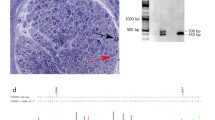
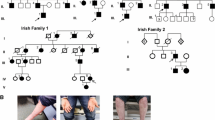
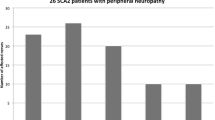
Abstract
Dominant intermediate Charcot-Marie-Tooth neuropathy subtype C (DI-CMTC) was associated with mutations in the YARS gene, encoding tyrosyl-tRNA synthetase, in two large unrelated Bulgarian and US pedigrees and one sporadic case. Here for the first time we describe the clinical, neurophysiological and histopathological features, and phenotypic differences between these two DI-CMTC families. Twenty-one affected individuals from the US family and 27 from the Bulgarian family were evaluated. The mean age of onset in US subjects was 10.7 years in men and 7.3 years in women, while in the Bulgarian participants it was 18.2 years in men and 33.7 years in women. The course was slowly progressive. Extensor digitorum brevis atrophy was uniform. Atrophy and/or weakness of upper and lower limb muscles were found in over 50 % of the subjects. Nerve conduction studies (NCS) were abnormal in all US adults and five of six children and all Bulgarian patients except one asymptomatic 25-year-old man. Median motor NCS were in the range of 29.5–45.6 m/s in the US family and 24.7–57.8 m/s in the Bulgarian family. Sural sensory nerve action potentials were absent in 14/21 and 4/12 NCS from adult US and Bulgarian participants, respectively. Analysis of sural nerve biopsies from US patients revealed age-dependent morphological changes of axonal degeneration, absence of onion bulbs, and <10 % fibers with segmental remyelination. Our findings provide further insights into the diagnosis and pathology of intermediate CMT. They also extend the phenotypic spectrum of peripheral neuropathies associated with aminoacyl-tRNA synthetase mutations.


Similar content being viewed by others
References
Buchthal F, Behse F (1977) Peroneal muscular atrophy (PMA) and related disorders I. Clinical manifestations as related to biopsy findings, nerve conduction and electromyography. Brain 100(Pt 1):41–66
Dyck PJ, Lambert EH (1968) Lower motor and primary sensory neuron diseases with peroneal muscular atrophy I. Neurologic, genetic, and electrophysiologic findings in hereditary polyneuropathies. Arch Neurol 18(6):603–618
Thomas PK, Calne DB (1974) Motor nerve conduction velocity in peroneal muscular atrophy: evidence for genetic heterogeneity. J Neurol Neurosurg Psychiatry 37(1):68–75
Madrid R, Bradley WG, Davis CJ (1977) The peroneal muscular atrophy syndrome. Clinical, genetic, electrophysiological and nerve biopsy studies. Part 2. Observations on pathological changes in sural nerve biopsies. J Neurol Sci 32(1):91–122
Bradley WG, Madrid R, Davis CJ (1977) The peroneal muscular atrophy syndrome. Clinical genetic, electrophysiological and nerve biopsy studies. Part 3. Clinical, electrophysiological and pathological correlations. J Neurol Sci 32(1):123–136
Davis CJ, Bradley WG, Madrid R (1978) The peroneal muscular atrophy syndrome: clinical, genetic, electrophysiological and nerve biopsy studies I. Clinical, genetic and electrophysiological findings and classification. J Genet Hum 26(4):311–349
Rossi A, Paradiso C, Cioni R, Rizzuto N, Guazzi G (1985) Charcot-Marie-Tooth disease: study of a large kinship with an intermediate form. J Neurol 232(2):91–98
Villanova M, Timmerman V, De JP, Malandrini A, Rizzuto N, Van BC et al (1998) Charcot-Marie-Tooth disease: an intermediate form. Neuromuscul Disord 8(6):392–393
