Abstract
Background
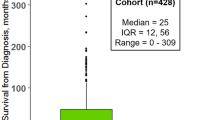
Primary lateral sclerosis is a progressive upper-motor-neuron disorder associated with markedly longer survival than ALS. In contrast to ALS, the genetic susceptibility, histopathological profile and imaging signature of PLS are poorly characterised. Suspected PLS patients often face considerable diagnostic delay and prognostic uncertainty.
Objective
To characterise the distinguishing clinical, genetic and imaging features of PLS in contrast to ALS and healthy controls.
Methods
A prospective population-based study was conducted with 49 PLS patients, 100 ALS patients and 100 healthy controls using genetic profiling, standardised clinical assessments and neuroimaging. Whole-brain and region-of-interest analyses were undertaken to evaluate patterns of grey and white matter degeneration.
Results
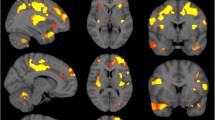
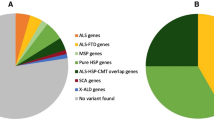
In PLS, disease burden in the motor cortex is more medial than in ALS consistent with its lower limb symptom-predominance. PLS is associated with considerable cerebellar white and grey matter degeneration and the extra-motor profile of PLS includes marked insular, inferior frontal and left pars opercularis pathology. Contrary to ALS, PLS spares the postcentral gyrus. The body and splenium of the corpus callosum are preferentially affected in PLS, in contrast to the genu involvement observed in ALS. Clinical measures show anatomically meaningful correlations with imaging metrics in a somatotopic distribution. PLS patients tested negative for C9orf72 repeat expansions, known ALS and HSP-associated genes.
Conclusions
Multiparametric imaging in PLS highlights disease-specific motor and extra-motor involvement distinct from ALS. In a condition where limited post-mortem data are available, imaging offers invaluable pathological insights. Anatomical correlations with clinical metrics confirm the biomarker potential of quantitative neuroimaging in PLS.





Similar content being viewed by others
Abbreviations
- AD:
-
Axial diffusivity
- ALS:
-
Amyotrophic lateral sclerosis
- C9orf72:
-
Chromosome 9 open reading frame 72
- CST:
-
Corticospinal tract
- DTI:
-
Diffusion tensor imaging
- EPI:
-
Echo-planar imaging
- FA:
-
Fractional anisotropy
- FDR:
-
False discovery rate
- FTD:
-
Frontotemporal dementia
- FOV:
-
Field-of-view
- FWE:
-
Familywise error
- GM:
-
Grey matter
- HARDI:
-
High angular resolution diffusion imaging
- HC:
-
Healthy control
- HSP:
-
Hereditary spastic paraplegia
- LMN:
-
Lower motor neuron
- MD:
-
Mean diffusivity
- MND:
-
Motor neuron disease
- MR:
-
Magnetic resonance
- PMC:
-
Primary motor cortex
- QBI:
-
q-Ball imaging
- RE:
-
Repeat expansion
- RD:
-
Radial diffusivity
- SC:
-
Spinal cord
- TBSS:
-
Tract-based spatial statistics
- TE:
-
Echo time
- TFCE:
-
Threshold-free cluster enhancement
- TR:
-
Repetition time
- UMN:
-
Upper motor neuron
- VBM:
-
Voxel-based morphometry
- WM:
-
White matter
References
Le Forestier N, Maisonobe T, Piquard A, Rivaud S, Crevier-Buchman L, Salachas F, Pradat PF, Lacomblez L, Meininger V (2001) Does primary lateral sclerosis exist? A study of 20 patients and a review of the literature. Brain J Neurol 124(Pt 10):1989–1999
Singer MA, Kojan S, Barohn RJ, Herbelin L, Nations SP, Trivedi JR, Jackson CE, Burns DK, Boyer PJ, Wolfe GI (2005) Primary lateral sclerosis: clinical and laboratory features in 25 patients. J Clin Neuromusc Dis 7(1):1–9. https://doi.org/10.1097/01.cnd.0000176974.61136.45
Almeida V, de Carvalho M, Scotto M, Pinto S, Pinto A, Ohana B, Swash M (2013) Primary lateral sclerosis: predicting functional outcome. Amyotroph Lateral Scler Frontotemporal Degener 14(2):141–145
Gordon PH, Cheng B, Katz IB, Pinto M, Hays AP, Mitsumoto H, Rowland LP (2006) The natural history of primary lateral sclerosis. Neurology 66(5):647–653
