J Aging Phys Act 24:559–566. https://doi.org/10.1123/japa.2015-0207
Sharif K, Watad A, Bragazzi NL, Lichtbroun M, Amital H, Shoenfeld Y (2018) Physical activity and autoimmune diseases: get moving and manage the disease. Autoimmun Rev 17:53–72. https://doi.org/10.1016/j.autrev.2017.11.010
Feter N, Freitas MP, Gonzales NG, Umpierre D, Cardoso RK, Rombaldi AJ (2018) Effects of physical exercise on myelin sheath regeneration: a systematic review and meta-analysis. Sci Sports 33:8–21. https://doi.org/10.1016/j.scispo.2017.06.009
Acknowledgements
Italian CIDP Database study group: Pietro Emiliano Doneddu, Giuseppe Liberatore, Francesca Gallia, and Eduardo Nobile-Orazio from the Department of Medical Biotechnology and Translational Medicine, Neuromuscular and Neuroimmunology Service, Humanitas Clinical and Research Institute, Milan University, Rozzano, Milan, Italy; Erdita Peci and Dario Cocito from the Department of Neuroscience, University of Turin, Turin, Italy. Daniele Velardo, Stefano Tronci and Raffaella Fazio from the Division of Neuroscience, Department of Neurology, Institute of Experimental Neurology (INSPE), San Raffaele Scientific Institute, Milan, Italy; Fiore Manganelli, Emanuele Spina, Antonietta Topa and Lucio Santoro from the Department of Neuroscience, Reproductive Sciences and Odontostomatology, University of Naples ‘Federico II’, Naples, Italy; Marta Ruiz and Chiara Briani from the Neurology Unit, Department of Neuroscience, University of Padua, Padua, Italy. Stefano Cotti Piccinelli, Alice Todeschini and Massimiliano Filosto from the Center for Neuromuscular Diseases and Neuropathies, Unit of Neurology ASST ‘Spedali Civili’, University of Brescia, Brescia, Italy; Corrado Cabona, Angela Zuppa and Luana Benedetti from the IRCCS Ospedale Policlinico San Martino, Genova, Italy; Antonio Toscano, Luca Gentile and Anna Mazzeo from the Department of Clinical and Experimental Medicine, Unit of Neurology, University of Messina, Messina, Italy; Giorgia Mataluni and Girolama Alessandra Marfia from the Disimmune Neuropathies Unit, Department of Systems Medicine, Tor Vergata University of Rome, Rome, Italy; Giuseppe Cosentino, Laura Piccolo, Ilaria Callegari and Andrea Cortese from the University of Pavia, IRCCS Foundation C. Mondino, Pavia, Italy; Elena Pinuccia Verrengia and Stefano Jann from the Department of Neuroscience, Niguarda Ca’ Granda Hospital, Milan, Italy; Elisa Bianchi and Ettore Beghi from the Laboratorio di Malattie Neurologiche, IRCCS-Istituto Mario Negri, Milan, Italy; Angelo Maurizio Clerici from the Neurology Unit, Circolo and Macchi Foundation Hospital, Insubria University, DBSV, Varese, Italy; Federica Scrascia and Marinella Carpo from the ASST Bergamo Ovest-Ospedale Treviglio, Treviglio, Italy; Martina Garnero and Angelo Schenone from the Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health, University of Genoa and IRCCS AOU San Martino-IST, Genoa, Italy; Marco Luigetti from Fondazione Policlinico Universitario Agostino Gemelli IRCCS, UOC Neurologia, Universita’ Cattolica del Sacro Cuore, Roma, Italy; Mario Sabatelli from Centro Clinico NEMO Adulti, Universita’ Cattolica del Sacro Cuore, Roma, Italy; Patrizia Dacci and Giuseppe Lauria from the Unit of Neuroalgology, IRCCS Foundation “Carlo Besta” Neurological Institute, Milan, Italy; Luca Leonardi and Giovanni Antonini from the Unit of Neuromuscular Diseases, Department of Neurology Mental Health and Sensory Organs (NESMOS), Faculty of Medicine and Psychology, ‘Sapienza’ University of Rome, Sant’Andrea Hospital, Rome, Italy; Tiziana Rosso from the Azienda UL.SS. 8 Asolo, Castelfranco Veneto, Italy; Erika Schirinzi and Gabriele Siciliano from the Neurology Unit, Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy; Claudia Balducci and Guido Cavaletti from the School of Medicine and Surgery and Experimental Neurology Unit, University of Milano-Bicocca, Monza, Italy.
Funding
The study was supported by a grant from Regione Lombardia, Italy, for patients from this region and subsequently extended to other Italian centers. The study was also supported by unrestricted grants from Kedrion Biopharma (Italy), CSL Behring (Italy), Humanitas Clinical and Research Institute (Milan, Italy), and GBS-CIDP Foundation International (USA). The funders had no role in study design, data collection and analysis, decision to publish, or in the preparation of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
PED designed and conceptualized the study, had a major role in the acquisition of data, analyzed the data, and wrote the first draft of the manuscript. EBianchi designed and executed the statistical analysis, contributed to the conception, organization, and execution of the research project, and reviewed and commented on the statistical analysis and the report. DC, FM, RF, MF, EBeghi, AM, GC, AC, SJ, AMC, GA, GS, GAM, CB, GL, TR, GC, MC, LB, AS, GL, EP, ES, ST, SCP, AT, LG, LP, EPV, LL, ES, GM, MR, MS and LS contributed to the study conception and design, had a major role inacquisition and interpretation of data and revised the manuscript for intellectual content. ENO conceived, organized and designed the study, reviewed and commented on the statistical analysis and reviewed the report.
Corresponding author
Ethics declarations
Conflicts of interest
Pietro Emiliano Doneddu has received travel grants to attend scientific meetings from CSL Behring and Kedrion. Dario Cocito has received honoraria for lecturing fromShire, CSL Behring, and Kedrion and travel grants to attend scientific meeting from Shire, Kedrion, and CSL Behring. Fiore Manganelli reports personal fees for scientific events from CSL Behring and has received travel grants to attend scientific meetings from CSL Behring and Kedrion. Raffaella Fazio has served on scientific advisory boards for CSL Behring and has received travel grants from Kedrion and CSL Behring to attend scientific meeting. Chiara Briani has served on scientific advisory boards for Pfizer, Alnylam, and Akcea, and has received travel grants from Kedrion and CSL Behring to attend scientific meeting. Massimiliano Filosto has served on scientific advisory boards for CSL Behring and has received travel grants from Kedrion, Baxter and CSL Behring to attend scientific meeting. Stefano Jann has received research grants from Grifols, outside this work, and travel grants from Grifols and Kedrion. Anna Mazzeo has received travel grants from Kedrion and CSL Behring to attend scientific meeting. Giuseppe Cosentino has received travel grants to attend scientific meetings from CSL Behring and Kedrion. Andrea Cortese has received travel grants to attend scientific meetings from Kedrion. Marinella Carpo has received travel grants to attend scientific meetings from Kedrion. Guido Cavaletti has received honoraria for lecturing and travel grants to attend scientific meetings from Kedrion. Ettore Beghi reports grants from UCB-Pharma, grants from Shire, grants from EISAI, personal fees from Viropharma, grants from Italian Ministry of Health, grants from Fondazione Borgonovo, grants from Associazione IDIC 15 and grants from European Union, outside the submitted work. Giuseppe Liberatore has received travel grants to attend scientific meetings from CSL Behring and Kedrion. Lucio Santoro reports personal fees for scientific events from CSL Behring and has received travel grants to attend scientific meetings from CSL Behring and Kedrion. Erdita Peci has received travel grants to attend scientific meetings from CSL Behring. Eduardo Nobile Orazio reports personal fees for Advisory or Scientific Board from Kedrion, Italy, Baxter, Italy, Novartis, Switzerland, CSL-Behring, Italy, Astellas, the Netherlands, outside the submitted work and travel grants to attend Scientific Meeting from Baxter, Grifols, Kedrion, and Novartis, Italy. The other authors declare no conflict of interest.
Ethics approval
The study was approved by the Ethical Committee of each participating center.
Consent to participate
Written informed consent was obtained from all participants at enrollment.
Rights and permissions
About this article
Cite this article
Doneddu, P.E., Bianchi, E., Cocito, D. et al. Impact of environmental factors and physical activity on disability and quality of life in CIDP. J Neurol 267, 2683–2691 (2020). https://doi.org/10.1007/s00415-020-09916-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-09916-y