Abstract
Purpose
Detection and prediction of the rate of brain volume loss with age is a significant unmet need in patients with primary progressive multiple sclerosis (PPMS). In this study we construct detailed brain volume maps for PPMS patients. These maps compare age-related changes in both cortical and sub-cortical regions with those in healthy individuals.
Methods
We conducted retrospective analyses of brain volume using T1-weighted Magnetic Resonance Imaging (MRI) scans of a large cohort of PPMS patients and healthy subjects. The volume of brain parenchyma (BP), cortex, white matter (WM), deep gray matter, thalamus, and cerebellum were measured using the robust SynthSeg segmentation tool. Age- and gender-related regression curves were constructed based on data from healthy subjects, with the 95% prediction interval adopted as the normality threshold for each brain region.
Results
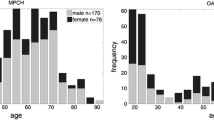
We analyzed 495 MRI scans from 169 PPMS patients, aged 20–79 years, alongside 563 exams from healthy subjects aged 20–86. Compared to healthy subjects, a higher proportion of PPMS patients showed lower than expected brain volumes in all regions except the cerebellum. The most affected areas were BP, WM, and thalamus. Lower brain volumes correlated with longer disease duration for BP and WM, and higher disability for BP, WM, cortex, and thalamus.
Conclusions
Constructing age- and gender-related brain volume maps enabled identifying PPMS patients at a higher risk of brain volume loss. Monitoring these high-risk patients may lead to better treatment decisions and improve patient outcomes.





Similar content being viewed by others
Data availability
The IXI dataset used for MRI scans of healthy subjects is publicly available at https://brain-development.org/ixi-dataset/. The MRI data and clinical information for PPMS patients used in this study are not publicly available due to privacy and ethical restrictions, as they were obtained from the SMCMS Data Registry, which contains sensitive patient information and is subject to strict confidentiality agreements.
References
Browne P, Chandraratna D, Angood C et al (2014) Atlas of Multiple Sclerosis 2013: A growing global problem with widespread inequity. Neurology 83:1022–1024. https://doi.org/10.1212/WNL.0000000000000768
Dobson R, Giovannoni G (2019) Multiple sclerosis - a review. Eur J Neurol 26:27–40. https://doi.org/10.1111/ene.13819
Gorman MP, Healy BC, Polgar-Turcsanyi M, Chitnis T (2009) Increased relapse rate in pediatric-onset compared with adult-onset multiple sclerosis. Arch Neurol 66:54–59. https://doi.org/10.1001/archneurol.2008.505
Compston A, Coles A (2008) Multiple sclerosis. Lancet 372:1502–1517. https://doi.org/10.1016/S0140-6736(08)61620-7
Miller DH, Leary SM (2007) Primary-progressive multiple sclerosis. Lancet Neurol 6:903–912. https://doi.org/10.1016/S1474-4422(07)70243-0
Fox EJ, Markowitz C, Applebee A et al (2018) Ocrelizumab reduces progression of upper extremity impairment in patients with primary progressive multiple sclerosis: Findings from the phase III randomized ORATORIO trial. Mult Scler 24:1862–1870. https://doi.org/10.1177/1352458518808189
Filippi M, Preziosa P, Barkhof F et al (2021) Diagnosis of Progressive Multiple Sclerosis From the Imaging Perspective: A Review. JAMA Neurol 78:351–364. https://doi.org/10.1001/jamaneurol.2020.4689
Kremenchutzky M, Lee D, Rice GP, Ebers GC (2000) Diagnostic brain MRI findings in primary progressive multiple sclerosis. Mult Scler 6:81–85. https://doi.org/10.1177/135245850000600205
Montalban X, Sastre-Garriga J, Filippi M et al (2009) Primary progressive multiple sclerosis diagnostic criteria: a reappraisal. Mult Scler 15:1459–1465. https://doi.org/10.1177/1352458509348422
Rocca MA, Absinta M, Filippi M (2012) The role of advanced magnetic resonance imaging techniques in primary progressive MS. J Neurol 259:611–621. https://doi.org/10.1007/s00415-011-6195-6
Smolinski L, Litwin T, Redzia-Ogrodnik B et al (2019) Brain volume is related to neurological impairment and to copper overload in Wilson’s disease. Neurol Sci 40:2089–2095. https://doi.org/10.1007/s10072-019-03942-z
