Abstract
The valence state of Fe plays a vital role in setting and recording the oxidation state of magmas, commonly expressed in terms of oxygen fugacity (\(f_{\textrm{O}_{2}}\)). However, our knowledge of how and why \(f_{\textrm{O}_{2}}\) varies within and between magmatic systems remains patchy because of diverse challenges associated with estimating the valence state of Fe in glasses and minerals routinely. Here we investigate Fe valence systematics in magmatic clinopyroxene crystals from ocean island basalts (OIBs) erupted in Iceland and the Azores to explore whether they record information about magma Fe\(^{3+}\) contents and magmatic \(f_{\textrm{O}_{2}}\) conditions. Although many studies assume that all Fe in augitic clinopyroxene crystals from OIBs occurs as Fe\(^{2+}\), we find that up to half of the total Fe present can occur as Fe\(^{3+}\), with crystals from alkali systems typically containing more Fe\(^{3+}\) than those from tholeiitic systems. Thus, Fe\(^{3+}\) is a major if under-appreciated constituent of augitic clinopyroxene crystals erupted from ocean island volcanoes. Most Fe\(^{3+}\) in these crystals is hosted within esseneite component (CaFe\(^{3+}\)AlSiO\(_{6}\)), though some may be hosted in aegirine component (NaFe\(^{3+}\)Si\(_{2}\)O\(_{6}\)) in crystals from alkali systems. Observations from samples containing quenched matrix glasses suggest that the incorporation of Fe\(^{3+}\) is related to the abundance of tetrahedrally coordinated Al (\(\mathrm {^{IV}}\)Al), implying some steric constraints over Fe\(^{3+}\) partitioning between clinopyroxene and liquid (i.e., \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) values), though this may not be an equilibrium relationship. For example, \(\mathrm {^{IV}}\)Al-rich \(\{hk0\}\) prism sectors of sector-zoned crystals contain more Fe\(^{3+}\) than \(\mathrm {^{IV}}\)Al-poor \(\{\bar{1}11\}\) hourglass sectors. Moreover, \(\mathrm {^{IV}}\)Al-rich compositions formed during disequilibrium crystallisation are enriched in Fe\(^{3+}\). Apparent clinopyroxene-liquid Fe\(^{2+}\)–Mg exchange equilibria (i.e., \(K\mathrm{{_{D, {Fe^{2+}-Mg}}^{cpx-liq}}}\) values) are similarly affected by disequilibrium crystallisation in our samples. Nonetheless, it is possible to reconcile our observed clinopyroxene compositions with glass Fe valence systematics estimated from olivine-liquid equilibria if we assume that \(K\mathrm{{_{D, {Fe^{2+}-Mg}}^{cpx-liq}}}\) values lies closer to experimentally reported values of 0.24\(-\)0.26 than values of \(\sim\)0.28 returned from a general model. In this case, olivine-liquid and clinopyroxene-liquid equilibria record equivalent narratives, with one of our glassy samples from Iceland recording evolution under \(f_{\textrm{O}_{2}}\) conditions about one log unit above fayalite-magnetite-quartz (FMQ) equilibrium (i.e., \(\sim\)FMQ+1) and our glassy Azorean sample recording evolution under significantly more oxidising conditions (\(\ge\)FMQ+2.5) before experiencing syn-eruptive reduction, likely as a result of SO\(_{2}\) degassing; our other glassy sample from Iceland was also affected by reductive SO\(_{2}\) degassing. Overall, our findings demonstrate that the Fe valence systematics of clinopyroxene crystals can record information about the conditions under which OIBs evolve, but that further experimental work is required to properly disentangle the effects of magma composition, disequilibrium and \(f_{\textrm{O}_{2}}\) conditions on clinopyroxene-liquid equilibria involving Fe\(^{2+}\) and Fe\(^{3+}\).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Magmas erupted in different tectonic settings often record evolution under different oxygen fugacity (\(f_{\textrm{O}_{2}}\)) conditions (Wood et al. 1990; Carmichael 1991; Cottrell et al. 2022), where \(f_{\textrm{O}_{2}}\) reflects the chemical potential of O in a magma. These different \(f_{\textrm{O}_{2}}\) conditions ultimately reflect differences in the pressure–temperature–composition (PTX) conditions under which magmas evolve in different settings. In particular, the valence state of Fe plays a central role in setting magmatic \(f_{\textrm{O}_{2}}\) because Fe is by far the most abundant multivalent element in terrestrial magmas (Frost 1991). Specifically, the greater the ratio of ferric-to-total Fe (Fe\(^{3+}\)/\(\Sigma\)Fe, where \(\Sigma\)Fe = Fe\(^{2+}\) + Fe\(^{3+}\)) in any given magma, the greater its \(f_{\textrm{O}_{2}}\), as described by various (P)TX-dependent parametrisations (Kress and Carmichael 1991; Berry et al. 2018; Borisov et al. 2018). Moreover, understanding coupled variations between the \(f_{\textrm{O}_{2}}\) and Fe\(^{3+}\)/\(\Sigma\)Fe content of magmas is is important because of the first-order control these variables exert over magmatic phase equilibria (e.g., Toplis and Carroll 1995; Feig et al. 2010), volatile solubilities (e.g., Jugo 2009; O’Neill 2021; Hughes et al. 2023) and equilibrium vapour compositions (e.g., Burgisser and Scaillet 2007; Oppenheimer et al. 2011). Magma Fe\(^{3+}\)/\(\Sigma\)Fe contents thus determine the trajectories along which magmas evolve, the nature of the minerals they crystallise, and the abundance and speciation of the volatiles they release, which in turn control ore formation processes and mediate long-term planetary habitability (Holland 2002; Evans and Tomkins 2011; Gaillard et al. 2011). Generating internally consistent datasets of magma Fe\(^{3+}\)/\(\Sigma\)Fe contents is thus a vital step towards improving our understanding of Earth’s evolution and behaviour through geological time. We aim to further this endeavour by studying the valence state of iron in clinopyroxene crystals, significant but poorly understood hosts of Fe\(^{3+}\) in magmatic systems.
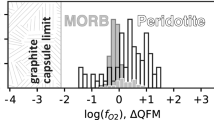
It is generally, though not universally (cf., Lee et al. 2005, 2012), accepted that arc basalts have higher Fe\(^{3+}\)/\(\Sigma\)Fe contents and evolve under more oxidising conditions (approximately one log unit above fayalite-magnetite-quartz equilibrium; \(\sim\)FMQ+1) than mid-ocean ridge basalts (MORBs; \(\sim\)FMQ), even if the reasons for this remain contested (Evans 2006; Kelley and Cottrell 2009; Gaetani 2016; Brounce et al. 2019; Cottrell et al. 2022; Evans and Tomkins 2022). Many ocean island basalts (OIBs) also appear to evolve under more oxidising conditions than MORBs, though global differences between MORBs and OIBs are decidedly more ambiguous than those between MORBs and arc basalts. Observations from Lanzarote and El Hierro in the Canary Islands, Fogo in Cape Verde and Erebus in Antarctica suggest that some OIBs evolve at \(\sim\)FMQ+2 or higher depending on the oxybarometer used (Moussallam et al. 2014, 2019; Taracsák et al. 2022; Nicklas et al. 2022). Observations from Laki in Iceland and Kīlaeua and Mauna Kea in Hawaii also imply evolution under somewhat elevated \(f_{\textrm{O}_{2}}\) conditions that extend up to \(\sim\)FMQ+1 (Moussallam et al. 2016; Hartley et al. 2017; Helz et al. 2017; Brounce et al. 2017). In contrast, observations from Piton de la Fournaise on Réunion imply evolution at or below FMQ–comparable \(f_{\textrm{O}_{2}}\) conditions to those experienced by MORBs (Brounce et al. 2022; Nicklas et al. 2022). While Réunion’s divergence from other OIB systems may initially appear confusing, Brounce et al. (2022) propose an explanation based on known geochemical differences between ocean island systems. Specifically, they suggest that relatively oxidised OIBs originate from mantle sources rich in recycled components and thus Fe\(^{3+}\) (EMI, EMII and HIMU), while relatively reduced OIBs originate from mantle sources poor in recycled components and Fe\(^{3+}\)(C/FOZO/PREMA; Zindler and Hart 1986; Stracke 2012; Weis et al. 2023). Nevertheless, the internally consistent estimates of magma Fe\(^{3+}\)/\(\Sigma\)Fe contents and magmatic \(f_{\textrm{O}_{2}}\) conditions across different ocean island volcanoes needed to verify or refute this suggestion remain elusive. We seek to address this, at least in part, by investigating the valence state of Fe in volcanic glasses and clinopyroxene crystals from Iceland and the Azores that erupt samples with affinities towards C/FOZO/PREMA and EMII reservoirs, respectively.
Considerable progress has been made in using glass and mineral compositions to estimate magmatic \(f_{\textrm{O}_{2}}\) conditions over recent decades, with two-oxide oxybarometry and Fe-XANES spectroscopy of volcanic glasses being especially fruitful (Cottrell et al. 2022, and references therein). However, the former depends on the presence of equilibrium magnetite and ilmenite pairs (e.g., Bacon and Druitt 1988; Ghiorso and Evans 2008), and the latter on the presence of pristine volcanic glass and access to a synchrotron radiation source (e.g., Cottrell et al. 2009; Muth and Cottrell 2023). Furthermore, the reduction of magmatic liquids by SO\(_{2}\) degassing during magma ascent and the sensitivity of hydrous glasses to beam damage add further complexity to interpreting results from Fe-XANES spectroscopy (Moussallam et al. 2016; Brounce et al. 2017; Cottrell et al. 2018). Glass and mineral Fe\(^{3+}\)/\(\Sigma\)Fe contents can also be determined by Mössbauer spectroscopy (e.g., McCammon 2021). However, laboratory-based Mössbauer spectroscopy is ill suited to achieving high sample throughputs and synchrotron-based Mössbauer spectroscopy can only be performed at a limited number of facilities, limiting the overall utility of the approach. Fortunately, different \(f_{\textrm{O}_{2}}\)-sensitive mineral-liquid or mineral-mineral equilibria can allow us to circumvent these issues and recover better estimates of magmatic \(f_{\textrm{O}_{2}}\), especially as minerals respond more slowly to syn- or post-eruptive changes in \(f_{\textrm{O}_{2}}\) than magmatic liquids. For example, recent re-evaluations of olivine-liquid equilibria that present high-precision parametrisations of Fe\(^{2+}\)–Mg exchange equilibria (i.e., predict \(K\mathrm {_{D, {Fe^{2+}-Mg}}^{{ol-liq}}}\) values) provide a potential avenue for estimating magmatic \(f_{\textrm{O}_{2}}\) without needing to perform Fe-XANES spectroscopy (Blundy et al. 2020; Davis and Cottrell 2021; Saper et al. 2022). While developing similar tools based on clinopyroxene-liquid equilibria is complicated by steric constraints associated with the crystal structure of clinopyroxene, we aim to exploit comparatively well understood olivine-liquid equilibria to gain new insights into Fe\(^{3+}\) partitioning and Fe\(^{2+}\)–Mg exchange equilibria between clinopyroxene crystals and their carrier liquids. A key goal of this work is to start building a better understanding how clinopyroxene compositions reflect magmatic \(f_{\textrm{O}_{2}}\) conditions.
Magmatic clinopyroxene crystals incorporate both Fe\(^{2+}\) and Fe\(^{3+}\) by virtue of hosting three distinct cation sites: clinopyroxene has the general formula M2(R\(^{2+}\))M1(R\(^{2+}\))T\(_{2}\)(2R\(^{4+}\))O\(_{6}\), where R is a metal cation, M2 is a distorted octahedral site, M1 is a regular octahedral site and T is a tetrahedral site typically occupied by Si forming the Si\(_{2}\)O\(_{6}\) chains that define the pyroxene structure (Morimoto et al. 1988). As a consequence, clinopyroxene crystals should be inherently sensitive to magma Fe\(^{3+}\)/\(\Sigma\)Fe contents and magmatic \(f_{\textrm{O}_{2}}\) conditions. However, little is known about Fe valence systematics in magmatic clinopyroxene crystals, which fundamentally limits our ability to explore relationships between \(f_{\textrm{O}_{2}}\) and clinopyroxene compositions. This in turn restricts our capacity to describe phase equilibria relations in natural and experimental systems, as well as calibrate the thermodynamic datasets that underpin algorithms like MELTS, THERMOCALC and MAGEMin that are widely used to model magmatic processes (Sack and Ghiorso 1994; Ghiorso and Sack 1995; Holland and Powell 1998; Powell et al. 1998; Jennings and Holland 2015; Holland et al. 2018; Riel et al. 2022).
