Abstract
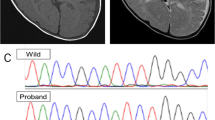
Allan-Herndon-Dudley syndrome (AHDS) is a very rare, X-linked psychomotor disability syndrome with delayed myelination, almost exclusively affecting boys. We present a case of a 4-year-old boy with AHDS who was found cyanotic, with intermittent vomiting and paroxysmal convulsions about 4 h after his parents went out, and was then taken to the hospital, where he eventually died the next day. The autopsy revealed foreign bodies in the tiny bronchi and alveoli of the deceased, congestion, and punctate hemorrhage in multiple organs, consistent with the diagnosis of asphyxia. Compared with a normally developing 4-year-old boy, the deceased showed cerebral atrophy and cerebral edema, and Luxol Fast Blue (LFB) stain indicated delayed cerebellar, hippocampal, and basal ganglia development and myelination. A novel frameshift mutation c.584delG in the SLC16A2 gene was detected. Family lineage investigation showed that the mutation was also detected in the deceased’s 8-year-old brother and biological mother. The present work enriches the profile mutations in SLC16A2 related to AHDS and emphasizes the importance of autopsy and postmortem genetic analysis in such cases.




Similar content being viewed by others
References
Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S (2004) A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet 74:168–175. https://doi.org/10.1086/380999
Allan W, Herndon CN, Dudley FC (1944) Some examples of the inheritance of mental deficiency: apparently sex-linked idiocy and microcephaly. Am J Ment Defic 48:325–334
Schwartz CE, May MM, Carpenter NJ et al (2005) Allan–Herndon–Dudley syndrome and the monocarboxylate transporter 8 (MCT8) gene. Am J Hum Genet 77:41–53. https://doi.org/10.1086/431313
Schwartz CE, Stevenson RE (2007) The MCT8 thyroid hormone transporter and Allan-Herndon-Dudley syndrome. Best Pract Res Clin Endocrinol Metab 21(2):307–321. https://doi.org/10.1016/j.beem.2007.03.009
Friesema EC, Grueters A, Biebermann H, Krude H, von Moers A, Reeser M, Barrett TG, Mancilla EE, Svensson J, Kester MH, Kuiper GG, Balkassmi S, Uitterlinden AG, Koehrle J, Rodien P, Halestrap AP, Visser TJ (2004) Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 364(9443):1435–7. https://doi.org/10.1016/S0140-6736(04)17226-7
Vaurs-Barrière C, Deville M, Sarret C et al (2009) Pelizaeus–Merzbacher-like disease presentation of MCT8 mutated male subjects. Ann Neurol 65:114–118. https://doi.org/10.1002/ana.21579
Namba N, Etani Y, Kitaoka T et al (2008) Clinical phenotype and endocrinological investigations in a patient with a mutation in the MCT8 thyroid hormone transporter. Eur J Pediatr 167:785–791. https://doi.org/10.1007/s00431-007-0589-6
Gika AD, Siddiqui A, Hulse AJ et al (2010) White matter abnormalities and dystonic motor disorder associated with mutations in the SLC16A2 gene. Dev Med Child Neurol 52:475–482. https://doi.org/10.1111/j.1469-8749.2009.03471.x
Friesema EC, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ (2003) Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem 278:40128–40135. https://doi.org/10.1074/jbc.M300909200
Visser WE, Vrijmoeth P, Visser FE, Arts WF, van Toor H, Visser TJ (2013) Identification, functional analysis, prevalence and treatment of monocarboxylate transporter 8 (MCT8) mutations in a cohort of adult patients with mental retardation. Clin Endocrinol (Oxf) 78(2):310–315. https://doi.org/10.1111/cen.12023
Biebermann H, Ambrugger P, Tarnow P, von Moers A, Schweizer U, Grueters A (2005) Extended clinical phenotype, endocrine investigations and functional studies of a loss-of-function mutation A150V in the thyroid hormone specific transporter MCT8. Eur J Endocrinol 153:359–366. https://doi.org/10.1530/eje.1.01980
