Abstract
Objectives
We aimed to volumetrically quantify endolymph and perilymph spaces of the inner ear in order to establish a methodological basis for further investigations into the pathophysiology and therapeutic monitoring of Menière’s disease.
Methods
Sixteen patients (eight females, aged 38–71 years) with definite unilateral Menière’s disease were included in this study. Magnetic resonance (MR) cisternography with a T2-SPACE sequence was combined with a Real reconstruction inversion recovery (Real-IR) sequence for delineation of inner ear fluid spaces. Machine learning and automated local thresholding segmentation algorithms were applied for three-dimensional (3D) reconstruction and volumetric quantification of endolymphatic hydrops. Test–retest reliability was assessed by the intra-class coefficient; correlation of cochlear endolymph volume ratio with hearing function was assessed by the Pearson correlation coefficient.
Results
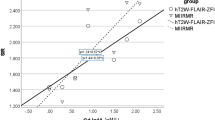
Endolymph volume ratios could be reliably measured in all patients, with a mean (range) value of 15 % (2–25) for the cochlea and 28 % (12–40) for the vestibulum. Test-retest reliability was excellent, with an intra-class coefficient of 0.99. Cochlear endolymphatic hydrops was significantly correlated with hearing loss (r = 0.747, p = 0.001).
Conclusions
MR imaging after local contrast application and image processing, including machine learning and automated local thresholding, enable the volumetric quantification of endolymphatic hydrops. This allows for a quantitative assessment of the effect of therapeutic interventions on endolymphatic hydrops.
Key Points
• Endolymphatic hydrops is the pathological hallmark of Menière’s disease.
• Endolymphatic hydrops can be visualized by locally enhanced ultra-high-resolution MR imaging.
• Computer-aided image processing enables quantification of endolymphatic hydrops.
• Endolymphatic hydrops correlates with hearing loss in patients with Menière’s disease.
• Therapeutic trials in Menière’s disease can be monitored with this quantitative approach.




Similar content being viewed by others
References
Havia M, Kentala E, Pyykko I (2005) Prevalence of Meniere's disease in general population of Southern Finland. Otolaryngol Head Neck Surg 133(5):762–768
Alexander TH, Harris JP (2010) Current epidemiology of Meniere's syndrome. Otolaryngol Clin North Am 43(5):965–970
Hallpike CS, Cairns H (1938) Observations on the Pathology of Meniere's Syndrome: (Section of Otology). Proc Roy Soc Med 31(11):1317–1336
Yamakawa K (1938) Über die pathologische Veränderung bei einem Menière-Kranken. Proceedings of 42nd Annual Meeting Oto-Rhino-Laryngol Soc Japan. J Otolaryngol Soc Jpn 4:2310–2312
AAO-HNS (1995) Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere's disease. American Academy of Otolaryngology-Head and Neck Foundation, Inc. Otolaryngol Head Neck Surg 113(3):181–185
Zou J, Pyykko I, Bjelke B, Dastidar P, Toppila E (2005) Communication between the perilymphatic scalae and spiral ligament visualized by in vivo MRI. Audiol Neuro-Otol 10(3):145–152
Nakashima T, Naganawa S, Sugiura M et al (2007) Visualization of Endolymphatic Hydrops in Patients With Meniere's Disease. Laryngoscope 117(3):415–420
Naganawa S, Satake H, Kawamura M, Fukatsu H, Sone M, Nakashima T (2008) Separate visualization of endolymphatic space, perilymphatic space and bone by a single pulse sequence; 3D-inversion recovery imaging utilizing real reconstruction after intratympanic Gd-DTPA administration at 3 Tesla. Eur Radiol 18(5):920–924