Floeter MK, Mills R (2009) Progression in primary lateral sclerosis: a prospective analysis. Amyotroph Lateral Sclerosis 10(5–6):339–346
Finegan E, Chipika RH, Shing SLH, Hardiman O, Bede P (2019) Primary lateral sclerosis: a distinct entity or part of the ALS spectrum? Amyotroph Lateral Sclerosis Frontotemporal Degener. https://doi.org/10.1080/21678421.2018.1550518
Iwata NK, Kwan JY, Danielian LE, Butman JA, Tovar-Moll F, Bayat E, Floeter MK (2011) White matter alterations differ in primary lateral sclerosis and amyotrophic lateral sclerosis. Brain J Neurol 134(9):2642–2655. https://doi.org/10.1093/brain/awr178
Müller HP, Gorges M, Kassubek R, Dorst J, Ludolph AC, Kassubek J (2018) Identical patterns of cortico-efferent tract involvement in primary lateral sclerosis and amyotrophic lateral sclerosis: a tract of interest-based MRI study. NeuroImage Clin 18:762–769. https://doi.org/10.1016/j.nicl.2018.03.018
Unrath A, Muller H-P, Riecker A, Ludolph AC, Sperfeld A-D, Kassubek J (2010) Whole brain-based analysis of regional white matter tract alterations in rare motor neuron diseases by diffusion tensor imaging. Hum Brain Mapp 31(11):1727–1740
Agosta F, Galantucci S, Riva N, Chio A, Messina S, Iannaccone S, Calvo A, Silani V, Copetti M, Falini A, Comi G, Filippi M (2014) Intrahemispheric and interhemispheric structural network abnormalities in PLS and ALS. Hum Brain Mapp 35(4):1710–1722. https://doi.org/10.1002/hbm.22286
Butman JA, Floeter MK (2007) Decreased thickness of primary motor cortex in primary lateral sclerosis. Am J Neuroradiol 28(1):87–91
Schuster C, Kasper E, Machts J, Bittner D, Kaufmann J, Benecke R, Teipel S, Vielhaber S, Prudlo J (2013) Focal thinning of the motor cortex mirrors clinical features of amyotrophic lateral sclerosis and their phenotypes: a neuroimaging study. J Neurol 260(11):2856–2864
Paganoni S, Alshikho MJ, Zürcher NR, Cernasov P, Babu S, Loggia ML, Chan J, Chonde DB, Garcia DI, Catana C, Mainero C, Rosen BR, Cudkowicz ME, Hooker JM, Atassi N (2018) Imaging of glia activation in people with primary lateral sclerosis. NeuroImage Clin 17:347–353. https://doi.org/10.1016/j.nicl.2017.10.024
Bede P, Hardiman O (2014) Lessons of ALS imaging: pitfalls and future directions—a critical review. NeuroImage Clin 4:436–443. https://doi.org/10.1016/j.nicl.2014.02.011
Clark MG, Smallwood Shoukry R, Huang CJ, Danielian LE, Bageac D, Floeter MK (2018) Loss of functional connectivity is an early imaging marker in primary lateral sclerosis. Amyotroph Lateral Sclerosis Frontotemporal Degener 19(7–8):562–569. https://doi.org/10.1080/21678421.2018.1517180
Van Weehaeghe D, Ceccarini J, Delva A, Robberecht W, Van Damme P, Van Laere K (2016) Prospective validation of 18F-FDG brain PET discriminant analysis methods in the diagnosis of amyotrophic lateral sclerosis. J Nucl Med 57(8):1238–1243. https://doi.org/10.2967/jnumed.115.166272
Bede P, Bokde A, Elamin M, Byrne S, McLaughlin RL, Jordan N, Hampel H, Gallagher L, Lynch C, Fagan AJ, Pender N, Hardiman O (2013) Grey matter correlates of clinical variables in amyotrophic lateral sclerosis (ALS): a neuroimaging study of ALS motor phenotype heterogeneity and cortical focality. J Neurol Neurosurg Psychiatry 84(7):766–773. https://doi.org/10.1136/jnnp-2012-302674
Bede P, Elamin M, Byrne S, Hardiman O (2013) Sexual dimorphism in ALS: exploring gender-specific neuroimaging signatures. Amyotroph Lateral Sclerosis Frontotemporal Degener. https://doi.org/10.3109/21678421.2013.865749
Floeter MK, Katipally R, Kim MP, Schanz O, Stephen M, Danielian L, Wu T, Huey ED, Meoded A (2014) Impaired corticopontocerebellar tracts underlie pseudobulbar affect in motor neuron disorders. Neurology 83(7):620–627. https://doi.org/10.1212/wnl.0000000000000693