Experiments performed on a Martian (i.e., shergottites-nahklites-chassignites (SNC)-group) meteorite composition under relatively reducing conditions (\(\sim\)FMQ−4.7 to \(\sim\)FMQ\(+\)0.3) suggest that \(f_{\textrm{O}_{2}}\) correlates with clinopyroxene Fe\(^{3+}\)/\(\Sigma\)Fe, though this inference is based on highly uncertain determinations of Fe\(^{3+}\)/\(\Sigma\)Fe in clinopyroxene crystals (McCanta et al. 2004). Indeed, the reducing conditions under which these experiments were performed and the poor precision of unoriented Fe-XANES spectroscopy performed on experimentally produced clinopyroxene crystals may have masked the true nature of relationships between \(f_{\textrm{O}_{2}}\) and clinopyroxene Fe\(^{3+}\)/\(\Sigma\)Fe. More generally, we speculate that our poor understanding of clinopyroxene Fe\(^{3+}\)/\(\Sigma\)Fe contents in magmatic systems largely reflects the challenges associated with determining Fe valence in mineral samples, whether by bulk methods or in situ microanalysis requiring access to a Mössbauer spectrometer or synchrotron radiation source (Canil and O’Neill 1996; Dyar et al. 2002; McCammon 2021; Steven et al. 2022).
Neave et al. (2024) recently re-evaluated previously discredited approaches for estimating clinopyroxene Fe\(^{3+}\) contents from stoichiometric constraints (cf., McGuire et al. 1989; Canil and O’Neill 1996). By optimising the electron probe microanalysis (EPMA) technique used to measure clinopyroxene crystals, they demonstrated that clinopyroxene Fe\(^{3+}\)/\(\Sigma\)Fe contents can be determined by stoichiometry using the approach of Droop (1987) with a precision approaching that achievable with Mössbauer spectroscopy. They also demonstrated that more than half of the Fe present in natural augite crystals can occur as Fe\(^{3+}\), in line with the sparse Mössbauer analyses of augite crystals from mafic alkaline rocks that are available in the literature (McGuire et al. 1989; Weis et al. 2015). They also proposed a new scheme for assigning clinopyroxene components that explicitly accounts for the presence of Fe\(^{3+}\). Specifically, they assigned most Fe\(^{3+}\) to esseneite component (Es, CaFe\(^{3+}\)AlSiO\(_{6}\)) via a Tschermak-type substitution, with some Fe\(^{3+}\) potentially being assigned to aegirine component (Ae, NaFe\(^{3+}\)Si\(_{2}\)O\(_{6}\)) formed from any Na remaining after forming jadeite component (Jd, NaAlSi\(_{2}\)O\(_{6}\)). We seek to provide the first systematic survey of Fe valence and Fe\(^{3+}\)-sensitive clinopyroxene componentry in OIB samples. Only by developing this deeper understanding of clinopyroxene Fe\(^{3+}\)/\(\Sigma\)Fe systematics will it eventually become possible to investigate how clinopyroxene compositions and phase equilibria involving clinopyroxene reflect magma Fe\(^{3+}\)/\(\Sigma\)Fe contents and ultimately magmatic \(f_{\textrm{O}_{2}}\) conditions.
Here we present high-precision analyses of clinopyroxene crystals in OIB samples from Iceland and the Azores in which clinopyroxene Fe\(^{3+}\)/\(\Sigma\)Fe contents have been estimated by stoichiomery following the approach of Droop (1987). By applying the revised scheme for assigning clinopyroxene components proposed by Neave et al. (2024), we show how Fe\(^{3+}\) is an important constituent of clinopyroxene crystals from diverse natural magmas that cannot readily be ignored in our treatment of clinopyroxene compositions. Finally, we use our improved understanding of magmatic clinopyroxene Fe\(^{3+}\)/\(\Sigma\)Fe contents to explore Fe\(^{3+}\) partitioning and Fe\(^{2+}\)–Mg exchange equilibria between clinopyroxene crystals and mafic liquids in OIB samples that evolved under different \(f_{\textrm{O}_{2}}\) conditions.
Samples
We studied six OIB samples erupted from well-characterised but geochemically distinct magmatic systems in the North Atlantic (Fig. 1). We investigated samples ranging from tholeiitic and alkali basalts to ankaramites and tephites in order to evaluate major element controls on clinopyroxene compositions and the effects of anticipated variations in magmatic \(f_{\textrm{O}_{2}}\) conditions on clinopyroxene Fe\(^{3+}\)/\(\Sigma\)Fe contents (Moussallam et al. 2019; Cottrell et al. 2022). Lava samples were cut into polished thin sections and tephra samples were mounted in epoxy prior to imaging and microanalysis.
Tholeiitic and moderately primitive (\(\sim\)7 wt% MgO) pillow basalt samples (HOR-11-01 and SKU-11-18) were collected from Skuggafjöll mountain in the Eastern Volcanic Zone (EVZ) of southern Iceland. The petrology of these samples and Skuggafjöll more generally is described by Neave et al. (2014). A tholeiititc and moderately primitive (\(\sim\)7 wt% MgO) basaltic tephra sample was collected from 2014 to 2015 Holuhraun eruption in the Northern Volcanic Zone (NVZ) of central Iceland. Our sample is equivalent to sample H14 described by Halldórsson et al. (2018), who also described the petrological evolution of the wider eruption. A tholeiitic and moderately evolved (\(\sim\)4.5 wt% MgO) basaltic lava sample (LAK-04) was collected from the Laki lava flow in the EVZ southern Iceland. The petrology of LAK-04 and the Laki lava flow as a whole is described by Passmore et al. (2012) and Neave et al. (2013).
Three ankaramite (highly olivine- and clinopyroxene-phyric alkali basalts) lava samples (HVAM13-01, ARN13-01 and HLTS13-01, from Hvammsmúli, Arnarholl and Holtsdalur, respectively) were collected from Eyjafjalljökull in the the EVZ of southern Iceland. The petrology of some of these ankaramite localities is described by Loughlin (1995) and Nikkola et al. (2019). An alkali basalt lava sample (PI-011) was collected from the northern flank of Pico volcano in the Azores while a trachybasaltic-to-tephritic tephra sample (PI-041) was collected from a vent on the Panalto da Achada fissure system on the southeastern flank. The petrology of Pico is discussed in detail by França et al. (2006), Zanon et al. (2020) and van Gerve et al. (2024).
Methods
In preparation for EPMA, backscattered electron (BSE) images were collected from epoxy mounts and thin sections using a FEI Quanta 650F scanning electron microscope (SEM) in the Department of Earth and Environmental Sciences at the University of Manchester. Imaging was performed to characterise compositional variability within and between our samples, with a particular focus on clinopyroxene crystals often characterised by complex sector and concentric zoning (e.g., Leung 1974; Dowty 1976; Ubide et al. 2019; Neave et al. 2019).
Quantitative analyses of clinopyroxene, olivine, plagioclase, oxides and glass were performed by EPMA using a JEOL JXA8530F instrument in the School of Earth Sciences at the University of Bristol operated with Probe for EPMA (https://www.probesoftware.com/). The following primary standards were used for calibration: Si, albite; Ti, TiO\(_{2}\); Al, sanidine; Cr, Cr\(_{2}\)O\(_{3}\); Fe, hematite; Mn, Mn metal; Mg, St. John’s olivine; Ca, wollastonite; Na, albite; K, sanidine; P, Durango apatite; and Ni, Ni metal.
Different analytical conditions were used for different phases, with particular care taken over clinopyroxene analyses to ensure that Fe\(^{3+}\)/\(\Sigma\)Fe could be determined by stoichiometry (Neave et al. 2024). We used an accelerating voltage of 15 kV and a spot size of 1 \(\upmu\)m to analyse clinopyroxene, olivine and oxides. The following elements were analysed with a current of 10 nA (on-peak counting times in seconds are shown in parentheses; background counting times were half on-peak counting times): Si(20), Ti(20), Al(20), Fe(40), Mg(40), Ca(20) and K(40); and the following elements were analysed with a current of 40 nA: Cr(60), Mn(30), Na(60), P(60) and Ni(30). We used this approach to maximise the precision of minor element analyses in clinopyroxene that affect the precision of stoichiometric determinations of Fe\(^{3+}\)/\(\Sigma\)Fe (Neave et al. 2024). All elements in plagioclase and glass were analysed with an accelerating voltage of 15 kV, a defocused 10 \(\upmu\)m spot and a current of 10 nA (on-peak counting times in seconds are shown in parentheses; background counting times were half on-peak counting times): Si(10), Ti(50), Al(10), Cr(10), Fe(30), Mn(30), Mg(50), Ca(10), Na(10), K(10) and P(40).
Accuracy was monitored by analysing the following secondary standards: in-house diopside, Cr-diopside and labradorite; and international kk1 kaersutite (Reay et al. 1989) and BCR-2G basaltic glass (Jochum et al. 2005). Major element (e.g., SiO\(_{2}\) and MgO in glass and clinopyroxene) concentrations were typically within 2% of preferred values (based on published values for international standards or longitudinal data for in-house standards), while minor element (e.g., Cr\(_{2}\)O\(_{3}\) and Na\(_{2}\)O in Cr-diopside and FeO\(_{\mathrm{{T}}}\) in plagioclase) concentrations were typically within 6% of preferred values. Counting statistics from analyses of unknown clinopyroxene crystals indicate that major (SiO\(_{2}\), Al\(_{2}\)O\(_{3}\), FeO\(_{\mathrm{{T}}}\), MgO and CaO) and minor (TiO\(_{2}\), Cr\(_{2}\)O\(_{3}\) and Na\(_{2}\)O) elements were determined with 1\(\upsigma\) precisions better than 1% and 5%, respectively; only MnO was measured with a worse 1\(\upsigma\) precision of \(\sim\)8%. Olivine, plagioclase, oxides and glass were measured with comparable 1\(\upsigma\) precisions; better than 2% for major elements and better than 5% for most minor elements. Full details of secondary standard analyses and analytical precision are provided alongside all EPMA data in the Supplementary Material.
Clinopyroxene textures in ocean island basalt samples from Iceland and the Azores. Labels are as follows: cpx, clinopyroxene; ol, olivine; plag, plagioclase; gl, glass; ilm, ilmenite; and ves, vesicle. A Backscattered electron (BSE) image of clinopyroxene in pillow basalt sample HOR-11-01 from Skuggafjöll. An interface between high-BSE-intensity \(\{hk0\}\) prism sectors and low-BSE-intensity \(\{\bar{1}11\}\) hourglass sectors is highlighted with an arrow. B BSE image of ophitic clinopyroxene in a basaltic tephra sample from Holuhraun. C Photomicrograph of clinopyroxene in ophitic arrangement in basaltic lava sample LAK-04 from Laki. D Photomicrograph of large clinopyroxene macrocrysts in ankaramitic lava sample HVAM13-01 from Eyjafjallajökull. The arrow shows rims undergoing dissolution (Neave and Maclennan 2020). E Photomicrograph of a large clinopyroxene macrocrysts in basaltic lava sample PI-011 from Pico, Azores. Patchy zoning is reflected in the variable colour of the clinopyroxene macrocryst. F BSE image of large clinopyroxene macrocysts in basaltic tephra sample PI-041 from Pico, Azores. A prominent concentric zoning boundary is highlighted with the arrow
Results
Petrography
Tholeiitic pillow basalt samples HOR-11-01 and SKU-11-18 from Skuggafjöll contain macrocrysts of plagioclase, clinopyroxene and olivine in a glassy-to-microcrytalline and somewhat vesicular groundmass (Neave et al. 2014). Macrocrysts are defined as being >100 \(\upmu\)m in length and texturally distinct from the groundmass (e.g., Thomson and Maclennan 2013). Clinopyroxene macrocrysts are up to \(\sim\)500 \(\upmu\)m in length and often occur in ophitic arrangement with plagioclase. They typically show weak oscillatory zoning and prominent hourglass sector zoning with low-BSE-intensity \(\{\bar{1}11\}\) hourglass sectors and high-BSE-intensity \(\{hk0\}\) prism sectors (Fig. 2A; Neave et al. 2019).
The tholeiitic basalt tephra sample from Holuhraun contains macrocrysts of plagioclase, clinopyroxene and olivine in a glassy and highly vesicular groundmass (Halldórsson et al. 2018). Clinopyroxene macrocrysts are up to \(\sim\)250 \(\upmu\)m in length and often occur in ophitic arrangement with plagioclase, and also sometimes in association with olivine. Most show weak oscillatory and hourglass sector zoning, though this is not well resolved at the resolution of our BSE images given the small size of the crystals examined here (Fig. 2B); Halldórsson et al. (2018) report larger crystals from other lava and tephra samples.