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL, ACMG Laboratory Quality Assurance Committee (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17(5):405–24. https://doi.org/10.1038/gim.2015.30
Gereben B, Zeöld A, Dentice M, Salvatore D, Bianco AC (2008) Activation and inactivation of thyroid hormone by deiodinases: local action with general consequences. Cell Mol Life Sci 65(4):570–590. https://doi.org/10.1007/s00018-007-7396-0
Remerand G, Boespflug-Tanguy O, Tonduti D, Touraine R, Rodriguez D, Curie A, Perreton N, Des Portes V, Sarret C, RMLX/AHDS Study Group (2019) Expanding the phenotypic spectrum of Allan-Herndon-Dudley syndrome in patients with SLC16A2 mutations. Dev Med Child Neurol 61(12):1439–1447. https://doi.org/10.1111/dmcn.14332
Groeneweg S, van Geest FS, Abacı A, Alcantud A, Ambegaonkar GP, Armour CM et al (2020) Disease characteristics of MCT8 deficiency: an international, retrospective, multicentre cohort study. Lancet Diabetes Endocrinol 8(7):594–605. https://doi.org/10.1016/S2213-8587(20)30153-4
Geddes JF, Tasker RC, Hackshaw AK, Nickols CD, Adams GG, Whitwell HL, Scheimberg I (2003) Dural haemorrhage in non-traumatic infant deaths: does it explain the bleeding in ‘shaken baby syndrome’? Neuropathol Appl Neurobiol 29:14–22. https://doi.org/10.1046/j.1365-2990.2003.00434.x
Pollanen MS (2011) Subdural hemorrhage in infancy: keep an open mind. Forensic Sci Med Pathol 7(3):298–300. https://doi.org/10.1007/s12024-011-9238-5
Calzà L, Fernández M, Giardino L (2015) Role of the thyroid system in myelination and neural connectivity. Compr Physiol 5(3):1405–1421. https://doi.org/10.1002/cphy.c140035
van Tilborg E, de Theije CGM, van Hal M, Wagenaar N, de Vries LS, Benders MJ, Rowitch DH, Nijboer CH (2018) Origin and dynamics of oligodendrocytes in the developing brain: implications for perinatal white matter injury. Glia 66(2):221–238. https://doi.org/10.1002/glia.23256 (Epub 2017 Nov 14)
Schiffmann R, van der Knaap MS (2009) Invited article: an MRI-based approach to the diagnosis of white matter disorders. Neurology 72(8):750–9. https://doi.org/10.1212/01.wnl.0000343049.00540.c8
Pouwels PJ, Vanderver A, Bernard G, Wolf NI, Dreha-Kulczewksi SF, Deoni SC, Bertini E, Kohlschütter A, Richardson W, Ffrench-Constant C, Köhler W, Rowitch D, Barkovich AJ (2014) Hypomyelinating leukodystrophies: translational research progress and prospects. Ann Neurol 76(1):5–19. https://doi.org/10.1002/ana.24194
Charzewska A, Wierzba J, Iżycka-Świeszewska E, Bekiesińska-Figatowska M, Jurek M, Gintowt A, Kłosowska A, Bal J, Hoffman-Zacharska D (2016) Hypomyelinating leukodystrophies - a molecular insight into the white matter pathology. Clin Genet 90(4):293–304. https://doi.org/10.1111/cge.12811
Klintschar M, Bilkenroth U, Arslan-Kirchner M, Schmidtke J, Stiller D (2009) Marfan syndrome: clinical consequences resulting from a medicolegal autopsy of a case of sudden death due to aortic rupture. Int J Legal Med 123(1):55–58. https://doi.org/10.1007/s00414-008-0288-5
Hugar BS, Praveen S, Kainoor SK, Shetty AR (2014) Sudden death in Marfan syndrome. J Forensic Sci 59:1126–1128. https://doi.org/10.1111/1556-4029.12415
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent
Written informed consent for publishing this scientific report was obtained from the direct relative of the decedent in this case.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Z., Zhao, S., Chen, J. et al. A novel frameshift mutation in Allan-Herndon-Dudley syndrome. Int J Legal Med 136, 1181–1187 (2022). https://doi.org/10.1007/s00414-022-02823-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-022-02823-z