Naganawa S, Yamazaki M, Kawai H, Bokura K, Sone M, Nakashima T (2010) Visualization of endolymphatic hydrops in Meniere's disease with single-dose intravenous gadolinium-based contrast media using heavily T(2)-weighted 3D-FLAIR. Magn Reson Med Sci 9(4):237–242
Nakashima T, Naganawa S, Pyykkö I et al (2009) Grading of endolymphatic hydrops using magnetic resonance imaging. Acta Otolaryngol 129(s560):5–8
Naganawa S, Ishihara S, Iwano S, Sone M, Nakashima T (2010) Three-Dimensional (3D) Visualization of Endolymphatic Hydrops after Intratympanic Injection of Gd-DTPA: Optimization of a 3D-Real Inversion-Recovery Turbo Spin-Echo (TSE) Sequence and Application of a 32-Channel Head Coil at 3 T. J Magn Reson Imaging 31(1):210–214
Naganawa S, Yamazaki M, Kawai H, Bokura K, Sone M, Nakashima T (2013) Three-dimensional Visualization of Endolymphatic Hydrops after Intravenous Administration of Single-dose Gadodiamide. Magn Reson Med Sci 12(2):147–151
Yamazaki M, Naganawa S, Kawai H, Sone M, Nakashima T (2012) Gadolinium distribution in cochlear perilymph: differences between intratympanic and intravenous gadolinium injection. Neuroradiology 54(10):1161–1169
Studholme C, Hawkes DJ, & Hill DL (198) A normalized entropy measure for multimodality image alignment. Proc SPIE Med Imaging (3338):132-143.
Iannuccelli E, Mompart F, Gellin J, Lahbib-Mansais Y, Yerle M, Boudier T (2010) NEMO: a tool for analyzing gene and chromosome territory distributions from 3D-FISH experiments. Bioinformatics 26(5):696–697
Gonzalez RC, Woods RE (2002) Digital Image Processing. Prentice Hall, Upper Saddle River
Zuiderveld K (1994) Contrast limited adaptive histogram equalization. Graphics gems IV, ed Paul SH (Academic Press Professional, Inc.), pp 474-485.
Pisano ED, Zong S, Hemminger BM et al (1998) Contrast limited adaptive histogram equalization image processing to improve the detection of simulated spiculations in dense mammograms. J Digit Imag 11(4):193–200
Breiman L (2001) Random forests. Mach Learn 45:5–32
Hamprecht CSaCSaUKaFA (2011) ilastik: Interactive Learning and Segmentation Toolkit. in 8th IEEE International Symposium on Biomedical Imaging (ISBI 2011).
Breiman L (1996) Bagging predictors. Mach Learn 24(2):123–140
Amit Y, Geman D (1997) Shape quantization and recognition with randomized trees. Neural Comput 9(7):1545–1588
Demsar J (2006) Statistical comparisons of classifiers over multiple data sets. J Mach Learn Res 7:1–30
Hand DJ, Till RJ (2001) A simple generalisation of the area under the ROC curve for multiple class classification problems. Mach Learn 45(2):171–186
Meyer D, Leisch F, Hornik K (2003) The support vector machine under test. Neurocomputing 55(1–2):169–186
Sezgin M, Sankur B (2004) Survey over image thresholding techniques and quantitative performance evaluation. J Electron Imaging 13(1):146–168
Sauvola J, Pietikainen M (2000) Adaptive document image binarization. Pattern Recogn 33(2):225–236
Trier OD, Jain AK (1995) Goal-Directed Evaluation of Binarization Methods. Ieee T Pattern Anal 17(12):1191–1201
Landini G. (Image J, Auto Local Threshold (U. S. National Institutes of Health, Bethesda, Maryland, USA).
Hallpike CS (1956) The caloric tests. J Laryngol Otol 70(1):15–28 (in eng)
Jongkees LB, Maas JP, Philipszoon AJ (1962) Clinical nystagmography. A detailed study of electro-nystagmography in 341 patients with vertigo. Pract Otorhinolaryngol (Basel) 24:65–93 (in eng)
Hopkins WG (2000) Measures of reliability in sports medicine and science. Sports Med 30(1):1–15 (in eng)
Bernsen J (1986) Dynamic thresholding of gray level images. Proc. Intl. Conf. on Pattern Recognition, pp 1251-1255
Davies E (1990) Machine Vision: Theory, Algorithms and Practicalities (Academic Press).