The tholeiitic basalt lava sample LAK04 from Laki contains macrocrysts of plagioclase, clinopyroxene and olivine in a microcrystalline and vesicular groundmass (Passmore et al. 2012; Neave et al. 2013). Clinopyroxene macrocrysts up to \(\sim\)800 \(\upmu\)m in length occur in ophitic arrangement with plagioclase, most often in multiphase glomerocrysts that also contain olivine (Fig. 2C). Most clinopyroxne crystals also show concentric and hourglass sector zoning like that observed in our samples from Skuggafjöll.
Ankaramitic lava samples HVAM13-01, ARN13-01 and HLTS13-01 from Eyjafjallajökull are dominated by large macrocrysts of olivine and clinopyroxene and smaller oxide macrocrysts in microcrystalline groundmasses, though some plagioclase macrocrysts occur in ARN13-01. Clinopyroxene macrocrysts up to \(\sim\)5 mm in length typically occur as individual, euhedral-to-subhedral crystals, with some occurring in association with olivine (Fig. 2D). Most clinopyroxene crystals are characterised by concentric core-to-rim zoning, though crystals in HVAM13-01 appear to show reaction rims consistent with dissolution prior to final solidification (Neave and Maclennan 2020).
The alkali basalt lava PI-011 from Pico contains large macrocrysts of clinopyroxene, olivine, plagioclase, magnetite and ilmenite in a microcrystalline and vesicular groundmass that also contains microcrysts (crystals <100 \(\upmu\)m in length that are distinct from the groundmass) of the same phases. Clinopyroxene macrocrysts up to \(\sim\)4 mm in length generally occur as individual, euhedral crystals, though some occur in glomerocrysts with olivine and/or plagioclase. Most crystals show slight core-to-rim zoning imprinted on complex patchy zoning (Fig. 2E).
The trachybasaltic-to-tephritic tephra sample PI-041 from Pico contains macrocrysts of clinopyroxene, olivine, plagioclase, magnetite and ilmenite in a glassy and highly vesicular groundmass that also contains microcrysts of the same phases. Clinopyroxene macrocrysts up to \(\sim\)2 mm in length generally occur as individual, euhedral to subhedral crystals, though some occur in association with olivine and ilmenite. Clinopyroxene crystals show evidence for both core-to-rim zoning and complex patchy zoning (Fig. 2F).
Summary of clinopyroxene compositions in ocean island basalt samples from Iceland and the Azores. A Pyroxene quadrilateral showing clinopyroxene quadrilateral components (diopside, Di; hedenbergite, Hd; enstatite, En; and ferrosilite, Fs) calculated following Morimoto et al. (1988) where the Fe component (\(\Sigma\)Fe*) is equal to the sum of Fe\(^{2+}\), Fe\(^{3+}\) and Mn. B Pyroxene quadrilateral showing adjusted clinopyroxene quadrilateral components (diopside, Di†; hedenbergite, Hd†; enstatite, En†; and ferrosilite, Fs†) calculated following Morimoto et al. (1988) where the Fe component (\(\Sigma\)Fe†) is equal to the sum of Fe\(^{2+}\) and Mn, with Fe\(^{2+}\) determined by stoichiometry following Droop (1987)
Major element chemistry
The major element chemistry of clinopyroxene crystals in our OIB samples is summarised in a series of pyroxene quadrilateral diagrams in Fig. 3; all crystals plot firmly in the quadrilateral field of a Q–J diagram (Morimoto et al. 1988). In Fig. 3A we follow the established convention of Morimoto et al. (1988) in calculating the Fe component (\(\Sigma\)Fe*) as the sum of Fe\(^{2+}\), Fe\(^{3+}\) and Mn. However, Fe\(^{3+}\) is not incorporated into any of the quadrilateral end-member components (diopside, Di, CaMgSi\(_{2}\)O\(_{6}\); hedenbergite, Hd, CaFe\(^{2+}\)Si\(_{2}\)O\(_{6}\); enstatite, En, MgSiO\(_{3}\) or ferrosilite, Fs, Fe\(^{2+}\)SiO\(_{3}\)); quadrilateral components only incorporate divalent cations on their M2 and M1 sites. In Fig. 3B we therefore show pyroxene quadrilaterals plotted with a modified Fe component (\(\Sigma\)Fe†) calculated from the sum of Fe\(^{2+}\) and Mn, with Fe\(^{2+}\) determined following Droop (1987). Clinopyroxene crystals from each of our OIB samples define distinct populations in both Fig. 3A and B.
Clinopyroxene crystals in tholeiitic samples from Laki, Holuhraun and Skuggafjöll define an approximate crystal line of descent (Fig. 3A), with those from Skuggafjöll being the most primitive (i.e., having the highest Di contents), those from Holuhraun being slightly more evolved, and those from Laki being the most evolved (i.e., having the lowest Di contents). Although Di and Hd dominate, all clinopyroxene crystals from tholeiitic basalts contain some En and Fs that increase in abundance as Mg and Fe contents decrease and increase, respectively, during magmatic evolution. Clinopyroxene crystals from Skuggafjöll show the greatest variability in DiHd (Di+Hd) and EnFs (En+Fs) contents, consistent with the presence of sector zoning described in greater detail below (Neave et al. 2019). Clinopyroxene crystals from Laki show the greatest overall spread in compositions, consistent with the protracted magmatic history recorded by these crystals (Neave et al. 2013). Removing Fe\(^{3+}\) from the calculation of quadrilateral components has no significant effect beyond moving all data from tholeiitic samples slightly towards the Di vertex.
Despite erupting from two geographically distinct systems, clinopyroxene crystals in alkali samples from Pico and Eyjafjallajökull also define an approximate crystal line of descent (Fig. 3A). Although the most primitive clinopyroxene crystals from Eyjafjallajökull and Pico (and PI-011 in particular) lie close to some crystals from the tholeiitic Skuggafjöll eruption in quadrilateral space, evolved clinopyroxene crystals from Eyjafjallajökull and Pico (PI-041 in particular) lie much closer to the Di-Hd tie line than evolved clinopyroxene crystals from the tholeiitic Holuhraun and Laki eruptions. That is, clinopyroxene crystals from alkali samples evolve towards higher DiHd, while those from tholeiitic samples evolve to lower DiHd. This effect is especially pronounced when Fe\(^{3+}\) is removed from the calculation of quadrilateral components and clinopyroxene compositions move towards the Di vertex (Fig 3B). In this case, some clinopyroxene crystals from PI-041 fall above the Di-Hd tie line, likely because of the greater contribution of Ca to non-quadrilateral components in clinopyroxene crystals from alkali samples with respect to those from tholeiitic samples (e.g., Robinson 1980).
Systematics of major elements hosted in non-quadrilateral clinopyroxene components in ocean island samples from Iceland and the Azores; 1\(\upsigma\) analytical uncertainties are shown. A Variation in Na\(_{2}\)O as a function of MgO. B Variation in Cr\(_{2}\)O\(_{3}\) as a function of MgO. C Covariation of Ti and Al atoms per formula unit (apfu) calculated on a six-oxygen basis
Clinopyroxene crystals from alkali samples contain higher Na\(_{2}\)O contents than those from tholeiitic samples (\(\sim\)0.30\(-\)0.60 wt% versus \(\sim\)0.15\(-\)0.30 wt%; Fig. 4A). Overall, there is a strong correlation between MgO and Na\(_{2}\)O, though clinopyroxene crystals from different eruptions fall on slightly offset arrays. Although significant (>1 wt%) concentrations of Cr\(_{2}\)O\(_{3}\) are only found in relatively primitive clinopyroxene crystals (Fig. 4B), analyses from both alkali and tholeitiic samples define the same broad trend; evolved clinopyroxene crystals contain negligible Cr\(_{2}\)O\(_{3}\). Clinopyroxene crystals from different eruptions are characterised by different Ti:Al values that are often remarkably constant within the products of any individual eruption. In general, clinopyroxene crystals from alkali samples have higher Ti:Al values than those from tholeiitic samples, with the exception of clinopyroxene crystals from the evolved and tholeiitic Laki eruption that overlap with clinopyroxene crystals in alkali ankaramite samples from Eyjafjallajökull.
Iron concentration and valence systematics in clinopyroxene crystals from Iceland and the Azores; 1\(\upsigma\) analytical uncertainties are shown. A MgO versus total Fe expressed as FeO (FeO\(_{\mathrm{{T}}}\)). B MgO versus FeO determined by stoichiometry following Droop (1987). C MgO versus Fe\(_{2}\)O\(_{3}\) determined by stoichiometry following Droop (1987). D MgO versus Fe\(^{3+}\)/\(\Sigma\)Fe determined by stoichiometry following Droop (1987)
Iron valence systematics
The concentration and valence systematics of Fe in clinopyroxene crystals from our OIB samples are summarised in Fig. 5. Distinct crystal lines of descent identified on pyroxene quadrilaterals are reproduced on a plot of MgO versus total Fe expressed as FeO (FeO\(_{\mathrm{{T}}}\); Fig. 5A). Clinopyroxene crystals in tholeiitic samples from Skuggafjöll, Holuhraun and Laki define a relatively steep trend in increasing FeO\(_{\mathrm{{T}}}\) with decreasing MgO (with the highest FeO\(_{\mathrm{{T}}}\) contents found in clinopyroxene rims from Laki), while clinopyroxene crystals in alkali samples from Pico define a shallower trend; crystals from Eyjafjallajökull fall between these two trends. However, relationships are weaker when MgO is plotted against FeO determined by stoichiometry following Droop (1987) (Fig. 5B). For example, clinopyroxene crystals in samples from Skuggafjöll and Laki contain \(\sim\)1 and \(\sim\)2 wt% less FeO than FeO\(_{\mathrm{{T}}}\), respectively, while FeO and FeO\(_{\mathrm{{T}}}\) are broadly comparable in the sample from Holuhraun. Conversely, the enrichment in FeO with decreasing MgO is markedly less coherent than the enrichment in FeO\(_{\mathrm{{T}}}\).
Clinopyroxene Fe\(_{2}\)O\(_{3}\) contents determined following Droop (1987) are summarised as a function of MgO in Fig. 5C. Clinopyroxene Fe\(_{2}\)O\(_{3}\) contents vary from within uncertainty of 0 wt%, where 1\(\upsigma\) uncertainties of \(\sim\)0.35 wt% were obtained using a Monte Carlo approach described by Neave et al. (2024), to \(\sim\)4.5 wt%, highlighting that Fe\(_{2}\)O\(_{3}\) is a significant constituent of many clinopyroxene crystals from OIBs. Alongside Al, Fe\(^{3+}\) is therefore probably the most abundant cation in magmatic clinopyroxene crystals after those responsible for forming quadrilateral components (i.e., Si, Ca, Mg and Fe\(^{2+}\)). Overall, Fe\(_{2}\)O\(_{3}\) contents are much lower in primitive clinopyroxene crystals with high MgO contents (\(\sim\)1 wt% at 17 wt% MgO) than evolved clinopyroxene crystals with low MgO contents (\(\sim\)4 wt% at 13 wt% MgO). To first order, clinopyroxene crystals from tholeiitic and alkali systems lie on the same MgO-Fe\(_{2}\)O\(_{3}\) trend. In detail some finer structure arises. For example, clinopyroxene crystals in the Holuhraun sample contain the lowest Fe\(_{2}\)O\(_{3}\) contents, which rarely exceed 1 wt.% and are often within uncertainty of 0 wt%; clinopyroxene crystals from Skuggafjöll contain \(\sim\)1 wt% more Fe\(_{2}\)O\(_{3}\) than those from Holuhraun but are notably larger in size and found in lava rather than tephra samples. Many clinopyroxene crystals in alkali samples are relatively evolved and contain high Fe\(_{2}\)O\(_{3}\) contents that overlap with values from Skuggafjöll and Laki (1–2 wt%) at their lower limits but extend to much higher values (\(\sim\)4.5 wt%) at their upper limits. On average, clinopyroxene crystals in samples from Eyjafjallajökull reach any given Fe\(_{2}\)O\(_{3}\) content at a higher MgO content than those from Pico. Rim and microcryst compositions from sample PI-041 extend from the main trend to low Fe\(_{2}\)O\(_{3}\) (within uncertainty of 0 wt%) at a constant MgO (\(\sim\)13 wt%).