Meoded A, Morrissette AE, Katipally R, Schanz O, Gotts SJ, Floeter MK (2015) Cerebro-cerebellar connectivity is increased in primary lateral sclerosis. NeuroImage Clin 7:288–296
Meoded A, Kwan JY, Peters TL, Huey ED, Danielian LE, Wiggs E, Morrissette A, Wu T, Russell JW, Bayat E, Grafman J, Floeter MK (2013) Imaging findings associated with cognitive performance in primary lateral sclerosis and amyotrophic lateral sclerosis. Dementia Geriatr Cognit Disord Extra 3(1):233–250
Canu E, Agosta F, Galantucci S, Chio A, Riva N, Silani V, Falini A, Comi G, Filippi M (2013) Extramotor damage is associated with cognition in primary lateral sclerosis patients. PLoS One [Electronic Resource] 8(12):e82017
Tu S, Menke RAL, Talbot K, Kiernan MC, Turner MR (2019) Cerebellar tract alterations in PLS and ALS. Amyotroph Lateral Sclerosis Frontotemporal Degener 20(3–4):281–284. https://doi.org/10.1080/21678421.2018.1562554
Bede P, Querin G, Pradat PF (2018) The changing landscape of motor neuron disease imaging: the transition from descriptive studies to precision clinical tools. Curr Opin Neurol 31(4):431–438. https://doi.org/10.1097/wco.0000000000000569
Dickson DW, Josephs KA, Amador-Ortiz C (2007) TDP-43 in differential diagnosis of motor neuron disorders. Acta Neuropathol 114(1):71–79
Kosaka T, Fu YJ, Shiga A, Ishidaira H, Tan CF, Tani T, Koike R, Onodera O, Nishizawa M, Kakita A, Takahashi H (2012) Primary lateral sclerosis: upper-motor-predominant amyotrophic lateral sclerosis with frontotemporal lobar degeneration–immunohistochemical and biochemical analyses of TDP-43. Neuropathol Off J Japan Society Neuropathol 32(4):373–384
Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron D (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Sclerosis Other Motor Neuron Disord Off Publ World Feder Neurol Res Group Motor Neuron Dis 1(5):293–299
Woo JH, Wang S, Melhem ER, Gee JC, Cucchiara A, McCluskey L, Elman L (2014) Linear associations between clinically assessed upper motor neuron disease and diffusion tensor imaging metrics in amyotrophic lateral sclerosis. PLoS One [Electronic Resource] 9(8):e105753
Bohannon RW, Smith MB (1987) Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 67(2):206–207
Pinto-Grau M, Burke T, Lonergan K, McHugh C, Mays I, Madden C, Vajda A, Heverin M, Elamin M, Hardiman O, Pender N (2017) Screening for cognitive dysfunction in ALS: validation of the Edinburgh Cognitive and Behavioural ALS Screen (ECAS) using age and education adjusted normative data. Amyotroph Lateral Sclerosis Frontotemporal Degener 18(1–2):99–106. https://doi.org/10.1080/21678421.2016.1249887
Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 169(1–2):13–21
Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnetjournal 17:10–12
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, Genome Project Data Processing S (2009) The sequence alignment/map format and SAMtools. Bioinformatics (Oxford, England) 25(16):2078–2079. https://doi.org/10.1093/bioinformatics/btp352
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PIW, Daly MJ, Sham PC (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81(3):559–575. https://doi.org/10.1086/519795
Cingolani P, Platts A, Wang LL, Coon M, Nguyen T, Wang L, Land SJ, Lu X, Ruden DM (2012) A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 6(2):80–92. https://doi.org/10.4161/fly.19695
Paila U, Chapman BA, Kirchner R, Quinlan AR (2013) GEMINI: integrative exploration of genetic variation and genome annotations. PLoS Comput Biol 9(7):e1003153. https://doi.org/10.1371/journal.pcbi.1003153
Project Min EALSSC (2018) Project MinE: study design and pilot analyses of a large-scale whole-genome sequencing study in amyotrophic lateral sclerosis. Eur J Hum Genet EJHG 26(10):1537–1546. https://doi.org/10.1038/s41431-018-0177-4
