Abstract
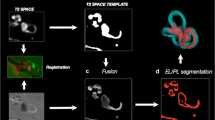
Intravenous contrast agent-enhanced magnetic resonance imaging of the endolymphatic space (ELS) of the inner ear permits direct, in-vivo, non-invasive visualization of labyrinthine structures and thus verification of endolymphatic hydrops (ELH). However, current volumetric assessment approaches lack normalization. The aim of this study was to develop a probabilistic atlas of the inner ear’s bony labyrinth as a first step towards an automated and reproducible volume-based quantification of the ELS. The study included three different datasets: a source dataset (D1) to build the probabilistic atlas and two testing sets (D2, D3). D1 included 24 right-handed patients (12 females; mean age 51.5 ± 3.9 years) and D2 5 patients (3 female; mean age 48.8 ± 5.01 years) with vestibular migraine without ELH or any measurable vestibular deficits. D3 consisted of five patients (one female; mean age 46 ± 5.2 years) suffering from unilateral Menière’s disease and ELH. Data processing comprised three steps: preprocessing using an affine and deformable fusion registration pipeline, computation of an atlas for the left and right inner ear using a label-assisted approach, and validation of the atlas based on localizing and segmenting previously unseen ears. The three-dimensional probabilistic atlas of the inner ear’s bony labyrinth consisted of the internal acoustic meatus and inner ears (including cochlea, otoliths, and semicircular canals) for both sides separately. The analyses showed a high level of agreement between the atlas-based segmentation and the manual gold standard with an overlap of 89% for the right ear and 86% for the left ear (measured by dice scores). This probabilistic in vivo atlas of the human inner ear’s bony labyrinth and thus of the inner ear’s total fluid space for both ears represents a necessary step towards a normalized, easily reproducible and reliable volumetric quantification of the perilymphatic and endolymphatic space in view of MR volumetric assessment of ELH. The proposed atlas lays the groundwork for state-of-the-art approaches (e.g., deep learning) and will be provided to the scientific community.



Similar content being viewed by others
Abbreviations
- 3D:
-
Three dimensional
- CISS:
-
Constructive interference in steady state
- DS:
-
Dice score
- ELH:
-
Endolymphatic hydrops
- ELS:
-
Endolymphatic space
- FLAIR:
-
Fluid-attenuated inversion recovery
- GRAPPA:
-
Generalized auto-calibrating partially parallel acquisition
- MR:
-
Magnetic resonance
- iv:
-
Intravenous
- it:
-
Intratympanal
- iMRI:
-
Gadolinium-enhanced high-resolution magnetic resonance imaging of the inner ear
- itMRI:
-
Intratympanal applied iMRI
- ivMRI:
-
Intravenous applied delayed iMRI
- L:
-
Left
- MD:
-
Menière’s disease
- MRI:
-
Magnetic resonance imaging
- R:
-
Right
- RH:
-
Right handed
- RMSE:
-
Root-mean-square error
- SD:
-
Standard deviation
- SVV:
-
Subjective visual vertical
- TNOV:
-
Total number of voxels
- TV:
-
Total volume
- vHIT:
-
Videooculography during head impulse test
- VM:
-
Vestibular migraine
References
Fraysse BG, Alonso A, House WF (1980) Menière’s disease and endolymphatic hydrops: clinical-histopathological correlations. Ann Otol Rhinol Laryngol Suppl 89:2–22
Nakashima T, Pyykkö I, Arroll MA et al (2016) Meniere’s disease. Nat Rev Dis Prim 2:16028. https://doi.org/10.1038/nrdp.2016.28
Rauch SD, Merchant SN, Thedinger BA (1989) Meniere’s syndrome and endolymphatic hydrops. Double-blind temporal bone study. Ann Otol Rhinol Laryngol 98:873–883
Ishiyama G, Lopez IA, Sepahdari AR, Ishiyama A (2015) Meniere’s disease: histopathology, cytochemistry, and imaging. Ann N Y Acad Sci 1343:49–57. https://doi.org/10.1111/nyas.12699
Pyykkö I, Zou J, Gürkov R et al (2019) Imaging of temporal bone. Adv Otolaryngol 82:12–31