Kennerson ML, Zhu D, Gardner RJ, Storey E, Merory J, Robertson SP et al (2001) Dominant intermediate Charcot-Marie-Tooth neuropathy maps to chromosome 19p12–p13.2. Am J Hum Genet 69(4):883–888
Mastaglia FL, Nowak KJ, Stell R, Phillips BA, Edmondston JE, Dorosz SM et al (1999) Novel mutation in the myelin protein zero gene in a family with intermediate hereditary motor and sensory neuropathy. J Neurol Neurosurg Psychiatry 67(2):174–179
Nicholson G, Myers S (2006) Intermediate forms of Charcot-Marie-Tooth neuropathy: a review. Neuromol Med 8(1–2):123–130
Malandrini A, Ceuterick C, Villanov M, Gambelli S, Berti G, Rossi A et al (2001) Ultrastructural findings in the peripheral nerve in a family with the intermediate form of Charcot-Marie-Tooth disease. J Submicrosc Cytol Pathol 33(1–2):59–63
Zuchner S, Noureddine M, Kennerson M, Verhoeven K, Claeys K, De JP et al (2005) Mutations in the pleckstrin homology domain of dynamin 2 cause dominant intermediate Charcot-Marie-Tooth disease. Nat Genet 37(3):289–294
Claeys KG, Zuchner S, Kennerson M, Berciano J, Garcia A, Verhoeven K et al (2009) Phenotypic spectrum of dynamin 2 mutations in Charcot-Marie-Tooth neuropathy. Brain 132(Pt 7):1741–1752
Fabrizi GM, Ferrarini M, Cavallaro T, Cabrini I, Cerini R, Bertolasi L et al (2007) Two novel mutations in dynamin-2 cause axonal Charcot-Marie-Tooth disease. Neurology 69(3):291–295
Bitoun M, Stojkovic T, Prudhon B, Maurage CA, Latour P, Vermersch P et al (2008) A novel mutation in the dynamin 2 gene in a Charcot-Marie-Tooth type 2 patient: clinical and pathological findings. Neuromuscul Disord 18(4):334–338
Boyer O, Nevo F, Plaisier E, Funalot B, Gribouval O, Benoit G et al (2011) INF2 mutations in Charcot-Marie-Tooth disease with glomerulopathy. N Engl J Med 365(25):2377–2388
Soong BW, Huang YH, Tsai PC, Huang CC, Pan HC, Lu YC et al (2013) Exome sequencing identifies GNB4 mutations as a cause of dominant intermediate Charcot-Marie-Tooth disease. Am J Hum Genet 92(3):422–430
Jordanova A, Thomas FP, Guergueltcheva V, Tournev I, Gondim FA, Ishpekova B et al (2003) Dominant intermediate Charcot-Marie-Tooth type C maps to chromosome 1p34-p35. Am J Hum Genet 73(6):1423–1430
Jordanova A, Irobi J, Thomas FP, Van DP, Meerschaert K, Dewil M et al (2006) Disrupted function and axonal distribution of mutant tyrosyl-tRNA synthetase in dominant intermediate Charcot-Marie-Tooth neuropathy. Nat Genet 38(2):197–202
Merkies IS, Schmitz PI, van der Meche FG, van Doorn PA (2000) Psychometric evaluation of a new sensory scale in immune-mediated polyneuropathies. Inflammatory Neuropathy Cause and Treatment (INCAT) Group. Neurology 54(4):943–949
Parano E, Uncini A, De Vivo DC, Lovelace RE (1993) Electrophysiologic correlates of peripheral nervous system maturation in infancy and childhood. J Child Neurol 8(4):336–338
Miller RG, Kuntz NL (1986) Nerve conduction studies in infants and children. J Child Neurol 1(1):19–26
Martinez AC, Ferrer MT, Conde MC, Bernacer M (1978) Motor conduction velocity and H-reflex in infancy and childhood II. Intra and extrauterine maturation of the nerve fibres. Development of the peripheral nerve from 1 month to 11 years of age. Electromyogr Clin Neurophysiol 18(1):11–27
Thomas FP, Lebo RV, Rosoklija G, Ding XS, Lovelace RE, Latov N et al (1994) Tomaculous neuropathy in chromosome 1 Charcot-Marie-Tooth syndrome. Acta Neuropathol 87(1):91–97
Jacobs JM, Love S (1985) Qualitative and quantitative morphology of human sural nerve at different ages. Brain 108(Pt 4):897–924
Storkebaum E, Leitao-Goncalves R, Godenschwege T, Nangle L, Mejia M, Bosmans I et al (2009) Dominant mutations in the tyrosyl-tRNA synthetase gene recapitulate in Drosophila features of human Charcot-Marie-Tooth neuropathy. Proc Natl Acad Sci USA 106(28):11782–11787
Nicholson GA (1991) Penetrance of the hereditary motor and sensory neuropathy Ia mutation: assessment by nerve conduction studies. Neurology 41(4):547–552
Rossi A, Paradiso C, Dell’Anna P, Mondelli M (1985) Short latency somatosensory evoked potentials in Charcot-Marie-Tooth disease. A family with an intermediate form. Acta Neurol Scand 71(2):156–163
Hahn AF, Ainsworth PJ, Naus CC, Mao J, Bolton CF (2000) Clinical and pathological observations in men lacking the gap junction protein connexin 32. Muscle Nerve Suppl 9:S39–S48
Antonellis A, Ellsworth RE, Sambuughin N, Puls I, Abel A, Lee-Lin SQ et al (2003) Glycyl tRNA synthetase mutations in Charcot-Marie-Tooth disease type 2D and distal spinal muscular atrophy type V. Am J Hum Genet 72(5):1293–1299
Latour P, Thauvin-Robinet C, Baudelet-Mery C, Soichot P, Cusin V, Faivre L et al (2010) A major determinant for binding and aminoacylation of tRNA(Ala) in cytoplasmic Alanyl-tRNA synthetase is mutated in dominant axonal Charcot-Marie-Tooth disease. Am J Hum Genet 86(1):77–82
Vester A, Velez-Ruiz G, McLaughlin HM, Lupski JR, Talbot K, Vance JM et al (2013) A loss-of-function variant in the human histidyl-tRNA synthetase (HARS) gene is neurotoxic in vivo. Hum Mutat 34(1):191–199
Gonzalez M, McLaughlin H, Houlden H, Guo M, Yo-Tsen L, Hadjivassilious M et al (2013) Exome sequencing identifies a significant variant in methionyl-tRNA synthetase (MARS) in a family with late-onset CMT2. J Neurol Neurosurg Psychiatry 84(11):1247–1249
McLaughlin HM, Sakaguchi R, Liu C, Igarashi T, Pehlivan D, Chu K et al (2010) Compound heterozygosity for loss-of-function lysyl-tRNA synthetase mutations in a patient with peripheral neuropathy. Am J Hum Genet 87(4):560–566
Acknowledgments
The American team received support for design and conduct of the study and collection of data from The Neuropathy Association and the St. Louis Chapter of The Neuropathy Association. The Bulgarian team received support for collection of data from the Research Fund of Sofia Medical University and National Science Fund of the Bulgarian Ministry of Education and Science. The Belgian team received support for design and conduct of the study and collection of data from the Fund for Scientific Research (FWO-Flanders), the Research Fund of the University of Antwerp (BOF-UA), the Medical Foundation Queen Elisabeth (GSKE), the Association Belge contre les Maladies Neuro-Musculaires (ABMM), and the US Muscular Dystrophy Association. The authors thank the study participants for their cooperation, Professors Mitko Abadjiev and Veneta Georgieva for pedigree data, and the Bulgarian Association for Neuromuscular Disorders and Ethnic Minorities Health Problems Foundation for assistance during patient visits.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical standards
All human studies were approved by the ethics committees of St. Louis University and Medical University-Sofia and were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Additional information
F. P. Thomas and V. Guergueltcheva have contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Thomas, F.P., Guergueltcheva, V., Gondim, F.A.A. et al. Clinical, neurophysiological and morphological study of dominant intermediate Charcot-Marie-Tooth type C neuropathy. J Neurol 263, 467–476 (2016). https://doi.org/10.1007/s00415-015-7989-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7989-8