Gonzalez R, Woods R (1992) Digital Image Processing. Addison-Wesley Longman Publishing Co., Inc., Boston, MA, USA
Chow CK, Kaneko T (1972) Automatic Boundary Detection of Left Ventricle from Cineangiograms. Comput Biomed Res 5(4):388
Jain AK (1989) Fundamentals of Digital Image Processing (Prentice Hall)
Rasband WS (1997-2012) Image J (U. S. National Institutes of Health, Bethesda, Maryland, USA)
Niblack W (1986) An introduction to Digital Image Processing (Prentice-Hall)
Gürkov R, Flatz W, Ertl-Wagner B, Krause E (2013) Endolymphatic hydrops in the horizontal semicircular canal: A morphologic correlate for canal paresis in Meniere's disease. Laryngoscope 123:503–506
Gürkov R, Flatz W, Louza J, Strupp M, Ertl-Wagner B, Krause E (2012) In vivo visualized endolymphatic hydrops and inner ear functions in patients with electrocochleographically confirmed Meniere's disease. Otology & Neurotology 33(6):1040–1045
Gürkov R, Flatz W, Louza J, Strupp M, Ertl-Wagner B, Krause E (2012) Herniation of the membranous labyrinth into the horizontal semicircular canal is correlated with impaired caloric response in Meniere's disease. Otol Neurotol 33(8):1375–1379
Gürkov R, Flatz W, Louza JP, Strupp M, Krause E (2011) In-vivo visualization of endolyphatic hydrops in patients with Meniere's disease: correlation with audiovestibular function. Eur Arch Otorhinolaryngol 268:1743–1748
Katayama N, Yamamoto M, Teranishi M et al (2010) Relationship between endolymphatic hydrops and vestibular-evoked myogenic potential. Acta Otolaryngol 130(8):917–923
Hornibrook J, Coates M, Goh A, Gourley J, Bird P (2012) Magnetic resonance imaging for Meniere's disease: correlation with tone burst electrocochleography. J Laryngol Otol 126(2):136–141
Jerin C, Berman A, Krause E, Ertl-Wagner B, Gürkov R (2014) Ocular vestibular evoked myogenic potential frequency tuning in certain Meniere's disease. Hear Res 310:54–59
Pullens B, Giard JL, Verschuur HP, & van Benthem PP (2010) Surgery for Meniere's disease. Cochrane Database Syst Rev (1):CD005395
Thirlwall AS & Kundu S (2006) Diuretics for Meniere's disease or syndrome. Cochrane Database Syst Rev 3:CD003599
James AL, Burton MJ (2001) Betahistine for Meniere's disease or syndrome. Cochrane Database Syst Rev 1, CD001873
Phillips JS & Westerberg B (2011) Intratympanic steroids for Meniere's disease or syndrome. Cochrane Database Syst Rev (7):CD008514
Pullens B, van Benthem PP (2011) Intratympanic gentamicin for Meniere's disease or syndrome. Cochrane Database Syst Rev 3, CD008234
Postema RJ, Kingma CM, Wit HP, Albers FW, Van Der Laan BF (2008) Intratympanic gentamicin therapy for control of vertigo in unilateral Menire's disease: a prospective, double-blind, randomized, placebo-controlled trial. Acta Otolaryngol 128(8):876–880 (in eng)
Yoshioka M, Naganawa S, Sone M, Nakata S, Teranishi M, Nakashima T (2009) Individual differences in the permeability of the round window: evaluating the movement of intratympanic gadolinium into the inner ear. Otol Neurotol 30(5):645–648
Naganawa S, Koshikawa T, Fukatsu H, Ishigaki T, Fukuta T (2001) MR cisternography of the cerebellopontine angle: comparison of three-dimensional fast asymmetrical spin-echo and three-dimensional constructive interference in the steady-state sequences. AJNR Am J Neuroradiol 22(6):1179–1185
Kojima S, Suzuki K, Hirata M, Shinohara H, Ueno E (2013) Depicting the semicircular canals with inner-ear MRI: a comparison of the SPACE and TrueFISP sequences. J Magn Reson Imaging 37(3):652–659