Lopez-Escamez JA, Attyé A (2019) Magnetic resonance imaging of endolymphatic hydrops: controversies and common ground. J Vestib Res. https://doi.org/10.3233/VES-180663
Naganawa S, Satake H, Kawamura M et al (2008) Separate visualization of endolymphatic space, perilymphatic space and bone by a single pulse sequence; 3D-inversion recovery imaging utilizing real reconstruction after intratympanic Gd-DTPA administration at 3 Tesla. Eur Radiol 18:920–924. https://doi.org/10.1007/s00330-008-0854-8
Naganawa S, Yamazaki M, Kawai H et al (2010) Visualization of endolymphatic hydrops in Ménière’s disease with single-dose intravenous gadolinium-based contrast media using heavily T(2)-weighted 3D-FLAIR. Magn Reson Med Sci 9:237–242
Attyé A, Eliezer M, Galloux A et al (2017) Endolymphatic hydrops imaging: differential diagnosis in patients with Meniere disease symptoms. Diagn Interv Imaging 98:699–706. https://doi.org/10.1016/j.diii.2017.06.002
Imai T, Uno A, Kitahara T et al (2017) Evaluation of endolymphatic hydrops using 3-T MRI after intravenous gadolinium injection. Eur Arch Oto-Rhino-Laryngol 274:4103–4111. https://doi.org/10.1007/s00405-017-4739-9
Karch-Georges A, Veillon F, Vuong H et al (2019) MRI of endolymphatic hydrops in patients with vestibular schwannomas: a case-controlled study using non-enhanced T2-weighted images at 3 Teslas. Eur Arch Oto-Rhino-Laryngol 276:1591–1599. https://doi.org/10.1007/s00405-019-05395-8
Kirsch V, Becker-Bense S, Berman A et al (2018 Oct) (2018) Transient endolymphatic hydrops after an attack of vestibular migraine: a longitudinal single case study. J Neurol 265(Suppl 1):51–53. https://doi.org/10.1007/s00415-018-8870-3 (Epub 2018 Apr 25)
Sun W, Liang Q, Kuang S et al (2019) 3D-real IR MRI detects serendipity of inner ear in enlarged vestibular aqueduct syndrome. Acta Otolaryngol 139:233–237. https://doi.org/10.1080/00016489.2018.1563719
Conte G, Caschera L, Tuscano B et al (2018) Three-Tesla magnetic resonance imaging of the vestibular endolymphatic space: a systematic qualitative description in healthy ears. Eur J Radiol 109:77–82. https://doi.org/10.1016/j.ejrad.2018.10.023
Lobo D, Tuñón M, Villarreal I et al (2018) Intratympanic gadolinium magnetic resonance imaging supports the role of endolymphatic hydrops in the pathogenesis of immune-mediated inner-ear disease. J Laryngol Otol 132:554–559. https://doi.org/10.1017/S0022215118000749
Nakada T, Yoshida T, Suga K et al (2014) Endolymphatic space size in patients with vestibular migraine and Ménière’s disease. J Neurol 261:2079–2084. https://doi.org/10.1007/s00415-014-7458-9
Neff BA, Staab JP, Egger SD et al (2012) Auditory and vestibular symptoms and chronic subjective dizziness in patients with Ménière’s disease, vestibular migraine, and Ménière’s disease with concomitant vestibular migraine. Otol Neurotol 33:1235–1244. https://doi.org/10.1097/MAO.0b013e31825d644a
Gürkov R, Berman A, Dietrich O et al (2015) MR volumetric assessment of endolymphatic hydrops. Eur Radiol 25:585–595. https://doi.org/10.1007/s00330-014-3414-4
Homann G, Vieth V, Weiss D et al (2015) Semi-quantitative vs. volumetric determination of endolymphatic space in Menière’s disease using endolymphatic hydrops 3T-HR-MRI after intravenous gadolinium injection. PLoS O ne 10:e0120357. https://doi.org/10.1371/journal.pone.0120357
Naganawa S, Suzuki K, Nakamichi R et al (2013) Semi-quantification of endolymphatic size on MR imaging after intravenous injection of single-dose gadodiamide: comparison between two types of processing strategies. Magn Reson Med Sci 12:261–269. https://doi.org/10.2463/mrms.2013-0019
Zwergal A, Kirsch V, Gerb J et al (2018) Neuro-otology: at the borders of ear and brain. Nervenarzt 89:1106–1114. https://doi.org/10.1007/s00115-018-0598-x