Clinopyroxene Fe\(^{3+}\)/\(\Sigma\)Fe contents estimated following the stoichiometric approach of Droop (1987) are summarised as a function of MgO in Fig. 5D. These Fe\(^{3+}\)/\(\Sigma\)Fe contents are typically subject to 1\(\upsigma\) uncertainties of \(\sim\)0.03 according to a Monte Carlo approach described by Neave et al. (2024). Clinopyroxene Fe\(^{3+}\)/\(\Sigma\)Fe contents extend from \(\sim\)0.0\(-\)0.3 in primitive crystals in samples from Skuggafjöll and Holuhraun, with the latter having lower values mostly limited to 0.0\(-\)0.1, to \(\sim\)0.4 and \(\sim\)0.5 in evolved crystals in samples from Eyjafjallajökull and Pico, respectively. Importantly, these high values are consistent with high-precision analyses of clinopyroxene crystals in mafic alkali rocks from the Canary Islands by Mössbauer spectroscopy that yielded Fe\(^{3+}\)/\(\Sigma\)Fe contents of 0.49\(-\)0.63 (Weis et al. 2015). Variations in Fe\(^{3+}\)/\(\Sigma\)Fe are nonetheless considerable at any given MgO for any given eruption (\(\ge\)0.3). However, in contrast with McGuire et al. (1989) who ascribed similarly variable clinopyroxene Fe\(^{3+}\)/\(\Sigma\)Fe contents to inadequacies in stoichiometric determinations of Fe\(^{3+}\), we believe that much of this variability is geological in origin. Our reasons for this are threefold: firstly, the close correspondence between Fe\(^{3+}\)/\(\Sigma\)Fe contents determined by Mössbauer and stoichiometry in endmember and single-crystal clinopyroxenes suggests that the latter can be reliable when EPMA is performed with sufficient care (Neave et al. 2024); secondly, Monte Carlo-derived estimates of uncertainties in Fe\(^{3+}\)/\(\Sigma\)Fe are smaller than the variability observed (1\(\upsigma\) uncertainties of \(\sim\)0.03 are an order of magnitude smaller than natural variability of \(\ge\)0.3 at any given MgO); and finally, our knowledge of clinopyroxene chemistry has grown considerably in recent decades. In particular, we now have a much greater understanding of how kinetics and sector zone development generate compositional variability independently of magmatic evolution and magma storage conditions, especially when considering the abundance and systematics of trivalent Al that may also mediate the incorporation of similarly trivalent Fe\(^{3+}\) (Mollo et al. 2010, 2018; Neave et al. 2019; Ubide et al. 2019; Di Fiore et al. 2021; MacDonald et al. 2023; Neave et al. 2024).
Chemical systematics of clinopyroxene crystals with hourglass sector zoning in samples from Skuggafjöll, Iceland. Light- and dark-rimmed symbols show core-to-rim profiles from high- and low-backscattered electron (BSE)-intensity zones associated with prism and hourglass sectors, respectively; 1\(\upsigma\) analytical uncertainties are shown. Vertical lines show the positions of crystal rims. Hourglass sectors (A-A\(^{\prime }\) profiles and dark-rimmed symbols) are poorer in Al\(_{2}\)O\(_{3}\), Fe\(^{3+}\) and esseneite (Es, CaFe\(^{3+}\)AlSiO\(_{6}\)) component than prism sectors (B-B\(^{\prime }\) profiles and light-rimmed symbols)
Chemical systematics of clinopyroxene crystals with concentric core-to-rim zoning in samples from Pico (PI-041), Azores. Vertical lines show the positions of crystal rims and grey shading shows the extent of rim zones identified from backscattered electron (BSE) images; 1\(\upsigma\) analytical uncertainties are shown. Rims are primarily reflected in changes in Mg# and TiO\(_{2}\) content rather than systematic changes in Fe\(^{3+}\)/\(\Sigma\)Fe and esseneite (Es, CaFe\(^{3+}\)AlSiO\(_{6}\)) component
Compositional zoning in major elements and iron valence
Magmatic clinopyroxene crystals are often characterised by complex internal zoning (Dowty 1976; Caricchi et al. 2020; Musu et al. 2023). Compositional variations associated with three types of clinopyoxene zoning observed in our OIB samples are summarised in Figs. 6, 7 and 8. Hourglass sector zoning occurs in diverse magmatic clinopyroxene crystals, and is generally characterised by the differential partitioning of elements between \(\{hk0\}\) prism sectors and \(\{\bar{1}11\}\) hourglass sectors, though the nature and extent of this differential partitioning depends on bulk composition (i.e., tholeiitic versus alkali), cooling rate and the degree of undercooling, (Leung 1974; Kouchi et al. 1983; Neave et al. 2019; Ubide et al. 2019; Mollo et al. 2023). Although some recent studies have incorporated Fe\(^{3+}\) into discussions of clinopyroxene zoning (Mollo et al. 2018; Di Fiore et al. 2021), the interplay between sector zoning and Fe valence systematics remains to be explored in detail.
Paired compositional profiles through hourglass and prism sectors in clinopyroxene crystals from our Skuggafjöll samples are shown in Fig. 6; similar zoning is present in clinopyroxene crystals from the Holuhraun sample but is less intense. Hourglass sectors are characterised by consistently lower Al\(_{2}\)O\(_{3}\) and Fe\(^{3+}\)/\(\Sigma\)Fe contents than prism sectors, in line with published observations from geochemically similar samples from the Holuhraun eruption (Neave et al. 2019; Caricchi et al. 2020) and considerably more alkaline samples from Mt Etna, Italy (Ubide et al. 2019; MacDonald et al. 2023; Musu et al. 2023).
Chemical systematics of clinopyroxene crystals with patchy zoning reflected by variations in BSE intensity in samples from Pico (PI-011), Azores. Vertical lines show the positions of crystal rims; 1\(\upsigma\) analytical uncertainties are shown. There is considerable variability in Mg#, TiO\(_{2}\), Fe\(^{3+}\)/\(\Sigma\)Fe and esseneite (Es, CaFe\(^{3+}\)AlSiO\(_{6}\)) component contents in crystals with patchy zoning but this variability is less systematic than that observed in crystals with hourglass sector zoning or concentric core-to-rim zoning (Figs. 6 and 7)
Compositional profiles through clinopyroxene crystals with concentric (i.e., core-to-rim) zoning in sample PI-041 from Pico are shown in Fig. 7. Crystal interiors only show modest variability in Mg# (where Mg# = molar Mg/(Mg+Fe\(^{2+}\))) or Fe\(^{3+}\)/\(\Sigma\)Fe contents that are largely within propagated analytical uncertainties. Core-to-rim zoning in these crystals is most clearly reflected in changes in TiO\(_{2}\) contents; changes in Mg# and Al\(_{2}\)O\(_{3}\) are more subtle. Moreover, there are no coherent changes in Fe\(^{3+}\)/\(\Sigma\)Fe contents across core-rim boundaries, suggesting that Fe\(^{3+}\)/\(\Sigma\)Fe zoning can be decoupled from zoning in other components.
Coarse compositional profiles through large clinopyroxene crystals with patchy zoning in sample PI-011 from Pico are shown in Fig. 8. Mg#, TiO\(_{2}\) and Al\(_{2}\)O\(_{3}\) show coupled variability beyond analytical uncertainty. Variability in Fe\(^{3+}\)/\(\Sigma\)Fe also exceeds analytical uncertainty though there is no clear relationship between Al\(_{2}\)O\(_{3}\) and Fe\(^{3+}\)/\(\Sigma\)Fe like there is in the crystals with hourglass sector zoning described above and shown in Fig. 6. Nonetheless, correlated variability in TiO\(_{2}\) and Al\(_{2}\)O\(_{3}\) suggests that the patchy zoning in these crystals may reflect similar crystallographic controls as hourglass sector zoning (Leung 1974; Ubide et al. 2019), even if the extent to which this variability is coupled to Fe\(^{3+}\)/\(\Sigma\)Fe differs between samples.
Clinopyroxene components in ocean island samples from Iceland and the Azores summarised as functions of clinopyroxene Mg#, where Mg# = molar Mg/(Mg+Fe\(^{2+}\)). The scheme for calculating the following components was described by Neave et al. (2024) and is summarised in the appendix. A jadeite, Jd; B aegirine, Ae; C esseneite, Es; D Ca-Tschermak’s component, CaTs; E titanian Ca-Tschermak’s component, CaTi; F chromian Ca-Tschermak’s component, CrCaTs; G diopside and hedenbergite, DiHd; and H enstatite and ferrosilite, EnFs
Discussion
Clinopyroxene chemical systematics
Clinopyroxene components calculated following the scheme described by Neave et al. (2024) are plotted as functions of clinopyroxene Mg# in Fig. 9 (a summary of the scheme is provided in the appendix). There is considerable variability within and between clinopyroxene crystals from different OIB samples. Clinopyroxene crystals from tholeiitic samples contain similar amounts of Jd, while the Jd content of crystals from alkali samples is larger and more variable (Fig. 9A). Although it is unsurprising that clinopyroxene crystals from alkali-rich systems contain more Na-bearing Jd than those from tholeiitic systems, differences in Jd contents also reflect differences in magma storage pressures. Both Jd-based clinopyroxene-liquid barometry and clinopyroxene-independent volatile saturation barometry indicate that the tholeiitic magmas erupted at Skuggafjöll, Holuhraun and Laki were stored at lower pressures prior to eruption (\(\sim\)200 MPa; Neave and Putirka 2017; Bali et al. 2018) than the alkali magmas erupted from Pico to produce PI-011 and PI-041 (200–600 MPa; van Gerve et al. 2024). The broadly intermediate Jd content of clinopyroxene crystals from Eyjafjallajökull reflects their intermediate storage depths (\(\sim\)300 MPa; Nikkola et al. 2019). As anticipated, relatively few clinopyroxene crystals contain much Ae (Fig. 9B), with any Ae present restricted to \(\mathrm {^{VI}}\)Al-poor analyses from Na-rich or low-Mg# crystals. Otherwise, there are no clear systematics in the Ae content of clinopyroxene crystals in our OIB samples. In contrast with the inferences of Lindsley (1983), Fe\(^{3+}\) does not appear to be hosted by Ae in most magmatic clinopyroxene crystals, aegirine and aegirine-augite crystals from evolved alkali systems notwithstanding (cf., Larsen 1976; White et al. 2005).
In line with their high Fe\(^{3+}\) but low Ae contents, we infer that Es is a major constituent of clinopyroxene crystals in our OIB samples (Fig. 9C). With the exception of some very Fe\(^{3+}\)-poor analyses in our Holuhraun sample (\(X_{{\textrm{Es}}}\) \(\sim\) 0) that are mainly found within \(\{\bar{1}11\}\) hourglass sectors (Fig. 6), the Es content of clinopyroxene crystals from tholeiitic and alkali samples largely overlap, with the latter extending to slightly higher values (\(\sim\)0.10 versus \(\sim\)0.07). Indeed, much of the spread in clinopyroxene Es contents at any given Mg# likely reflects the differential partitioning of Es between sectors as well as propagated uncertainties in clinopyroxene Fe\(^{3+}\) contents (Fig. 6; Ubide et al. 2019). The tempering of high Es contents in the highest-Fe\(^{3+}\) clinopyroxene crystals from alkali samples reflects the formation of minor Ae in some cases (Fig. 9B). Overall, it is likely that Es is the most abundant non-quadrilateral component in most clinopyroxene crystals from our OIB samples. Although the Es content of clinopyroxene crystals does not correlate straightforwardly with clinopyroxene Mg# (Fig. 9C), Fe\(_{2}\)O\(_{3}\) correlates negatively with MgO (Fig. 5C) on a sample-by-sample basis. While relationships between MgO and Fe\(_{2}\)O\(_{3}\) are complicated by the details of partitioning behaviour (see below), we note that a broad increase in clinopyroxene Fe\(_{2}\)O\(_{3}\) with increasing differentiation may partly reflect the progressive enrichment of magmas in Fe\(_{2}\)O\(_{3}\) by the crystallisation of Fe\(^{3+}\)-poor phases such as olivine and plagioclase. Indeed, such so-called auto-oxidation has been described in experiments on calc-alkaline magmas (Ulmer et al. 2018), and has been invoked to explain the progressive oxidation of evolving MORBs (O’Neill et al. 2018).