Pagani E, Rocca MA, Gallo A et al (2005) Regional brain atrophy evolves differently in patients with multiple sclerosis according to clinical phenotype. AJNR Am J Neuroradiol 26:341–346
Eshaghi A, Prados F, Brownlee WJ et al (2018) Deep gray matter volume loss drives disability worsening in multiple sclerosis. Ann Neurol 83:210–222. https://doi.org/10.1002/ana.25145
Andravizou A, Dardiotis E, Artemiadis A et al (2019) Brain atrophy in multiple sclerosis: mechanisms, clinical relevance and treatment options. Auto Immun Highlights 10:7. https://doi.org/10.1186/s13317-019-0117-5
Sepulcre J, Sastre-Garriga J, Cercignani M et al (2006) Regional gray matter atrophy in early primary progressive multiple sclerosis: a voxel-based morphometry study. Arch Neurol 63:1175–1180. https://doi.org/10.1001/archneur.63.8.1175
Eshaghi A, Marinescu RV, Young AL et al (2018) Progression of regional grey matter atrophy in multiple sclerosis. Brain 141:1665–1677. https://doi.org/10.1093/brain/awy088
Gur RC, Mozley PD, Resnick SM et al (1991) Gender differences in age effect on brain atrophy measured by magnetic resonance imaging. Proc Natl Acad Sci U S A 88:2845–2849. https://doi.org/10.1073/pnas.88.7.2845
Polman CH, Reingold SC, Banwell B et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302. https://doi.org/10.1002/ana.22366
Swanton JK, Rovira A, Tintore M et al (2007) MRI criteria for multiple sclerosis in patients presenting with clinically isolated syndromes: a multicentre retrospective study. Lancet Neurol 6:677–686. https://doi.org/10.1016/S1474-4422(07)70176-X
Li X, Morgan PS, Ashburner J et al (2016) The first step for neuroimaging data analysis: DICOM to NIfTI conversion. J Neurosci Methods 264:47–56. https://doi.org/10.1016/j.jneumeth.2016.03.001
Billot B, Greve DN, OulaPuonti c AT et al (2023) SynthSeg: Segmentation of brain MRI scans of any contrast and resolution without retraining. Med Image Anal 86:102789. https://doi.org/10.1016/j.media.2023.102789
Billot B, Magdamo C, Cheng Y et al (2023) Robust machine learning segmentation for large-scale analysis of heterogeneous clinical brain MRI datasets. Proc Natl Acad Sci U S A 120:e2216399120. https://doi.org/10.1073/pnas.2216399120
Caspi Y, Brouwer RM, Schnack HG et al (2020) Changes in the intracranial volume from early adulthood to the sixth decade of life: A longitudinal study. Neuroimage 220:116842. https://doi.org/10.1016/j.neuroimage.2020.116842
Iglesias JE, Ferraris S, Modat M et al (2017) Template-free estimation of intracranial volume: A preterm birth animal model study. 3–13. https://doi.org/10.1007/978-3-319-67561-9_1
Voevodskaya O, Simmons A, Nordenskjöld R et al (2014) The effects of intracranial volume adjustment approaches on multiple regional MRI volumes in healthy aging and Alzheimer’s disease. Front Aging Neurosci 6:264. https://doi.org/10.3389/fnagi.2014.00264
Hansen TI, Brezova V, Eikenes L et al (2015) How Does the Accuracy of Intracranial Volume Measurements Affect Normalized Brain Volumes? Sample Size Estimates Based on 966 Subjects from the HUNT MRI Cohort. AJNR Am J Neuroradiol 36:1450–1456. https://doi.org/10.3174/ajnr.A4299
Borgonovo E, Plischke E (2016) Sensitivity analysis: A review of recent advances. Eur J Oper Res 248:869–887. https://doi.org/10.1016/j.ejor.2015.06.032
Pollard TJ, Johnson AEW, Raffa JD, Mark RG (2018) An open source Python package for producing summary statistics for research papers. JAMIA Open 1:26–31. https://doi.org/10.1093/jamiaopen/ooy012
Seabold S, Perktold J (2010) Statsmodels: econometric and statistical modeling with python. pp 92–96. https://doi.org/10.25080/Majora-92bf1922-011
Virtanen P, Gommers R, Oliphant TE et al (2020) SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods 17:261–272. https://doi.org/10.1038/s41592-019-0686-2
Hunter JD (2007) Matplotlib: A 2D Graphics Environment. Comput Sci Eng 9:90–95. https://doi.org/10.1109/MCSE.2007.55
Waskom M (2021) seaborn: statistical data visualization. J Open Source Softw 6:3021. https://doi.org/10.21105/joss.03021
Carne RP, Vogrin S, Litewka L, Cook MJ (2006) Cerebral cortex: an MRI-based study of volume and variance with age and sex. J Clin Neurosci 13:60–72. https://doi.org/10.1016/j.jocn.2005.02.013
Enzinger C, Fazekas F, Matthews PM et al (2005) Risk factors for progression of brain atrophy in aging: six-year follow-up of normal subjects. Neurology 64:1704–1711. https://doi.org/10.1212/01.WNL.0000161871.83614.BB
Schuff N, Woerner N, Boreta L et al (2009) MRI of hippocampal volume loss in early Alzheimer’s disease in relation to ApoE genotype and biomarkers. Brain 132:1067–1077. https://doi.org/10.1093/brain/awp007
Sheline YI, Gado MH, Price JL (1998) Amygdala core nuclei volumes are decreased in recurrent major depression. NeuroReport 9:2023–2028. https://doi.org/10.1097/00001756-199806220-00021
Günther V, Lindner C, Dannlowski U et al (2016) Amygdalar Gray Matter Volume and Social Relating in Schizophrenia. Neuropsychobiology 74:139–143. https://doi.org/10.1159/000458528
Cagol A, Schaedelin S, Barakovic M et al (2022) Association of Brain Atrophy With Disease Progression Independent of Relapse Activity in Patients With Relapsing Multiple Sclerosis. JAMA Neurol 79:682–692. https://doi.org/10.1001/jamaneurol.2022.1025
Herting MM, Sowell ER (2017) Puberty and structural brain development in humans. Front Neuroendocrinol 44:122–137. https://doi.org/10.1016/j.yfrne.2016.12.003
Rovaris M, Gallo A, Falini A et al (2005) Axonal injury and overall tissue loss are not related in primary progressive multiple sclerosis. Arch Neurol 62:898–902. https://doi.org/10.1001/archneur.62.6.898
Chen X, Schädelin S, Lu P-J et al (2023) Personalized maps of T1 relaxometry abnormalities provide correlates of disability in multiple sclerosis patients. Neuroimage Clin 37:103349. https://doi.org/10.1016/j.nicl.2023.103349
Kolind S, Matthews L, Johansen-Berg H et al (2012) Myelin water imaging reflects clinical variability in multiple sclerosis. Neuroimage 60:263–270. https://doi.org/10.1016/j.neuroimage.2011.11.070
Kutzelnigg A, Lucchinetti CF, Stadelmann C et al (2005) Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128:2705–2712. https://doi.org/10.1093/brain/awh641
Mesaros S, Rocca MA, Pagani E et al (2011) Thalamic damage predicts the evolution of primary-progressive multiple sclerosis at 5 years. AJNR Am J Neuroradiol 32:1016–1020. https://doi.org/10.3174/ajnr.A2430
Minagar A, Barnett MH, Benedict RHB et al (2013) The thalamus and multiple sclerosis: modern views on pathologic, imaging, and clinical aspects. Neurology 80:210–219. https://doi.org/10.1212/WNL.0b013e31827b910b
Motl RW, Zivadinov R, Bergsland N, Benedict RHB (2016) Thalamus volume and ambulation in multiple sclerosis: a cross-sectional study. Neurodegener Dis Manag 6:23–29. https://doi.org/10.2217/nmt.15.71
Batista S, Zivadinov R, Hoogs M et al (2012) Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. J Neurol 259:139–146. https://doi.org/10.1007/s00415-011-6147-1
Siger M (2022) Magnetic Resonance Imaging in Primary Progressive Multiple Sclerosis Patients : Review. Clin Neuroradiol 32:625–641. https://doi.org/10.1007/s00062-022-01144-3
Anderson VM, Wheeler-Kingshott CAM, Abdel-Aziz K et al (2011) A comprehensive assessment of cerebellar damage in multiple sclerosis using diffusion tractography and volumetric analysis. Mult Scler 17:1079–1087. https://doi.org/10.1177/1352458511403528
Zivadinov R, Sepcic J, Nasuelli D et al (2001) A longitudinal study of brain atrophy and cognitive disturbances in the early phase of relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 70:773–780. https://doi.org/10.1136/jnnp.70.6.773
Acknowledgements
The authors extend their appreciation to the patients and caregivers whose essential contributions have been invaluable to this manuscript.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Internal Review Board of Sheba Medical Center. Informed consent was waived as the study was non-interventional and retrospective. Data was collected, coded, and analyzed according to the ethical standards of human experimentation.
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Warszawer, Y., Gurevich, M., Kerpel, A. et al. Mapping brain volume change across time in primary-progressive multiple sclerosis. Neuroradiology (2024). https://doi.org/10.1007/s00234-024-03354-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00234-024-03354-7