Gray KR, Aljabar P, Heckemann RA, Hammers A, Rueckert D (2011) Random Forest-Based Manifold Learning for Classification of Imaging Data in Dementia. Lect Notes Comput Sc 7009:159–166
Lempitsky V, Verhoek M, Noble JA, Blake A (2009) Random Forest Classification for Automatic Delineation of Myocardium in Real-Time 3D Echocardiography. Funct Imaging Model Heart, Proc 5528:447–456
Lindner C, Thiagarajah S, Wilkinson JM, Wallis GA, Cootes TF (2013) Fully Automatic Segmentation of the Proximal Femur Using Random Forest Regression Voting. IEEE Trans Med Imaging 32(8):1462–1472
Shili H, Romdhane LB, & Ayeb B (2013) Reliable Probabilistic Classification of Mammographic Masses using Random Forests. in The 9th International Conference on Data Mining (DMIN’2013) (Las Vegas, Nevada, USA)
Buckingham RA, Valvassori GE (2001) Inner ear fluid volumes and the resolving power of magnetic resonance imaging: can it differentiate endolymphatic structures? Ann Otol Rhinol Laryngol 110(2):113–117
Kendi TK, Arikan OK, Koc C (2005) Volume of components of labyrinth: magnetic resonance imaging study. OtolNeurotol 26(4):778–781
Liu F, Huang W, Meng X, Wang Z, Liu X, Chen Q (2012) Comparison of noninvasive evaluation of endolymphatic hydrops in Meniere's disease and endolymphatic space in healthy volunteers using magnetic resonance imaging. Acta Otolaryngol 132(3):234–240
Naganawa S, Suzuki K, Nakamichi R et al (2013) Semi-quantification of Endolymphatic Size on MR Imaging after Intravenous Injection of Single-dose Gadodiamide: Comparison between Two Types of Processing Strategies. Magn Reson Med Sci 12(4):261–269
Kato M, Teranishi M, Katayama N, Sone M, Naganawa S, Nakashima T (2011) Association between endolymphatic hydrops as revealed by magnetic resonance imaging and caloric response. Otol Neurotol 32(9):1480–1485
Acknowledgments
The scientific guarantor of this publication is Robert Gürkov. The authors of this manuscript declare no relationship with any companies whose products or services may be related to the subject matter of the article. This study has received funding by the German Ministry of Research and Education (BMBF, grant No. 01EO0901). No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Methodology: prospective, observational / experimental, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Additional information
R. Gürkov and A. Berman equally contributed as First Authors
D. Keeser and B. Ertl-Wagner equally contributed as Last Authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Volume rendering of right inner ear with moderate endolymphatic hydrops. Perilymph space is colored cyan, endolymph space is colored red. Cochlear endolymphatic space is most dilated in the apical region, whereas in the basal turn and ductus reuniens it appears not dilated. Vestibular endolymphatic space is moderately dilated, and there is no herniation into the non-ampullated crura of the semicircular canals. Rotation around yaw axis (Video 1) and around pitch axis (Video 2). (AVI 4223 kb)
ESM 2
(AVI 3496 kb)
Rights and permissions
About this article
Cite this article
Gürkov, R., Berman, A., Dietrich, O. et al. MR volumetric assessment of endolymphatic hydrops. Eur Radiol 25, 585–595 (2015). https://doi.org/10.1007/s00330-014-3414-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3414-4