The abundance of Ca-Tschermak’s component (CaTs, CaAlAlSiO\(_{6}\)) varies considerably between clinopyroxene crystals in different samples (Fig. 9D). Clinopyroxene crystals in our samples from Skuggafjöll and alkali systems have large ranges in CaTs contents (0.00\(-\)0.05), which reflects the prevalence of hourglass sector zoning and patchy zoning in these samples (Figs. 6 and 8). Such zoning is largely defined by the partitioning of quadrilateral and non-quadrilateral components between different sectors (Neave et al. 2019; Ubide et al. 2019). Crystals from our Laki sample are poor in CaTs because their relatively high Fe\(_{2}\)O\(_{3}\) contents mean that \(\mathrm {^{IV}}\)Al occurs alongside Fe\(^{3+}\) in Es rather than alongside \(\mathrm {^{VI}}\)Al in CaTs, consistent with the high FeO\(_{\mathrm{{T}}}\) content of the Laki lava as a whole (Passmore et al. 2012). In contrast, crystals from our Holuhraun sample are relatively rich in CaTs, consistent with their low Fe\(_{2}\)O\(_{3}\) contents. Although high CaTs contents can reflect disequilibrium crystallisation in some clinopyroxenes, this does not appear to be the case in crystals from Holuhraun (Neave et al. 2019); we return to the high CaTs and low Es contents of Holuhraun clinopyroxene crystals below.
The abundance of titanium pyroxene component (CaTi, CaTiAl\(_{2}\)O\(_{6}\)) varies significantly between samples (Fig. 9E). Clinopyroxene crystals in tholeiitic samples define an approximate differentiation trend at relatively low CaTi for any given Mg# (evolving from \(\sim\)0.01 at Mg# = 0.90 to \(\sim\)0.04 at Mg# = 0.75), while clinopyroxene crystals in alkali samples from Pico evolve towards much higher CaTi (from \(\sim\)0.02 at Mg# = 0.90 to \(\sim\)0.08 at Mg# = 0.75). Clinopyroxene crystals in samples from Eyjafjalljökull define a somewhat intermediate trend that partly overlaps with the trend defined by crystals in samples from Pico. These trends not only reflect the way in which clinopyroxene Ti contents track magmatic evolution, but also the close association between Ti and Al in clinopyroxene crystals from alkali magmas that is generally conserved across sector zones (Leung 1974; Downes 1974; Ubide et al. 2019). The incorporation of Ti alongside 2\(\mathrm {^{IV}}\)Al is consistent with the Ti:Al ratios in excess of 1:2 that are observed in all samples (Fig. 4C), where excess Al remaining after CaTi formation can form other components including Jd, Es and CaTs. We also note that calculating CaTi from Ti rather than residual \(\mathrm {^{IV}}\)Al as suggested by Putirka et al. (1996) is essential for recovering clinopyroxene Ti systematics accurately; calculating from \(\mathrm {^{IV}}\)Al could overestimate apparent CaTi contents when Es is not accounted for. Although there is no need to form other Ti-bearing components such as Ti-diopside (CaMgTi\(_{2}\)O\(_{6}\)), neptunite (NaFe\(^{2+}_{0.5}\)Ti\(_{0.5}\)Si\(_{2}\)O\(_{6}\)), its Mg-bearing equivalent (NaMg\(_{0.5}\)Ti\(_{0.5}\)Si\(_{2}\)O\(_{6}\)) or the fictive alumino-buffonite (CaMg\(_{0.5}\)Ti\(_{0.5}\)AlSiO\(_{6}\)) of Sack and Ghiorso (1994) to account for the compositions of our clinopyroxene crystals, there is ample scope for further substitutions that are not captured by the componentry scheme of Neave et al. (2024). Addressing this complexity would however require a thermodynamic approach that is beyond the scope of this manuscript.
Significant amounts of chromian Ca-Tschermak’s component (CrAlTs, CaCrAlSiO\(_{6}\)) only occur in high-Mg# clinopyroxene crystals (Fig. 9F). This is consistent with the relative compatibility of Cr in clinopyroxene and its relatively low abundance in OIB magmas. Thus, CrAlTs contents rapidly decrease from \(\sim\)0.030 to <0.005 over a Mg# window of \(\sim\)0.90 to 0.75 in clinopyroxene crystals from both tholeiitic and alkali samples. High CrAlTs contents are offset to higher Mg# in clinopyroxene crystals from Pico and Eyjafjallajökull then their tholeiitic equivalents because the higher Fe\(^{3+}\) content of these crystals leads to higher Mg# values at any given Mg content.
As anticipated, all clinopyroxene crystals in our OIB samples are dominated by DiHd (Fig. 9G). It is notable that the DiHd contents of clinopyroxene crystals from tholeiitic samples are generally lower than those from our alkali samples (0.64\(-\)0.76 versus 0.70\(-\)0.80, respectively). The DiHd content of clinopyroxene crystals from tholeiitic samples also decreases with decreasing Mg# in a manner consistent with the pyroxene solvus between DiHd and EnFs narrowing with decreasing temperature (Lindsley and Andersen 1983). In contrast, the DiHd content of clinopyroxene crystals from alkali samples remains broadly constant with decreasing Mg#. Importantly, calculating DiHd in this manner accounts for the incorporation of Ca into non-quadrilateral components, meaning we do not observe the excess DiHd implied from pyroxene quadrilaterals shown in Fig. 3B that account for Fe\(^{3+}\) but not non-quadrilateral Ca; the high and steady DiHd content with decreasing Mg# in clinopyroxene crystals from alkali samples appears to be real. Unsurprisingly, EnFs contents of our clinopyroxene crystals are almost perfectly antithetical to their DiHd contents, with the striking observation that \(X_{\mathrm{{EnFs}}}\) does not increase with decreasing Mg# in clinopyroxene crystals from alkali samples.
Ferric iron partitioning
The presence of significant Fe\(^{3+}\) in clinopyroxene crystals from diverse OIB samples demonstrates that the valence state of Fe can no longer be ignored in robust descriptions of magmatic evolution that draw on clinopyroxene chemistry. It also implies that clinopyroxene crystals may record widespread but currently under-exploited archives of magmatic \(f_{\textrm{O}_{2}}\) conditions. Indeed, the potential for clinopyroxene oxybarometry has been demonstrated for mantle samples investigated by Mössbauer spectroscopy (Luth and Canil 1993). However, poorly defined steric constraints on Fe\(^{3+}\) incorporation into clinopyroxene mean that it is currently impossible to extend oxybarometric approaches based on olivine-liquid Fe\(^{2+}\)–Mg exchange equilibria described by \(K\mathrm{{_{D, {Fe^{2+}-Mg}}^{ol-liq}}}\) values to clinopyroxene-liquid Fe\(^{2+}\)–Mg exchange equilibria (Blundy et al. 2020; Davis and Cottrell 2021; Saper et al. 2022). Thus, a vital step towards understanding the effects of magmatic \(f_{\textrm{O}_{2}}\) on clinopyroxene compositions involves exploring the systematics of Fe\(^{3+}\) partitioning between clinopyroxene crystals and magmatic liquids.
Samples from Holuhraun, Skuggafjöll and Pico (PI-041) contain clinopyroxene crystals in textural equilibrium with their host glasses and are thus suitable for investigating Fe\(^{3+}\) partitioning. Clinopyroxene analyses from Skuggafjöll and Holuhraun were filtered to only include high-Al\(_{2}\)O\(_{3}\) analyses from \(\{hk0\}\) prism sectors that Halldórsson et al. (2018) and Neave et al. (2019) interpreted as recording equilibrium magma storage conditions. Specifically, these authors observed that thermobarometric calculations performed on analyses from prism sectors returned pressures in line with estimates from volatile saturation barometers and geophysical observations while analyses from hourglass sectors returned implausible, negative pressures, implying that they do record equilibrium and are thus unsuitable for investigating Fe\(^{3+}\) partitioning. Nonetheless, we note that the degree to which different sectors may or may not record equilibrium appears to differ between tholeiitic and alkali systems, as discussed by Ubide et al. (2019) and Neave et al. (2019). Rim and microcryst analyses feasibly in equilibrium with the matrix glass of PI-041 were identified texturally; compositionally distinct rims in contact with glasses are clear in clinopyroxene crystals from PI-041 (Figs. 2F and 8) and appear unaffected by sector zoning. In any case, both prism and hourglass sectors in crystals from alkali systems appear capable of recording equilibrium magma storage conditions (MacDonald et al. 2023).
Variations in clinopyroxene Al\(_{2}\)O\(_{3}\) and Fe\(_{2}\)O\(_{3}\) contents in samples from Skuggaföll, Holuhraun and Pico (PI-041) that are textural in equilibrium with their host glasses. Although analyses in PI-041 and our samples from Skuggafjöll are more variable than those in our Holuhraun sample, much of the coupled variability across these samples can be explained by incorporating variable amounts of esseneite (Es, CaFe\(^{3+}\)AlSiO\(_{6}\)) component, as illustrated by the vector labelled +Es
The Al\(_{2}\)O\(_{3}\) and Fe\(_{2}\)O\(_{3}\) contents of clinopyroxene analyses in textural equilibrium with their host glasses are shown in Fig. 10; analyses with Fe\(_{2}\)O\(_{3}<\)0.35 were removed as they lie below the effective detection limit of stoichiometric determinations. Analyses from our Holuhraun sample show minimal variability, while those from both PI-041 and our Skuggafjöll samples are much more variable. Having eliminated effects from sector zoning, elevated and somewhat correlated Fe\(_{2}\)O\(_{3}\) and Al\(_{2}\)O\(_{3}\) contents likely reflect the enhanced incorporation of trivalent cations via Tschermak-type substitutions during disequilibrium crystallisation (Mollo et al. 2010; Ubide et al. 2019; Di Fiore et al. 2021). That is, while the clinopyproxene analyses we selected appear to be in textural equilibrium with their host glasses, only some of them are likely to have formed at conditions close to chemical equilibrium.
Estimating pre-eruptive magmatic oxygen fugacity (\(f_{\textrm{O}_{2}}\)) conditions recorded by olivine-liquid equilibria in samples from Skuggaföll, Holuhraun and Pico (PI-041). A Estimation of glass Mg# contents, where Mg# = Mg/(Mg+Fe\(^{2+}\)). Glass Fe\(^{2+}\) contents were estimated using the \(K\mathrm {_{D, {Fe^{2+}-Mg}}^{{ol-liq}}}\) model of Saper et al. (2022) that assumes all Fe occurs as Fe\(^{2+}\). Equilibrium olivine-liquid pairs were identified texturally from BSE images. B Conversion of estimated glass Fe\(^{3+}\)/\(\Sigma\)Fe contents into \(f_{\textrm{O}_{2}}\) conditions (expressed as log units relative to fayalite-magnetite-quartz equilibrium; \(\Delta\)FMQ) according to the model of Borisov et al. (2018). Large symbols show mean values that reflect our best estimates of magmatic \(f_{\textrm{O}_{2}}\) conditions
In order to convert our observed clinopyroxene Fe\(_{2}\)O\(_{3}\) contents into Fe\(^{3+}\) partition coefficients (i.e., \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) values) we estimated the Fe\(^{3+}\)/\(\Sigma\)Fe content of glasses in our Skuggaföll, Holuhraun and Pico (PI-041) samples from olivine-liquid equilibria (Fig. 11). Specifically, we determined glass Fe\(^{3+}\)/\(\Sigma\)Fe contents from olivine rims and microcrysts, using Equation 10 of Saper et al. (2022) to calculate appropriate \(K\mathrm{{_{D, {Fe^{2+}-Mg}}^{ol-liq}}}\) values from which glass Fe\(^{2+}\) contents and Mg# values could be estimated (Fig. 11A). Glass Fe\(^{3+}\)/\(\Sigma\)Fe contents were then converted into \(f_{\textrm{O}_{2}}\) conditions using the model of Borisov et al. (2018), and are reported as log-unit deviations from FMQ in Fig. 11B. Best estimates of glass Fe\(^{3+}\)/\(\Sigma\)Fe in our Skuggafjöll and Holuhraun samples of \(\sim\)0.19 are similar to values reported from other Icelandic tholeiites from Fe-XANES spectroscopy (up to 0.20 for Laki; Hartley et al. 2017). A glass Fe\(^{3+}\)/\(\Sigma\)Fe content of \(\sim\)0.19 corresponds to an \(f_{\textrm{O}_{2}}\) of FMQ+1, which is broadly consistent with S systematics in the products of the Holuhraun eruption that indicate an \(f_{\textrm{O}_{2}}\) of \(\sim\)FMQ+0.5 (Bali et al. 2018). Olivine crystals from PI-041 return higher glass Fe\(^{3+}\)/\(\Sigma\)Fe contents of \(\sim\)0.33 that correspond to an \(f_{\textrm{O}_{2}}\) of \(\sim\)FMQ+2.5, in line with glass Fe\(^{3+}\)/\(\Sigma\)Fe maxima observed in OIB samples erupted elsewhere (Brounce et al. 2017; Moussallam et al. 2019). These values are also comparable to the results of V-in-olivine oxybarometry performed on petrologically similar samples from El Hierro in the Canary Islands (Taracsák et al. 2022). While subject to analytical uncertainties, our olivine-based approach nonetheless provides plausible and internally consistent estimates of glass Fe\(^{3+}\)/\(\Sigma\)Fe that facilitate deeper investigations of clinopyroxene-liquid Fe\(^{3+}\) partitioning.