Abel O, Shatunov A, Jones AR, Andersen PM, Powell JF, Al-Chalabi A (2013) Development of a smartphone app for a genetics website: the amyotrophic lateral sclerosis online genetics database (ALSoD). JMIR mHealth uHealth 1(2):e18–e18. https://doi.org/10.2196/mhealth.2706
Klebe S, Stevanin G, Depienne C (2015) Clinical and genetic heterogeneity in hereditary spastic paraplegias: from SPG1 to SPG72 and still counting. Rev Neurol (Paris) 171(6–7):505–530. https://doi.org/10.1016/j.neurol.2015.02.017
Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O'Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, Tukiainen T, Birnbaum DP, Kosmicki JA, Duncan LE, Estrada K, Zhao F, Zou J, Pierce-Hoffman E, Berghout J, Cooper DN, Deflaux N, DePristo M, Do R, Flannick J, Fromer M, Gauthier L, Goldstein J, Gupta N, Howrigan D, Kiezun A, Kurki MI, Moonshine AL, Natarajan P, Orozco L, Peloso GM, Poplin R, Rivas MA, Ruano-Rubio V, Rose SA, Ruderfer DM, Shakir K, Stenson PD, Stevens C, Thomas BP, Tiao G, Tusie-Luna MT, Weisburd B, Won HH, Yu D, Altshuler DM, Ardissino D, Boehnke M, Danesh J, Donnelly S, Elosua R, Florez JC, Gabriel SB, Getz G, Glatt SJ, Hultman CM, Kathiresan S, Laakso M, McCarroll S, McCarthy MI, McGovern D, McPherson R, Neale BM, Palotie A, Purcell SM, Saleheen D, Scharf JM, Sklar P, Sullivan PF, Tuomilehto J, Tsuang MT, Watkins HC, Wilson JG, Daly MJ, MacArthur DG (2016) Analysis of protein-coding genetic variation in 60,706 humans. Nature 536(7616):285–291. https://doi.org/10.1038/nature19057
Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR (2015) A global reference for human genetic variation. Nature 526(7571):68–74. https://doi.org/10.1038/nature15393
Stenson PD, Mort M, Ball EV, Evans K, Hayden M, Heywood S, Hussain M, Phillips AD, Cooper DN (2017) The Human Gene Mutation Database: towards a comprehensive repository of inherited mutation data for medical research, genetic diagnosis and next-generation sequencing studies. Hum Genet 136(6):665–677. https://doi.org/10.1007/s00439-017-1779-6
Byrne S, Elamin M, Bede P, Shatunov A, Walsh C, Corr B, Heverin M, Jordan N, Kenna K, Lynch C, McLaughlin RL, Iyer PM, O'Brien C, Phukan J, Wynne B, Bokde AL, Bradley DG, Pender N, Al-Chalabi A, Hardiman O (2012) Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9orf72 repeat expansion: a population-based cohort study. Lancet Neurol 11(3):232–240. https://doi.org/10.1016/S1474-4422(12)70014-5
Kenna KP, McLaughlin RL, Byrne S, Elamin M, Heverin M, Kenny EM, Cormican P, Morris DW, Donaghy CG, Bradley DG, Hardiman O (2013) Delineating the genetic heterogeneity of ALS using targeted high-throughput sequencing. J Med Genet 50(11):776–783. https://doi.org/10.1136/jmedgenet-2013-101795
Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, James S, Voets N, Watkins K, Matthews PM, James A (2007) Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain J Neurol 130(Pt 9):2375–2386
Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS (2001) A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage 14(1 Pt 1):21–36
Fischl B (2012) FreeSurfer. NeuroImage 62(2):774–781. https://doi.org/10.1016/j.neuroimage.2012.01.021
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci 97(20):11050–11055. https://doi.org/10.1073/pnas.200033797
Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31(3):968–980. https://doi.org/10.1016/j.neuroimage.2006.01.021
Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van Zijl P, Mori S (2007) Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage 36(3):630–644. https://doi.org/10.1016/j.neuroimage.2007.02.049
Bede P, Elamin M, Byrne S, McLaughlin RL, Kenna K, Vajda A, Fagan A, Bradley DG, Hardiman O (2015) Patterns of cerebral and cerebellar white matter degeneration in ALS. J Neurol Neurosurg Psychiatry 86(4):468–470. https://doi.org/10.1136/jnnp-2014-308172
Schuster C, Elamin M, Hardiman O, Bede P (2016) The segmental diffusivity profile of amyotrophic lateral sclerosis associated white matter degeneration. Eur J Neurol 23(8):1361–1371. https://doi.org/10.1111/ene.13038
Bersano E, Sarnelli MF, Solara V, De Marchi F, Sacchetti GM, Stecco A, Corrado L, D'Alfonso S, Cantello R, Mazzini L (2018) A case of late-onset OCD developing PLS and FTD. Amyotroph Lateral Sclerosis Frontotemporal Degener 19(5–6):463–465. https://doi.org/10.1080/21678421.2018.1440405
de Vries BS, Rustemeijer LMM, van der Kooi AJ, Raaphorst J, Schröder CD, Nijboer TCW, Hendrikse J, Veldink JH, van den Berg LH, van Es MA (2017) A case series of PLS patients with frontotemporal dementia and overview of the literature. Amyotroph Lateral Sclerosis Frontotemporal Degener 18(7–8):534–548. https://doi.org/10.1080/21678421.2017.1354996
Budde MD, Xie M, Cross AH, Song SK (2009) Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. J Neurosci Off J Soc Neurosci 29(9):2805–2813. https://doi.org/10.1523/JNEUROSCI.4605-08.2009
Sun SW, Liang HF, Trinkaus K, Cross AH, Armstrong RC, Song SK (2006) Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med 55(2):302–308. https://doi.org/10.1002/mrm.20774
Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH (2002) Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage 17(3):1429–1436
Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC (2005) Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage 26(1):132–140. https://doi.org/10.1016/j.neuroimage.2005.01.028
Ulug AM, Grunewald T, Lin MT, Kamal AK, Filippi CG, Zimmerman RD, Beal MF (2004) Diffusion tensor imaging in the diagnosis of primary lateral sclerosis. J Magn Reson Imaging 19(1):34–39
Muller HP, Agosta F, Riva N, Spinelli EG, Comi G, Ludolph AC, Filippi M, Kassubek J (2018) Fast progressive lower motor neuron disease is an ALS variant: a two-centre tract of interest-based MRI data analysis. NeuroImage Clin 17:145–152. https://doi.org/10.1016/j.nicl.2017.10.008
Schuster C, Hardiman O, Bede P (2017) Survival prediction in amyotrophic lateral sclerosis based on MRI measures and clinical characteristics. BMC Neurol 17(1):73. https://doi.org/10.1186/s12883-017-0854-x
Schuster C, Hardiman O, Bede P (2016) Development of an automated MRI-based diagnostic protocol for amyotrophic lateral sclerosis using disease-specific pathognomonic features: a quantitative disease-state classification study. PLoS ONE 11(12):e0167331. https://doi.org/10.1371/journal.pone.0167331
Grollemund V, Pradat PF, Querin G, Delbot F, Le Chat G, Pradat-Peyre JF, Bede P (2019) Machine learning in amyotrophic lateral sclerosis: achievements, pitfalls, and future directions. Front Neurosci 13:135. https://doi.org/10.3389/fnins.2019.00135
Dickson DW (2008) TDP-43 immunoreactivity in neurodegenerative disorders: disease versus mechanism specificity. Acta Neuropathol 115(1):147–149. https://doi.org/10.1007/s00401-007-0323-5
Brettschneider J, Del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M, Suh E, Van Deerlin VM, Wood EM, Baek Y, Kwong L, Lee EB, Elman L, McCluskey L, Fang L, Feldengut S, Ludolph AC, Lee VM, Braak H, Trojanowski JQ (2013) Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol 74(1):20–38. https://doi.org/10.1002/ana.23937
Bede P, Hardiman O (2014) Lessons of ALS imaging: pitfalls and future directions—a critical review. NeuroImage Clin 4:436–443. https://doi.org/10.1016/j.nicl.2014.02.011
Turner MR, Agosta F, Bede P, Govind V, Lule D, Verstraete E (2012) Neuroimaging in amyotrophic lateral sclerosis. Biomark Med 6(3):319–337. https://doi.org/10.2217/bmm.12.26
Christidi F, Karavasilis E, Ferentinos P, Xirou S, Velonakis G, Rentzos M, Zouvelou V, Zalonis I, Efstathopoulos E, Kelekis N, Evdokimidis I (2018) Investigating the neuroanatomical substrate of pathological laughing and crying in amyotrophic lateral sclerosis with multimodal neuroimaging techniques. Amyotroph Lateral Sclerosis Frontotemporal Degener 19(1–2):12–20. https://doi.org/10.1080/21678421.2017.1386689
Stoodley CJ, Schmahmann JD (2010) Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 46(7):831–844. https://doi.org/10.1016/j.cortex.2009.11.008
Bede P, Finegan E (2018) Revisiting the pathoanatomy of pseudobulbar affect: mechanisms beyond corticobulbar dysfunction. Amyotroph Lateral Sclerosis Frontotemporal Degener 19(1–2):4–6. https://doi.org/10.1080/21678421.2017.1392578
Bede P, Bokde ALW, Byrne S, Elamin M, McLaughlin RL, Kenna K, Fagan AJ, Pender N, Bradley DG, Hardiman O (2013) Multiparametric MRI study of ALS stratified for the C9orf72 genotype. Neurology 81(4):361–369. https://doi.org/10.1212/WNL.0b013e31829c5eee
Mitsumoto H, Nagy PL, Gennings C, Murphy J, Andrews H, Goetz R, Floeter MK, Hupf J, Singleton J, Barohn RJ, Nations S, Shoesmith C, Kasarskis E, Factor-Litvak P (2015) Phenotypic and molecular analyses of primary lateral sclerosis. Neurol Genet. https://doi.org/10.1212/01.NXG.0000464294.88607.dd
Chipika RH, Finegan E, Li Hi Shing S, Hardiman O, Bede P (2019) tracking a fast-moving disease: longitudinal markers, monitoring, and clinical trial endpoints in ALS. Front Neurol 10:229. https://doi.org/10.3389/fneur.2019.00229
Schuster C, Elamin M, Hardiman O, Bede P (2015) Presymptomatic and longitudinal neuroimaging in neurodegeneration–from snapshots to motion picture: a systematic review. J Neurol Neurosurg Psychiatry 86(10):1089–1096. https://doi.org/10.1136/jnnp-2014-309888
Steinacker P, Feneberg E, Weishaupt J, Brettschneider J, Tumani H, Andersen PM, von Arnim CA, Bohm S, Kassubek J, Kubisch C, Lule D, Muller HP, Muche R, Pinkhardt E, Oeckl P, Rosenbohm A, Anderl-Straub S, Volk AE, Weydt P, Ludolph AC, Otto M (2016) Neurofilaments in the diagnosis of motoneuron diseases: a prospective study on 455 patients. J Neurol Neurosurg Psychiatry 87(1):12–20. https://doi.org/10.1136/jnnp-2015-311387
Acknowledgements
The authors are thankful for the kindness and generosity of all patients, their families and the healthy controls for participating in this research project. Without their support, this project would not have been possible. Peter Bede is supported by the Health Research Board (HRB—Ireland; HRB EIA-2017-019), the Andrew Lydon scholarship, the Irish Institute of Clinical Neuroscience IICN—Novartis Ireland Research Grant, the Iris O'Brien Foundation, and the Research Motor Neuron (RMN-Ireland) Foundation. Russell L McLaughlin is supported by the Motor Neurone Disease Association (957-799) and Science Foundation Ireland (17/CDA/4737). Mark A Doherty is supported by Science Foundation Ireland (15/SPP/3244). The sponsors of this study had no role in the design, analyses, presentation of this work or the decision to submit these findings for publication.
Author information
Authors and Affiliations
Contributions
Drafting the manuscript: EF, PB. Clinical assessments: EF, OH, RHC, CD, NP, PB. Neuroimaging analyses: EF, PB. Genetic analyses: MAD, JCH, AV, RLM. Conceptualisation of the study: EF, OH, PB. Revision of the manuscript for intellectual content: EF, RHC, SLHS, MAD, JCH, AV, CD, RLM, NP, OH, and PB.
Corresponding author
Ethics declarations
Conflicts of interest
The authors of this manuscript have no conflicts of interest to disclose.
Ethical Standards
This study was approved by the Institutional Ethics (Medical Research) Committee, and all participants provided informed consent prior to inclusion.
Rights and permissions
About this article
Cite this article
Finegan, E., Chipika, R.H., Li Hi Shing, S. et al. The clinical and radiological profile of primary lateral sclerosis: a population-based study. J Neurol 266, 2718–2733 (2019). https://doi.org/10.1007/s00415-019-09473-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09473-z