Penney GP, Weese J, Little JA et al (1998) A comparison of similarity measures for use in 2-D-3-D medical image registration. IEEE Trans Med Imaging 17:586–595. https://doi.org/10.1109/42.730403
Nakashima T, Naganawa S, Pyykko I et al (2009) Grading of endolymphatic hydrops using magnetic resonance imaging. Acta Otolaryngol Suppl. https://doi.org/10.1080/00016480902729827
Barath K, Schuknecht B, Naldi AM et al (2014) Detection and grading of endolymphatic hydrops in meniere disease using MR imaging. Am J Neuroradiol 35:1387–1392. https://doi.org/10.3174/ajnr.A3856
Carmichael OT, Aizenstein HA, Davis SW et al (2005) Atlas-based hippocampus segmentation in Alzheimer’s disease and mild cognitive impairment. Neuroimage 27:979–990. https://doi.org/10.1016/j.neuroimage.2005.05.005
Bilello M, Lao Z, Krejza J et al (2012) Atlas-based classification of hyperintense regions from MR diffusion-weighted images of the brain: preliminary results. Neuroradiol J 25:112–120. https://doi.org/10.1177/197140091202500115
Toga AW, Thompson PM (2001) The role of image registration in brain mapping. Image Vis Comput 19:3–24. https://doi.org/10.1016/S0262-8856(00)00055-X
Dieterich M, Bense S, Lutz S et al (2003) Dominance for vestibular cortical function in the non-dominant hemisphere. Cereb Cortex 13:994–1007
Mansour SL, Schoenwolf GC (2005) Morphogenesis of the inner ear. In: Kelley M, Wu D, Popper A, Fay R (eds) Development of the inner ear. Springer, New York, pp 43–84
Hatch EP, Noyes CA, Wang X et al (2007) Fgf3 is required for dorsal patterning and morphogenesis of the inner ear epithelium. Development 134:3615–3625. https://doi.org/10.1242/dev.006627
Dill T (2008) Contraindications to magnetic resonance imaging: non-invasive imaging. Heart 94:943–948. https://doi.org/10.1136/hrt.2007.125039
Lempert T (2013) Vestibular migraine. Semin Neurol 33:212–218. https://doi.org/10.1055/s-0033-1354596
Lopez-Escamez JA, Carey J, Chung W-H et al (2015) Diagnostic criteria for Menière’s disease. J Vestib Res 25:1–7. https://doi.org/10.3233/VES-150549
Dieterich M, Brandt T (1993) Ocular torsion and tilt of subjective visual vertical are sensitive brainstem signs. Ann Neurol 33:292–299. https://doi.org/10.1002/ana.410330311
Schneider E, Villgrattner T, Vockeroth J et al (2009) EyeSeeCam: an eye movement-driven head camera for the examination of natural visual exploration. Ann N Y Acad Sci 1164:461–467. https://doi.org/10.1111/j.1749-6632.2009.03858.x
Jongkees LB, Maas JP, Philipszoon AJ (1962) Clinical nystagmography. A detailed study of electro-nystagmography in 341 patients with vertigo. Pract Otorhinolaryngol (Basel) 24:65–93
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Salmaso D, Longoni AM (1985) Problems in the assessment of hand preference. Cortex 21:533–549. https://doi.org/10.1016/S0010-9452(58)80003-9
Kirsch V, Ertl-Wagner B, Berman A et al (2018) High-resolution MRI of the inner ear enables syndrome differentiation and specific treatment of cerebellar downbeat nystagmus and secondary endolymphatic hydrops in a postoperative ELST patient. J Neurol 265:48–50. https://doi.org/10.1007/s00415-018-8858-z
Fedorov A, Beichel R, Kalpathy-Cramer J et al (2012) Asymptotic stability of switching systems. Magn Reson Imaging 30:1323–1341. https://doi.org/10.1016/j.mri.2012.05.001
Schindelin J, Arganda-Carreras I, Frise E et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
Christensen GE, He J, Dill JA et al (2003) Automatic measurement of the labyrinth using image registration and a deformable inner ear atlas. Acad Radiol 10:988–999
Sharp GC, Li R, Wolfgang J, et al (2009) Plastimatch—an open source software suite for radiotherapy image processing. In: Proceedings of the XVIth international conference on the use of computers in radiotherapy (ICCR). Amsterdam, Netherlands
Sharp GC (2011) Deformable image registration using B-splines. Radiation Oncology-Massachusetts General Hospital, Boston, USA
Verma A, Mishra A (2015) Image compression using gaussian smoothing filter and median filter. Int J Recent Innov Trends Comput Commun 3:6344–6347
Cabezas M, Oliver A, Lladó X et al (2011) A review of atlas-based segmentation for magnetic resonance brain images. Comput Methods Progr Biomed 104:e158–e177. https://doi.org/10.1016/j.cmpb.2011.07.015
Zou KH, Warfield SK, Bharatha A et al (2004) Statistical validation of image segmentation quality based on a spatial overlap index. Acad Radiol 11:178–189. https://doi.org/10.1016/S1076-6332(03)00671-8
Liu F, Huang W, Meng X et al (2012) Comparison of noninvasive evaluation of endolymphatic hydrops in Meniere’s disease and endolymphatic space in healthy volunteers using magnetic resonance imaging. Acta Otolaryngol 132:234–240. https://doi.org/10.3109/00016489.2011.637232
Kendi TK, Arikan OK, Koc C (2005) Volume of components of labyrinth: magnetic resonance imaging study. Otol Neurotol 26:778–781
Morita N, Kariya S, Deroee AF et al (2009) Membranous labyrinth volumes in normal ears and Ménière disease: a three-dimensional reconstruction study NIH public access. Laryngoscope 119:2216–2220. https://doi.org/10.1002/lary.20723
Levy RB, Marquarding T, Reid AP et al (2019) Circuit asymmetries underlie functional lateralization in the mouse auditory cortex. Nat Commun 10:2783. https://doi.org/10.1038/s41467-019-10690-3
Tervaniemi M, Hugdahl K (2003) Lateralization of auditory-cortex functions. Brain Res Rev 43:231–246. https://doi.org/10.1016/j.brainresrev.2003.08.004
Sininger YS, Bhatara A (2012) Laterality of basic auditory perception. Laterality 17:129. https://doi.org/10.1080/1357650X.2010.541464
Janzen J, Schlindwein P, Bense S et al (2008) Neural correlates of hemispheric dominance and ipsilaterality within the vestibular system. Neuroimage 42:1508–1518. https://doi.org/10.1016/j.neuroimage.2008.06.026
Lopez C, Blanke O, Mast FW (2012) The human vestibular cortex revealed by coordinate-based activation likelihood estimation meta-analysis. Neuroscience 212:159–179. https://doi.org/10.1016/j.neuroscience.2012.03.028
Kirsch V, Boegle R, Keeser D et al (2018) Handedness-dependent functional organizational patterns within the bilateral vestibular cortical network revealed by fMRI connectivity based parcellation. Neuroimage 178:224–237. https://doi.org/10.1016/j.neuroimage.2018.05.018 (Epub 2018 May 19)
Bense S, Bartenstein P, Lutz S et al (2003) Three determinants of vestibular hemispheric dominance during caloric stimulation: a positron emission tomography study. Ann N Y Acad Sci 1004:440–445. https://doi.org/10.1111/j.1749-6632.2003.tb00256.x
Dieterich M, Kirsch V, Brandt T (2017) Right-sided dominance of the bilateral vestibular system in the upper brainstem and thalamus. J Neurol. https://doi.org/10.1007/s00415-017-8453-8
Mišić B, Betzel RF, Griffa A et al (2018) Network-based asymmetry of the human auditory system. Cereb Cortex 28:2655–2664. https://doi.org/10.1093/cercor/bhy101
Goni J, van den Heuvel MP, Avena-Koenigsberger A et al (2014) Resting-brain functional connectivity predicted by analytic measures of network communication. Proc Natl Acad Sci 111:833–838. https://doi.org/10.1073/pnas.1315529111
Andoh J, Matsushita R, Zatorre RJ (2015) Asymmetric interhemispheric transfer in the auditory network: evidence from TMS, resting-state fMRI, and diffusion imaging. J Neurosci 35:14602–14611. https://doi.org/10.1523/JNEUROSCI.2333-15.2015
Cammoun L, Thiran JP, Griffa A et al (2015) Intrahemispheric cortico-cortical connections of the human auditory cortex. Brain Struct Funct 220:3537–3553. https://doi.org/10.1007/s00429-014-0872-z
Boemio A, Fromm S, Braun A, Poeppel D (2005) Hierarchical and asymmetric temporal sensitivity in human auditory cortices. Nat Neurosci 8:389–395. https://doi.org/10.1038/nn1409
Dalca AV, Balakrishnan G, Guttag J, Sabuncu MR (2018) Unsupervised learning for fast probabilistic diffeomorphic registration. MICCAI: Medical image computing and computer assisted intervention. https://doi.org/10.1007/978-3-030-00928-1_82
Dalca AV, Yu E, Golland P et al (2019) Unsupervised deep learning for Bayesian brain MRI segmentation. MICCAI: Medical Image Computing and Computer Assisted Intervention. arXiv:1904.11319
Stojanov D, Aracki-Trenkic A, Benedeto-Stojanov D (2016) Gadolinium deposition within the dentate nucleus and globus pallidus after repeated administrations of gadolinium-based contrast agents—current status. Neuroradiology 58:433–441. https://doi.org/10.1007/s00234-016-1658-1
Moser FG, Watterson CT, Weiss S et al (2018) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: comparison between gadobutrol and linear gadolinium-based contrast agents. Am J Neuroradiol 39:421–426. https://doi.org/10.3174/ajnr.A5538
Boyken J, Frenzel T, Lohrke J et al (2018) Gadolinium accumulation in the deep cerebellar nuclei and globus pallidus after exposure to linear but not macrocyclic gadolinium-based contrast agents in a retrospective pig study with high similarity to clinical conditions. Investig Radiol 53:278–285. https://doi.org/10.1097/RLI.0000000000000440
Radtke A, von Brevern M, Neuhauser H et al (2012) Vestibular migraine: long-term follow-up of clinical symptoms and vestibulo-cochlear findings. Neurology 79:1607–1614. https://doi.org/10.1212/WNL.0b013e31826e264f
Ito T, Kitahara T, Inui H et al (2016) Endolymphatic space size in patients with Meniere’s disease and healthy controls. Acta Otolaryngol 6489:1–4. https://doi.org/10.3109/00016489.2016.1169556
Liu F, Huang W, Wang Z et al (2011) Noninvasive evaluation of endolymphatic space in healthy volunteers using magnetic resonance imaging. Acta Otolaryngol 131:247–257. https://doi.org/10.3109/00016489.2010.524938
Acknowledgements
Partially funded by the Society for the Advancement of Science and Research at the Medical Faculty of the Ludwig Maximilians University Munich (Verein zur Förderung von Wissenschaft und Forschung an der Medizinischen Fakultät der Ludwig-Maximilians-Universität München), the Friedrich-Baur-Stiftung (FBS), the Graduate School of Systemic Neurosciences (GSN), and the German Federal Ministry of Education and Research (German Center for Vertigo and Balance Disorders-IFBLMU, Grant code 01EO140). This is part of the dissertation of F. Nejatbakhshesfahani. We thank Gregory C. Sharp for his help in choosing the right applications in 3D Slicer, Gary E Christensen and his group for sending us a CT-template of the inner ear, and Albert Berman for introducing this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare they have no competing financial interests.
Ethical standard
All the procedures conducted with the participants of this study were carried out according to the Declaration of Helsinki. The protocol of the study was approved by the Institutional Review Board approval was obtained prior to the initiation of the study (No 641-15).
Informed consent
Each patient provided informed consent.
Additional information
This manuscript is part of a supplement sponsored by the German Federal Ministry of Education and Research within the funding initiative for integrated research and treatment centers.
V. Kirsch and F. Nejatbakhshesfahani share first authorship.
Rights and permissions
About this article
Cite this article
Kirsch, V., Nejatbakhshesfahani, F., Ahmadi, SA. et al. A probabilistic atlas of the human inner ear’s bony labyrinth enables reliable atlas-based segmentation of the total fluid space. J Neurol 266 (Suppl 1), 52–61 (2019). https://doi.org/10.1007/s00415-019-09488-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09488-6