Estimates of \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) from clinopyroxene-liquid pairs in samples from Skuggaföll, Holuhraun and Pico (PI-041) that are textural equilibrium. Clinopyroxene Fe\(^{3+}\) contents were determined by stoichiometry following Droop (1987) and liquid (i.e., glass) Fe\(^{3+}\) contents were estimated via olivine-liquid equilibria (Fig. 11). Published \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) estimates from McCanta et al. (2004), Mallmann and O’Neill (2009) and Davis and Cottrell (2021) are shown; compositional information is not available for McCanta et al. (2004) or Mallmann and O’Neill (2009). A Clinopyroxene Al\(_{2}\)O\(_{3}\) contents correlate weakly and positively with \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) in our samples, and our estimated \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) values span the same range as published estimates. B Clinopyroxene \(\mathrm {^{IV}}\)Al contents correlate positively and modestly with \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\), consistent with clinopyroxene Fe\(^{3+}\) contents being mediated by the abundance of esseneite (Es, CaFe\(^{3+}\)AlSiO\(_{6}\)) component and implying steric constraints on Fe\(^{3+}\) partitioning
Estimated \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) values are summarised as a function of clinopyroxene Al\(_{2}\)O\(_{3}\) contents in Fig. 12A. While each of the clinopyroxene populations considered form a distinct cluster on Fig. 12A, little overall structure is apparent. Analyses from our Holuhraun sample lie to low Al\(_{2}\)O\(_{3}\) and low \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\), while those from our Skuggafjöll samples lie to low Al\(_{2}\)O\(_{3}\) but with a large range in \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) and those from Pico span wide ranges in Al\(_{2}\)O\(_{3}\) and \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\). However, when \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) values are summarised as a function of \(\mathrm {^{IV}}\)Al, which forms Es alongside Fe\(^{3+}\) via the coupled substitution (Mg,Fe\(^{2+}\)) + Si = Fe\(^{3+}\) + \(\mathrm {^{IV}}\)Al, some structure can be resolved. Correlated variations in \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) and \(\mathrm {^{IV}}\)Al in our tholeiitic and alkali samples can be fitted with separate linear models of modest quality (\(r^{2}\) = 0.35 and 0.32, respectively). Nonetheless, our observations suggest that \(\mathrm {^{IV}}\)Al can play a role in mediating Fe\(^{3+}\) incorporation into magmatic clinopyroxene crystals (e.g., Di Fiore et al. 2021), and that the nature of this effect may depend on system composition. The shallower slope of the regression through crystals from alkali samples with respect to that through crystals from tholeiitic samples likely reflects the incorporation of greater amounts of Ti as CaTi (i.e., CaTiAl\(_{2}\)O\(_{6}\)) in the former.
Published estimates of \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) are shown in Fig. 12. Mallmann and O’Neill (2009) and Davis and Cottrell (2021) report values of 0.453±0.158 and 0.78±0.30, respectively, while McCanta et al. (2004) report values in the range 0.00\(-\)0.77. Iron was only present at trace levels in the (near-)CMAS experiments of Mallmann and O’Neill (2009), casting doubt on the suitability of using their estimated \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) value to describe Fe\(^{3+}\) partitioning in OIB samples where Fe\(_{2}\)O\(_{3}\) is a major constituent (>1 wt.%), despite overlapping with our observations from natural samples. Davis and Cottrell (2021) investigated Fe\(^{3+}\) partitioning in ultramafic but nevertheless Fe-poor systems, deriving \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) indirectly through Fe\(^{2+}\)–Mg exchange between olivine and clinopyroxene. While Davis and Cottrell (2021) inferred that \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) was not affected by \(f_{\textrm{O}_{2}}\) in their variable \(f_{\textrm{O}_{2}}\) experiments, there is insufficient chemical variability in their experimentally produced clinopyroxene crystals to identify potential chemical controls over Fe\(^{3+}\) partitioning. That is, the clinopyroxene crystals they used to estimate \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) have very similar Al\(_{2}\)O\(_{3}\) and \(\mathrm {^{IV}}\)Al contents, so any potential mediation of Fe\(^{3+}\) incorporation by Al\(_{2}\)O\(_{3}\) or \(\mathrm {^{IV}}\)Al cannot be evaluated. The \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) values reported by Davis and Cottrell (2021) are nonetheless consistent with our linear model through clinopyroxene analyses from tholeiitic samples (Fig. 12B).
McCanta et al. (2004) report values of 0.00\(-\)0.77 from their experiments on a SNC meteorite composition, with values of 0.00\(-\)0.09 being obtained under reducing conditions when Fe\(^{3+}\) was almost absent (\(\sim\)FMQ−4.7 to FMQ−2.7) and elevated values of 0.48\(-\)0.77 being obtained under conditions approaching those relevant for terrestrial magmatism (\(\sim\)FMQ−0.7 to \(\sim\)FMQ\(+\)0.3). Unfortunately McCanta et al. (2004) do not provide the compositional information required to assess whether their reported variations in \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) reflect changes in \(f_{\textrm{O}_{2}}\) alone, or whether clinopyroxene compositions also play a role. Moreover, the Fe\(^{3+}\) contents of augite crystals measured by McCanta et al. (2004) were subject to 1\(\upsigma\) uncertainties similar in magnitude to the Fe\(^{3+}\) contents themselves, and as such their reported \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) values should be considered similarly uncertain.
Overall, our observations indicate that \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) values in OIB systems likely range between 0.2 and 1.0 depending on the composition of the clinopyroxene and liquid in question. Clinopyroxene \(\mathrm {^{IV}}\)Al contents appear to play a role in meditating their Fe\(^{3+}\) contents, but the strength of this effect depends on system alkalinity, with clinopyroxene crystals in alkali systems incorporating less Fe\(^{3+}\) for any given increase in \(\mathrm {^{IV}}\)Al than those from tholeiitic systems, likely because of the coupled incorporation of Ti. Importantly, if Fe\(^{3+}\) partitioning is linked to the incorporation of \(\mathrm {^{IV}}\)Al, then clinopyroxene Fe\(_{2}\)O\(_{3}\) contents will be equally sensitive to disequilibrium crystallisation as their Al\(_{2}\)O\(_{3}\) and TiO\(_{2}\) contents (Mollo et al. 2010, 2013; Ubide et al. 2019). That is, coupled variations in clinopyroxene Fe\(_{2}\)O\(_{3}\), Al\(_{2}\)O\(_{3}\) and TiO\(_{2}\) contents likely result from disequilibrium processes such that apparent \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) values may not always truly reflect equilibrium Fe\(^{3+}\) partitioning behaviour. For example, prism sectors may record the best estimates of equilibrium \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) values values in our tholeiitic samples (Neave et al. 2019), while either prism or hourglass sectors may record equilibrium \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) values in our alkali samples (Ubide et al. 2019; MacDonald et al. 2023). Moreover, our evaluations assume that the clinopyroxene crystals analysed grew from liquids whose Fe\(^{3+}\)/\(\Sigma\)Fe contents were correctly estimated from olivine-liquid equilibria, which may not be the case for our Holuhraun sample for reasons discussed below.
Combining observations from Skuggafjöll prism sectors with observations from Pico samples as a whole suggests that equilibrium \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) may lie close to 0.6, with much of the variability we observe resulting from kinetic effects associated with variable Al\(_{2}\)O\(_{3}\) incorporation. While our estimated \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) values of \(\sim\)0.6 are broadly in line with the sparse experimental observations available, new experiments and analyses are required to develop robust and predictive descriptions of Fe\(^{3+}\) partitioning between clinopyroxene and liquid.
Iron-magnesium exchange equilibria
Clinopyroxene crystals primarily record magmatic evolution in a transition from high-Mg# compositions dominated by Di to low-Mg# compositions that are relatively enriched in Hd. As such, clinopyroxene-liquid equilibria are often summarised in terms of Fe\(^{2+}\)–Mg exchange (Wood and Blundy 1997; Putirka 2008). This convention at least partly reflects similarities between clinopyroxene-liquid equilibria described using \(K\mathrm{{_{D, {Fe^{2+}-Mg}}^{cpx-liq}}}\) values and olivine-liquid equilibria described using \(K\mathrm{{_{D, {Fe^{2+}-Mg}}^{ol-liq}}}\) values (Roeder and Emslie 1970; Blundy et al. 2020; Saper et al. 2022). Equation 35 of Putirka (2008) provides the most recent model capable of predicting \(K\mathrm{{_{D, {Fe-Mg}}^{cpx-liq}}}\) values that is feasibly applicable to OIB samples. Although this model incorporates the effect of temperature on \(K\mathrm{{_{D, {Fe-Mg}}^{cpx-liq}}}\), and returns a value of \(\sim\)0.28 at temperatures relevant to OIB evolution (\(\sim\)1200 \(^{\circ }\)C) that is close to \(K\mathrm{{_{D, {Fe^{2+}-Mg}}^{cpx-liq}}}\) values from experiments on mafic compositions (0.24\(-\)0.26; Sisson and Grove 1993; Pilet et al. 2010; Salazar-Naranjo and Vlach 2023), it assumes that all Fe occurs as Fe\(^{2+}\) in both clinopyroxene crystals and their host glasses and thus strictly describes Fe–Mg exchange rather than Fe\(^{2+}\)–Mg exchange. This assumption is clearly incorrect for basaltic liquids characterised by Fe\(^{3+}\)/\(\Sigma\)Fe contents that range from \(\sim\)0.1 to \(\sim\)0.3 across a naturally relevant range of \(f_{\textrm{O}_{2}}\) conditions (FMQ−1 to FMQ+2; Kress and Carmichael 1991; Borisov et al. 2018; Cottrell et al. 2022). Our findings also demonstrate that this assumption is incorrect from the crystal perspective, with clinopyroxene crystals in diverse OIB samples exhibiting Fe\(^{3+}\)/\(\Sigma\)Fe contents ranging from \(\sim\)0.0 to \(\sim\)0.5 (Fig. 5D). Given that Fe\(^{2+}\) and Fe\(^{3+}\) are incorporated into clinopyroxene within quadrilateral and non-quadrilateral components, respectively, Fe\(^{3+}\) is unlikely to exchange directly with Mg or Fe\(^{2+}\) (e.g., Ubide et al. 2019; Neave et al. 2024), meaning that Fe\(^{2+}\)–Mg exchange may be only indirectly affected by Fe\(^{3+}\). Improving our ability to interpret observations from clinopyroxene crystals in natural and experimental systems thus requires clinopyroxene-liquid Fe\(^{2+}\)–Mg exchange equilibria and \(K\mathrm{{_{D, {Fe^{2+}-Mg}}^{cpx-liq}}}\) systematics sensu stricto to be investigated in greater depth.
The effect of Fe\(^{3+}\) on Fe\(^{2+}\)–Mg exchange equilibria between clinopyroxene crystals and their host glasses in samples from: A Skuggafjöll, Iceland (B) Holuhraun, Iceland, and C Pico (PI-041), Azores. Coloured lines show \(K\mathrm{{_{D, {Fe^{2+}-Mg}}^{cpx-liq}}}\) values. A nominally equilibrium value of 0.28 determined with Equation 35 of Putirka (2008) at 1200\(^{\circ }\)C is shown as a thick, solid line; this equation assumes that all Fe is present as Fe\(^{2+}\) and thus returns \(K\mathrm{{_{D, {Fe-Mg}}^{cpx-liq}}}\) values. A arguably more appropriate \(K\mathrm{{_{D, {Fe^{2+}-Mg}}^{cpx-liq}}}\) value of 0.24 suggested by experiments on mafic alkaline magmas is shown as a thick, dashed line (Pilet et al. 2010; Salazar-Naranjo and Vlach 2023). Black symbols show clinopyroxene-liquid equilibria calculated assuming that all Fe occurs as Fe\(^{2+}\), as recommended by Wieser et al. (2023b). Coloured symbols show values calculated with clinopyroxene Fe\(^{2+}\) contents determined by stoichiometry following Droop (1987) and liquid (i.e., glass) Fe\(^{2+}\) contents fixed at a range of different oxygen fugacity (\(f_{\textrm{O}_{2}}\)) conditions according to the model of Borisov et al. (2018). Large symbols show mean clinopyroxene compositions at \(f_{\textrm{O}_{2}}\) conditions estimated via olivine-liquid equilibria (Fig. 11). Arrows and question marks indicate where clinopyroxene records of \(f_{\textrm{O}_{2}}\) may deviate from olivine records, likely as a result of reductive SO\(_{2}\) degassing in the cases of Holuhraun and PI-041 microcrysts
Samples from Skuggafjöll, Holuhraun and Pico (PI-041) contain clinopyroxene crystals in textural equilibrium with their host glasses and are suitable for investigating Fe\(^{2+}\)–Mg exchange. Combined glass and clinopyroxene Mg# systematics are summarised in Fig. 13. Clinopyroxene-liquid Fe–Mg exchange equilibria were first calculated following current recommendations that all Fe should be taken as Fe\(^{2+}\) in both clinopyroxene and liquid (Wieser et al. 2023a, b). These calculations return \(K\mathrm{{_{D, {Fe-Mg}}^{cpx-liq}}}\) values within uncertainty of (±0.08, 1\(\upsigma\)), but also consistently lower than, the \(\sim\)0.28 value calculated with Equation 35 of Putirka (2008) potentially as a result of ignoring of Fe\(^{3+}\). Indeed, we speculate that the large uncertainty in \(K\mathrm{{_{D, {Fe-Mg}}^{cpx-liq}}}\) values estimated from this equation (±0.08, 1\(\upsigma\)) compared with uncertainties in \(K\mathrm{{_{D, {Fe^{2+}-Mg}}^{ol-liq}}}\) values estimated with various recent models (typically ±0.03, 1\(\upsigma\), or less; Blundy et al. 2020; Saper et al. 2022) reflects the currently unconstrained influence of Fe\(^{3+}\) in clinopyroxene.
Clinopyroxene-liquid Fe\(^{2+}\)–Mg exchange equilibria were subsequently calculated with clinopyroxene Fe\(^{2+}\) contents determined by stoichiometry following Droop (1987) and liquid (i.e., glass) Fe\(^{2+}\) contents fixed at a range of relevant \(f_{\textrm{O}_{2}}\) conditions (FMQ to FMQ+3) according to the model of Borisov et al. (2018). Accounting for the presence of Fe\(^{3+}\) increases both glass and clinopyroxene Mg# values. However, the magnitudes of Mg# increases vary within and between samples because clinopyroxene Fe\(_{2}\)O\(_{3}\) contents also vary (Fig. 5C). Best estimates of clinopyroxene-liquid Fe\(^{2+}\)–Mg exchange equilibria were estimated by assuming that mean clinopyroxene compositions (from prism sectors for Skuggafjöll and Holuhraun) were in equilibrium with glass Fe\(^{3+}\)/\(\Sigma\)Fe contents estimated from olivine-liquid equilibria (Fig. 11), \(\sim\)0.19 for samples from Skuggafjöll and Holuhraun and \(\sim\)0.33 for sample PI-041 from Pico.
The best estimate of clinopyroxene-liquid Fe\(^{2+}\)–Mg equilibrium calculated from the mean clinopyroxene composition in our Skuggafjöll samples is consistent with a \(K\mathrm{{_{D, {Fe^{2+}-Mg}}^{cpx-liq}}}\) value of \(\sim\)0.23 (Fig. 13A), within model uncertainty of \(\sim\)0.28, but also much closer to experimentally reported values of \(\sim\)0.24\(-\)0.26 (Sisson and Grove 1993; Pilet et al. 2010; Salazar-Naranjo and Vlach 2023). If we assume, for now, that a \(K\mathrm{{_{D, {Fe^{2+}-Mg}}^{cpx-liq}}}\) value of \(\sim\)0.28 from Putirka (2008) is correct, our mean Skuggafjöll clinopyroxene composition would indicate \(f_{\textrm{O}_{2}}\) close to \(\sim\)FMQ+2.5. However, observations from elsewhere in Iceland and the Reykjanes Ridge suggest that such oxidising conditions are unrealistic for Icelandic tholeiites (Shorttle 2015; Hartley et al. 2017; Bali et al. 2018; Novella et al. 2020). If we instead assume that a \(K\mathrm{{_{D, {Fe^{2+}-Mg}}^{cpx-liq}}}\) value of \(\sim\)0.23 is more appropriate, as suggested by experimental observations, the mean clinopyroxene composition from Skuggafjöll would be consistent with an \(f_{\textrm{O}_{2}}\) close to \(\sim\)FMQ+1.0 (Fig. 13A), in broad agreement with estimates from the nearby Laki eruption (\(\sim\)FMQ+0.7; Hartley et al. 2017). If mean compositions were compromised to some degree by disequilibrium crystallisation such that excess Fe\(_{2}\)O\(_{3}\) had been incorporated alongside excess Al\(_{2}\)O\(_{3}\) in some analyses (e.g., Mollo et al. 2010; Di Fiore et al. 2021), then true \(K\mathrm{{_{D, {Fe^{2+}-Mg}}^{cpx-liq}}}\) values may be slightly higher and equilibrium \(f_{\textrm{O}_{2}}\) conditions slightly more reducing.
The best estimate of clinopyroxene-liquid Fe\(^{2+}\)–Mg equilibrium calculated from the mean clinopyroxene composition in our Holuhraun sample is consistent with a \(K\mathrm{{_{D, {Fe^{2+}-Mg}}^{cpx-liq}}}\) value of \(\sim\)0.28 (Fig. 13B). Assuming again that a \(K\mathrm{{_{D, {Fe^{2+}-Mg}}^{cpx-liq}}}\) value of \(\sim\)0.28 is appropriate, the mean clinopyroxene composition from Holuhraun would be consistent with an \(f_{\textrm{O}_{2}}\) close to \(\sim\)FMQ+1.0. However, if we assume that equilibrium \(K\mathrm{{_{D, {Fe^{2+}-Mg}}^{cpx-liq}}}\) values are closer to experimentally derived values (0.24\(-\)0.26), as strongly indicated by observations from Skuggafjöll, then our clinopyroxene crystals would record an \(f_{\textrm{O}_{2}}\) below FMQ (Fig. 13B). While such reducing conditions initially seem unlikely (cf., \(\sim\)FMQ+0.5 for Holuhraun; Bali et al. 2018), it is also possible that the crystals we analysed from Holuhraun formed after reductive SO\(_{2}\) degassing given their small size (Fig. 2B), especially when compared with the larger crystals reported from other eruption products (Halldórsson et al. 2018). Indeed, it is well documented that SO\(_{2}\) degassing during magma ascent leads to the reduction of magmatic liquids (Moussallam et al. 2016; Helz et al. 2017), with initially high-\(f_{\textrm{O}_{2}}\) OIBs (\(\sim\)FMQ+3) recording \(f_{\textrm{O}_{2}}\) conditions at or below FMQ at the point of eruption (Taracsák et al. 2022). As such, estimating glass Fe\(^{3+}\)/\(\Sigma\)Fe contents from olivine-liquid equilibria may have overestimated the true amount of Fe\(^{3+}\) present in the Holuhraun carrier liquid during clinopyroxene crystallisation. Indeed, the low \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) values estimated for Holuhraun probably also reflect an incorrect evaluation of liquid Fe\(^{3+}\)/\(\Sigma\)Fe and should not be considered as reliable equilibrium values.
Interpreting clinopyroxene-liquid Fe\(^{2+}\)–Mg equilibria in sample PI-041 from Pico is complex as different crystal populations appear to record growth under different conditions (Fig. 13C). If we initially assume a \(K\mathrm{{_{D, {Fe^{2+}-Mg}}^{cpx-liq}}}\) value of \(\sim\)0.28, the mean microcryst composition would record a plausible \(f_{\textrm{O}_{2}}\) of \(\sim\)FMQ+2.5 while the mean rim composition would record seemingly implausible and disequilibrium \(f_{\textrm{O}_{2}}\) conditions well in excess of FMQ+3. If we instead assume that equilibrium \(K\mathrm{{_{D, {Fe^{2+}-Mg}}^{cpx-liq}}}\) values are closer to the 0.24\(-\)0.25 values observed in the prod cuts of experiments on compositionally analogous systems (Pilet et al. 2010; Salazar-Naranjo and Vlach 2023), then the mean rim composition returns oxidising but plausible \(f_{\textrm{O}_{2}}\) conditions of \(\ge\)FMQ+2.5, while mean microcryst composition returns relatively reducing conditions of FMQ+1 (Fig. 13C). However, if rims and microcrysts formed before and after reductive SO\(_{2}\) degassing, respectively, the seemingly discordant \(f_{\textrm{O}_{2}}\) conditions they record can be reconciled. We hence speculate that the rims of clinopyroxene (and olivine) crystals in sample PI-041 from Pico formed under relatively oxidising \(f_{\textrm{O}_{2}}\) conditions (\(\ge\)FMQ+2.5) at depth prior to any significant SO\(_{2}\) degassing. We suggest that clinopyroxene microcrysts then formed after significant SO\(_{2}\) degassing occurred during ascent such that they recorded relatively reducing \(f_{\textrm{O}_{2}}\) conditions of \(\sim\)FMQ+1.
Overall, our results illustrate that accounting for the presence of Fe\(^{3+}\) significantly impacts how clinopyroxene-liquid Fe\(^{2+}\)–Mg exchange equilibria in OIB samples are evaluated. Nonetheless, \(K\mathrm{{_{D, {Fe-Mg}}^{cpx-liq}}}\) values calculated assuming that all Fe is present as Fe\(^{2+}\) are generally within the (large) ±0.08(1\(\sigma\)) uncertainty of values predicted with Equation 35 of Putirka (2008). Once Fe\(^{3+}\) is accounted for, \(K\mathrm{{_{D, {Fe^{2+}-Mg}}^{cpx-liq}}}\) values estimated from our natural samples generally lie closer to experimentally derived values of 0.24\(-\)0.26 than 0.28 (Sisson and Grove 1993; Pilet et al. 2010; Salazar-Naranjo and Vlach 2023). Moreover, different clinopyroxene populations within a single sample (PI-041 from Pico) record different Fe\(^{2+}\)–Mg systematics, potentially mirroring variations in \(f_{\textrm{O}_{2}}\) conditions reported from other OIB systems that result from SO\(_{2}\) degassing during ascent (Moussallam et al. 2016; Helz et al. 2017; Taracsák et al. 2022). Considerable uncertainties nevertheless remain in our interpretations. Targeted experiments are thus required to identify and disentangle the effects of composition, disequilibrium and \(f_{\textrm{O}_{2}}\) conditions on clinopyroxene-liquid Fe\(^{2+}\)–Mg exchange before we can calibrate a robust predictive model of \(K\mathrm{{_{D, {Fe^{2+}-Mg}}^{cpx-liq}}}\) for modelling and interpreting OIB samples.
Conclusions
By applying the stoichiometric approach for determining clinopyroxene Fe\(^{3+}\)/\(\Sigma\)Fe contents described by Droop (1987) to compositionally diverse OIB samples from Iceland and the Azores, we demonstrate that Fe\(^{3+}\) is an abundant if under-appreciated cation in magmatic clinopyroxene crystals from ocean island volcanoes. On average, clinopyroxene crystals from alkali systems have higher Fe\(^{3+}\)/\(\Sigma\)Fe contents than those from tholeiitic systems, which translate into higher Es contents and, in some cases, non-negligible Ae contents. The incorporation of Ca and \(\mathrm {^{IV}}\)Al in clinopyroxene crystals alongside Fe\(^{3+}\) has important consequences for the relative proportions of other clinopyroxene components such as DiHd and EnFs, and trends in clinopyroxene Jd, CaTi and CrAlTs contents can be variably linked to magma composition (i.e., alkalinity), storage pressure and the degree of magmatic evolution. Clinopyroxene Es contents are somewhat related to the \(f_{\textrm{O}_{2}}\) conditions under which their carrier liquids evolved, with Fe\(^{3+}\)-rich crystals from alkali samples that typically evolve under relatively oxidising conditions containing more Es than Fe\(^{3+}\)-poor crystals from tholeiitic samples. However, elevated clinopyroxene Es, CaTs and CaTi contents sometimes reflect disequilibrium crystallisation, including in \(\{\bar{1}11\}\) hourglass sectors from crystals in tholeiitic samples. Overall, Fe\(^{3+}\) shows similar behaviour to Al and Ti during clinopyroxene crystallisation and its behaviour may thus be interpreted within a growing corpus of knowledge about the textural and chemical evolution of clinopyroxene crystals.
By exploiting the ability to estimate glass Fe\(^{3+}\)/\(\Sigma\)Fe contents from olivine-liquid equilibria, we investigated Fe\(^{3+}\) partitioning in a subset of our OIB samples with quenched matrix glasses. Olivine-liquid equilibria in tholeiitic samples from Skuggafjöll and Holuhraun record glass compositions consistent with \(f_{\textrm{O}_{2}}\) conditions of \(\sim\)FMQ+1, while those in the alkali sample PI-041 from Pico record compositions consistent with relatively oxidising \(f_{\textrm{O}_{2}}\) conditions of \(\sim\)FMQ+2.5. These values are consistent with observations from a range of ocean island settings, and potentially reinforce suggestions that OIBs with EMII affinities, like those from the Azores, evolve under more oxidising conditions than those with C/FOZO/PREMA affinities, like those from Iceland. Estimated \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) values from clinopyroxene analyses in textural equilibrium with their host glasses fall in the range 0.2\(-\)1.0, and appear to be mediated by the abundance of \(\mathrm {^{IV}}\)Al. This implies that there are steric constraints on the incorporation of Fe\(^{3+}\) into clinopyroxene, though the exact nature of \(\mathrm {^{IV}}\)Al-\(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) relationships differs between tholeiitic and alkali compositions. Some of the variability in apparent \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) values may however reflect disequilibrium, with \(\mathrm {^{IV}}\)Al- and Fe\(^{3+}\)-rich analyses in samples from Pico and Skuggafjöll reflecting the enhanced incorporation of non-quadrilateral components during disequilibrium crystallisation; equilibrium \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) values during magmatic evolution may thus lie in the middle of the 0.2\(-\)1.0 range (i.e., \(\sim\)0.6) given that low values from Holuhraun may also reflect disequilibrium processes. While our findings are consistent with the few experimental determinations of \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) currently available, new experiments are required to disentangle the effects of system composition and disequilibrium crystallisation on clinopyroxene-liquid Fe\(^{3+}\) partitioning.
Extending our evaluation to clinopyroxene-liquid Fe\(^{2+}\)–Mg exchange equilibria suggests that \(K\mathrm{{_{D, {Fe^{2+}-Mg}}^{cpx-liq}}}\) values (as opposed to \(K\mathrm{{_{D, {Fe-Mg}}^{cpx-liq}}}\) values) lie close to experimental estimates (0.24\(-\)0.26) when \(f_{\textrm{O}_{2}}\) conditions are similar to those recorded by olivine-liquid equilibria in samples unaffected by SO\(_{2}\) degassing (i.e., \(\sim\)FMQ+1 for Skuggafjöll and \(\sim\)FMQ+2.5 for Pico). Differences in apparent \(K\mathrm{{_{D, {Fe^{2+}-Mg}}^{cpx-liq}}}\) values within and between different populations of clinopyroxene crystals we analysed reflect the same disequilibrium processes recorded in \(\mathrm {^{IV}}\)Al-Fe\(^{3+}\) systematics, and highlight the importance of evaluating whether phases are in chemical as well as textural equilibrium before interpreting the meaning of Fe\(^{2+}\)–Mg exchange equilibria. Thus, our findings demonstrate that the non-negligible abundance of Fe\(^{3+}\) in clinopyroxene crystals from OIB settings has a significant effect on clinopyroxene-liquid Fe\(^{2+}\)–Mg exchange equilibria. While our investigation suggests that \(D\mathrm {^{{cpx-liq}}_{{Fe_{2}O_{3}}}}\) and \(K\mathrm{{_{D, {Fe^{2+}-Mg}}^{cpx-liq}}}\) values offer potential routes for estimating glass Fe\(^{3+}\)/\(\Sigma\)Fe contents and magmatic \(f_{\textrm{O}_{2}}\) conditions, further experiments are required to robustly define clinopyroxene-liquid equilibria involving both Fe\(^{2+}\) and Fe\(^{3+}\). These experiments will also pave the way towards understanding equilibria between clinopyroxene and other minerals that will open new opportunities for determining and modelling the behaviour of Fe\(^{2+}\) and Fe\(^{3+}\) and thence \(f_{\textrm{O}_{2}}\) in glass-free systems.
Data availability
All EPMA data, including analyses of secondary standards, are provided in the Supplementary Material.
References
Bacon CR, Druitt TH (1988) Compositional evolution of the zoned calcalkaline magma chamber of Mount Mazama, Crater Lake, Oregon. Contrib Miner Petrol 98:224–256. https://doi.org/10.1007/BF00402114
Bali E, Hartley ME, Halldórsson SA et al (2018) Melt inclusion constraints on volatile systematics and degassing history of the 2014–2015 Holuhraun eruption, Iceland. Contrib Miner Petrol 173:9. https://doi.org/10.1007/s00410-017-1434-1
Berry AJ, Stewart GA, O’Neill HSC et al (2018) A re-assessment of the oxidation state of iron in MORB glasses. Earth Planet Sci Lett 483:114–123. https://doi.org/10.1016/j.epsl.2017.11.032
Blundy JD, Melekhova E, Ziberna L et al (2020) Effect of redox on Fe-Mg-Mn exchange between olivine and melt and an oxybarometer for basalts. Contrib Miner Petrol 7:103. https://doi.org/10.1007/s00410-020-01736-7
Borisov A, Behrens H, Holtz F (2018) Ferric/ferrous ratio in silicate melts: a new model for 1 atm data with special emphasis on the effects of melt composition. Contrib Miner Petrol 173(12):98. https://doi.org/10.1007/s00410-018-1524-8
Brounce M, Stolper E, Eiler J (2017) Redox variations in Mauna Kea lavas, the oxygen fugacity of the Hawaiian plume, and the role of volcanic gases in Earth’s oxygenation. Proc Natl Acad Sci 114(34):8997–9002. https://doi.org/10.1073/pnas.1619527114
Brounce M, Cottrell E, Kelley KA (2019) The redox budget of the Mariana subduction zone. Earth Planet Sci Lett 528:115859. https://doi.org/10.1016/j.epsl.2019.115859
Brounce M, Stolper E, Eiler J (2022) The mantle source of basalts from Reunion Island is not more oxidized than the MORB source mantle. Contrib Miner Petrol 177(1):7. https://doi.org/10.1007/s00410-021-01870-w
Burgisser A, Scaillet B (2007) Redox evolution of a degassing magma rising to the surface. Nature 445(7124):194–197. https://doi.org/10.1038/nature05509
Canil D, O’Neill HSC (1996) Distribution of ferric iron in some upper-mantle assemblages. J Petrol 37(3):609–635. https://doi.org/10.1093/petrology/37.3.609
Caricchi L, Petrelli M, Bali E et al (2020) A data driven approach to investigate the chemical variability of clinopyroxenes from the 2014–2015 Holuhraun-Bárdarbunga Eruption (Iceland). Front Earth Sci 8:1–15. https://doi.org/10.3389/feart.2020.00018
Carmichael ISE (1991) The redox states of basic and silicic magmas: a reflection of their source regions? Contrib Miner Petrol 106(2):129–141. https://doi.org/10.1007/BF00306429
Cottrell E, Kelley K, Lanzirotti A et al (2009) High-precision determination of iron oxidation state in silicate glasses using XANES. Chem Geol 268(3–4):167–179. https://doi.org/10.1016/j.chemgeo.2009.08.008
Cottrell E, Lanzirotti A, Mysen B et al (2018) A Mössbauer-based XANES calibration for hydrous basalt glasses reveals radiation-induced oxidation of Fe. Am Miner 103:489–501. https://doi.org/10.2138/am-2018-6268
Cottrell E, Birner SK, Brounce M, et al (2022) Oxygen Fugacity Across Tectonic Settings. In: Moretti R, Neuville D (eds) Magma Redox Geochimistry, Geophysical Monograph 266. John Wiley & Sons, Inc., pp 33–61. https://doi.org/10.1002/9781119473206.ch3
Davis FA, Cottrell E (2021) Partitioning of Fe2O3 in peridotite partial melting experiments over a range of oxygen fugacities elucidates ferric iron systematics in mid-ocean ridge basalts and ferric iron content of the upper mantle. Contrib Miner Petrol 176(9):67. https://doi.org/10.1007/s00410-021-01823-3
Di Fiore F, Mollo S, Vona A, MacDonald A, Ubide T, Nazzari M, Romano C, Scarlato P (2021) Kinetic partitioning of major and trace cations between clinopyroxene and phonotephritic melt under convective stirring conditions: new insights into clinopyroxene sector zoning and concentric zoning. Chem Geol 584:120531. https://doi.org/10.1016/j.chemgeo.2021.120531
Downes MJ (1974) Sector and oscillatory zoning in calcic augites from M. Etna, Sicily. Contrib Miner Petrol 47(3):187–196. https://doi.org/10.1007/BF00371538
Dowty E (1976) Crystal structure and crystal growth: II. Sector zoning in minerals. Am Miner 61:460–469
Droop GTR (1987) A general equation for Estimating Fe3+ concentrations in ferromagnesian silicates and oxides from microprobe analyses, using stoichiometric criteria. Mineral Mag 51(361):431–435. https://doi.org/10.1180/minmag.1987.051.361.10
Dyar MD, Gunter ME, Delany JS et al (2002) Systematics in the structure and XANES spectra of pyroxenes, amphiboles, and micas as derived from oriented single crystals. Can Mineral 40(5):1375–1393. https://doi.org/10.2113/gscanmin.40.5.1375
Evans KA, Tomkins AG (2022) Redox variables and mechanisms in subduction magmatism and volcanism. In: Moretti R, Neuville DR (eds) Geophysical monograph series, 1st edn. Wiley, pp 63–91. https://doi.org/10.1002/9781119473206.ch4
Evans KA (2006) Redox decoupling and redox budgets: conceptual tools for the study of earth systems. Geology 34(6):489–492. https://doi.org/10.1130/G22390.1
Evans KA, Tomkins AG (2011) The relationship between subduction zone redox budget and arc magma fertility. Earth Planet Sci Lett 308(3–4):401–409. https://doi.org/10.1016/j.epsl.2011.06.009
Feig ST, Koepke J, Snow JE (2010) Effect of oxygen fugacity and water on phase equilibria of a hydrous tholeiitic basalt. Contrib Miner Petrol 160(4):551–568. https://doi.org/10.1007/s00410-010-0493-3
França ZT, Tassinari CC, Cruz JV et al (2006) Petrology, geochemistry and Sr-Nd-Pb isotopes of the volcanic rocks from Pico Island-Azores (Portugal). J Volcanol Geoth Res 156(1–2):71–89. https://doi.org/10.1016/j.jvolgeores.2006.03.013
Frost BR (1991) Introduction to oxygen fugacity and its petrologic importance. Rev Mineral Geochem 25:1–9
Gaetani GA (2016) The behavior of Fe\(^{\rm 3+ }\)/\(\Sigma\)Fe during partial melting of spinel lherzolite. Geochim Cosmochim Acta 185:64–77. https://doi.org/10.1016/j.gca.2016.03.019
Gaillard F, Scaillet B, Arndt NT (2011) Atmospheric oxygenation caused by a change in volcanic degassing pressure. Nature 478(7368):229–232. https://doi.org/10.1038/nature10460
Ghiorso MS, Evans BW (2008) Thermodynamics of rhombohedral oxide solid solutions and a revision of the Fe-Ti two-oxide geothermometer and oxygen-barometer. Am J Sci 308(9):957–1039. https://doi.org/10.2475/09.2008.01
Ghiorso MS, Sack RO (1995) Chemical mass transfer in magmatic processes IV. A revised and internally consistent thermodynamic model for the interpolation and extrapolation of liquid-solid equilibria in magmatic systems at elevated temperatures and pressures. Contrib Miner Petrol 119(2–3):197–212. https://doi.org/10.1007/BF00307281
Halldórsson SA, Bali E, Hartley ME et al (2018) Petrology and geochemistry of the 2014–2015 Holuhraun eruption, central Iceland: compositional and mineralogical characteristics, temporal variability and magma storage. Contrib Miner Petrol 173:64. https://doi.org/10.1007/s00410-018-1487-9
Hartley ME, Shorttle O, Maclennan J et al (2017) Olivine-hosted melt inclusions as an archive of redox heterogeneity in magmatic systems. Earth Planet Sci Lett 479:192–205. https://doi.org/10.1016/j.epsl.2017.09.029
Helz RT, Cottrell E, Brounce MN et al (2017) Olivine-melt relationships and Syneruptive Redox variations in the 1959 eruption of Kīlauea volcano as revealed by XANES. J Volcanol Geoth Res 333–334:1–14. https://doi.org/10.1016/j.jvolgeores.2016.12.006
Holland HD (2002) