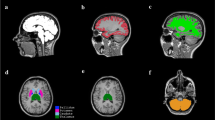
Abstract
Objectives
Although whole and individual regional brain volume loss have been separately reported to correlate with disability in multiple sclerosis (MS), hierarchical cluster analyses of the whole and regional brain to find their pattern in MS are few.
Methods
We cross-sectionally conducted high-resolution, T1-weighted volumetric MRI examinations in 75 MS patients and 21 healthy controls (HCs) to measure the volumes of whole brain and a total of 56 brain regions of interest. Using a hierarchical cluster analysis with multivariate imaging data, we classified the patients into clusters according to their brain-volume patterns. Principal component analysis was also applied. Clinical features and brain volumes were then compared among the MS clusters.
Results
The MS patients were categorized into three major clusters (Clusters 1, 2, and 3) with increasing disability in that order. Principal component analysis also identified Clusters 1, 2 and 3. Whole brain volume and supratentorial regional brain volumes, including thalamus and corpus callosum, decreased severely in Cluster 3 and moderately in Cluster 2, while equally preserved in Cluster 1 and the HCs. Only the volumes of the ventral diencephalon and T1 white matter hypointensities significantly differed in Clusters 1, 2 and 3 and HCs. In contrast, the volumes of the cerebellar cortex and brainstem were significantly different between Clusters 3 and 1, whereas there were no significant differences between Clusters 1 and 2 and Clusters 2 and 3.
Conclusion
We identified brain regions that exhibit different degree of atrophy in a background of global brain atrophy in MS.




Similar content being viewed by others
References
Reich DS, Lucchinetti CF, Calabresi PA (2018) Multiple sclerosis. N Engl J Med 378(2):169–180. https://doi.org/10.1056/NEJMra1401483
Vigeveno RM, Wiebenga OT, Wattjes MP, Geurts JJ, Barkhof F (2012) Shifting imaging targets in multiple sclerosis: from inflammation to neurodegeneration. J Magn Reson Imaging 36(1):1–19. https://doi.org/10.1002/jmri.23578
Rocca MA, Comi G, Filippi M (2017) The role of T1-weighted derived measures of neurodegeneration for assessing disability progression in multiple sclerosis. Front Neurol 8:433. https://doi.org/10.3389/fneur.2017.00433
Rocca MA, Battaglini M, Benedict RH, De Stefano N, Geurts JJ, Henry RG, Horsfield MA, Jenkinson M, Pagani E, Filippi M (2017) Brain MRI atrophy quantification in MS: from methods to clinical application. Neurology 88(4):403–413. https://doi.org/10.1212/wnl.0000000000003542
Eshaghi A, Prados F, Brownlee W, Altmann DR, Tur C, Cardoso MJ, De Angelis F, van de Pavert SH, Cawley N, De Stefano N, Stromillo ML, Battaglini M, Ruggieri S, Gasperini C, Filippi M, Rocca MA, Rovira A, Sastre-Garriga J, Vrenken H, Leurs CE, Killestein J, Pirpamer L, Enzinger C, Ourselin S, Wheeler-Kingshott C, Chard D, Thompson AJ, Alexander DC, Barkhof F, Ciccarelli O (2018) Deep grey matter volume loss drives disability worsening in multiple sclerosis. Ann Neurol. https://doi.org/10.1002/ana.25145
Vollmer T, Huynh L, Kelley C, Galebach P, Signorovitch J, DiBernardo A, Sasane R (2016) Relationship between brain volume loss and cognitive outcomes among patients with multiple sclerosis: a systematic literature review. Neurol Sci 37(2):165–179. https://doi.org/10.1007/s10072-015-2400-1
Granberg T, Martola J, Bergendal G, Shams S, Damangir S, Aspelin P, Fredrikson S, Kristoffersen-Wiberg M (2015) Corpus callosum atrophy is strongly associated with cognitive impairment in multiple sclerosis: results of a 17-year longitudinal study. Mult Scler (Houndmills, Basingstoke, England) 21(9):1151–1158. https://doi.org/10.1177/1352458514560928
Schoonheim MM, Hulst HE, Brandt RB, Strik M, Wink AM, Uitdehaag BM, Barkhof F, Geurts JJ (2015) Thalamus structure and function determine severity of cognitive impairment in multiple sclerosis. Neurology 84(8):776–783. https://doi.org/10.1212/wnl.0000000000001285
Weier K, Till C, Fonov V, Yeh EA, Arnold DL, Banwell B, Collins DL (2016) Contribution of the cerebellum to cognitive performance in children and adolescents with multiple sclerosis. Mult Scler (Houndmills, Basingstoke, England) 22(5):599–607. https://doi.org/10.1177/1352458515595132
Whitwell JL, Przybelski SA, Weigand SD, Ivnik RJ, Vemuri P, Gunter JL, Senjem ML, Shiung MM, Boeve BF, Knopman DS, Parisi JE, Dickson DW, Petersen RC, Jack CR Jr, Josephs KA (2009) Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: a cluster analysis study. Brain J Neurol 132(Pt 11):2932–2946. https://doi.org/10.1093/brain/awp232
Noh Y, Jeon S, Lee JM, Seo SW, Kim GH, Cho H, Ye BS, Yoon CW, Kim HJ, Chin J, Park KH, Heilman KM, Na DL (2014) Anatomical heterogeneity of Alzheimer disease: based on cortical thickness on MRIs. Neurology 83(21):1936–1944. https://doi.org/10.1212/wnl.0000000000001003
Uribe C, Segura B, Baggio HC, Abos A, Marti MJ, Valldeoriola F, Compta Y, Bargallo N, Junque C (2016) Patterns of cortical thinning in nondemented Parkinson's disease patients. Mov Disord 31(5):699–708. https://doi.org/10.1002/mds.26590
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O'Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69(2):292–302. https://doi.org/10.1002/ana.22366
Fischl B, Liu A, Dale AM (2001) Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging 20(1):70–80. https://doi.org/10.1109/42.906426
Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis I. Segmentation and surface reconstruction. NeuroImage 9(2):179–194. https://doi.org/10.1006/nimg.1998.0395
Segonne F, Pacheco J, Fischl B (2007) Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging 26(4):518–529. https://doi.org/10.1109/tmi.2006.887364
Dale AM, Sereno MI (1993) Improved Localizadon of Cortical Activity by Combining EEG and MEG with MRI Cortical Surface Reconstruction: a Linear Approach. J Cogn Neurosci 5(2):162–176. https://doi.org/10.1162/jocn.1993.5.2.162
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97(20):11050–11055. https://doi.org/10.1073/pnas.200033797
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33(3):341–355
Ward JH (1963) Hierarchical grouping to optimize an objective function. J Am Statist Assoc 58(301):236–244. https://doi.org/10.2307/2282967
Azevedo CJ, Overton E, Khadka S, Buckley J, Liu S, Sampat M, Kantarci O, Lebrun FC, Siva A, Okuda DT, Pelletier D (2015) Early CNS neurodegeneration in radiologically isolated syndrome. Neurol (R) Neuroimmunol Neuroinflamm 2(3):e102. https://doi.org/10.1212/nxi.0000000000000102
Jain S, Sima DM, Ribbens A, Cambron M, Maertens A, Van Hecke W, De Mey J, Barkhof F, Steenwijk MD, Daams M, Maes F, Van Huffel S, Vrenken H, Smeets D (2015) Automatic segmentation and volumetry of multiple sclerosis brain lesions from MR images. NeuroImage Clin 8:367–375. https://doi.org/10.1016/j.nicl.2015.05.003
Akaishi T, Nakashima I, Mugikura S, Aoki M, Fujihara K (2017) Whole brain and grey matter volume of Japanese patients with multiple sclerosis. J Neuroimmunol 306:68–75. https://doi.org/10.1016/j.jneuroim.2017.03.009
Fujimori J, Baba T, Meguro Y, Nakashima I, Mori E, Fujihara K, Aoki M (2015) Comparison of the rao brief repeatable neuropsychological battery with wechsler adult intelligence scale-III and Wechsler Memory Scale-revised in Japanese patients with multiple sclerosis. Clin Exp Neuroimmunol 6(3):306–308
De Stefano N, Airas L, Grigoriadis N, Mattle HP, O'Riordan J, Oreja-Guevara C, Sellebjerg F, Stankoff B, Walczak A, Wiendl H, Kieseier BC (2014) Clinical relevance of brain volume measures in multiple sclerosis. CNS Drugs 28(2):147–156. https://doi.org/10.1007/s40263-014-0140-z
Tao G, Datta S, He R, Nelson F, Wolinsky JS, Narayana PA (2009) Deep gray matter atrophy in multiple sclerosis: a tensor based morphometry. J Neurol Sci 282(1–2):39–46. https://doi.org/10.1016/j.jns.2008.12.035
Riccitelli G, Rocca MA, Pagani E, Martinelli V, Radaelli M, Falini A, Comi G, Filippi M (2012) Mapping regional grey and white matter atrophy in relapsing–remitting multiple sclerosis. Mult Scler (Houndmills, Basingstoke, England) 18(7):1027–1037. https://doi.org/10.1177/1352458512439239
Jacobsen C, Hagemeier J, Myhr KM, Nyland H, Lode K, Bergsland N, Ramasamy DP, Dalaker TO, Larsen JP, Farbu E, Zivadinov R (2014) Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry 85(10):1109–1115. https://doi.org/10.1136/jnnp-2013-306906
Rocca MA, Mesaros S, Pagani E, Sormani MP, Comi G, Filippi M (2010) Thalamic damage and long-term progression of disability in multiple sclerosis. Radiology 257(2):463–469. https://doi.org/10.1148/radiol.10100326
Granberg T, Bergendal G, Shams S, Aspelin P, Kristoffersen-Wiberg M, Fredrikson S, Martola J (2015) MRI-defined corpus callosal atrophy in multiple sclerosis: a comparison of volumetric measurements, corpus callosum area and index. J Neuroimaging 25(6):996–1001. https://doi.org/10.1111/jon.12237
Riva M, Ikonomidou VN, Ostuni JJ, van Gelderen P, Auh S, Ohayon JM, Tovar-Moll F, Richert ND, Duyn JH, Bagnato F (2009) Tissue-specific imaging is a robust methodology to differentiate in vivo T1 black holes with advanced multiple sclerosis-induced damage. AJNR Am J Neuroradiol 30(7):1394–1401. https://doi.org/10.3174/ajnr.A1573
Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ (2008) Decreased volume of the brain reward system in alcoholism. Biol Psychiat 64(3):192–202. https://doi.org/10.1016/j.biopsych.2008.01.018
Henry RG, Shieh M, Okuda DT, Evangelista A, Gorno-Tempini ML, Pelletier D (2008) Regional grey matter atrophy in clinically isolated syndromes at presentation. J Neurol Neurosurg Psychiatry 79(11):1236–1244. https://doi.org/10.1136/jnnp.2007.134825
Piccolo L, Kumar G, Nakashima I, Misu T, Kong Y, Wakerley B, Ryan S, Cavey A, Fujihara K, Palace J (2015) Multiple sclerosis in Japan appears to be a milder disease compared to the UK. J Neurol 262(4):831–836. https://doi.org/10.1007/s00415-015-7637-3
Funding
Kazuo Fujihara has received funding for travel and speaker honoraria from Bayer Schering Pharma, Biogen Idec, Eisai Inc., Mitsubishi Tanabe Pharma Corporation, Novartis Pharma, Astellas Pharma Inc., Takeda Pharmaceutical Company Limited, Asahi Kasei Medical Co., Daiichi Sankyo, and Nihon Pharmaceutical; Dr. Fujihara’s research is funded by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (22229008, 2010–2015; 26293205, 2014–2016) and by Grants-in-Aid for Scientific Research from the Ministry of Health, Welfare and Labor of Japan (2010 to present). Ichiro Nakashima is receiving research support from LSI Medience and is funded by JSPS KAKENHI Grant Number 17K09772. The funders had no role in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by JF, RO and TB. The first draft of the manuscript was written by JF and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Kazuo Fujihara serves on scientific advisory boards for Bayer Schering Pharma, Biogen Idec, Mitsubishi Tanabe Pharma Corporation, Novartis Pharma, Chugai Pharmaceutical, Ono Pharmaceutical, Nihon Pharmaceutical, Merck Serono, Alexion Pharmaceuticals, MedImmune, and Medical Review; serves as an editorial board member for Clinical and Experimental Neuroimmunology (2009 to present) and an advisory board member for the Sri Lanka Journal of Neurology; and has received research support from Bayer Schering Pharma, Biogen Idec Japan, Asahi Kasei Medical, The Chemo-Sero-Therapeutic Research Institute, Teva Pharmaceutical, Mitsubishi Tanabe Pharma, Teijin Pharma, Chugai Pharmaceutical, Ono Pharmaceutical, Nihon Pharmaceutical, and Genzyme Japan. Mike Wattjes reports speaker or consultancy fees from Bayer Healthcare, Biogen, Biologix, Celgene, Eisai, Genilac, Imcyse, Merck Serono, Novartis, Roche, and Sanofi Genzyme. Ichiro Nakashima is serving on scientific advisory boards for Biogen Japan and Novartis Pharma and is receiving honoraria for speaking engagements with Biogen Japan, Mitsubishi Tanabe Pharma, Novartis Pharma, Takeda Pharmaceutical, and Eisai. No other disclosures were reported.
Ethical standards
This study was approved by the institutional ethics committee and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
All patients provided written informed consent.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fujimori, J., Fujihara, K., Ogawa, R. et al. Patterns of regional brain volume loss in multiple sclerosis: a cluster analysis. J Neurol 267, 395–405 (2020). https://doi.org/10.1007/s00415-019-09595-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09595-4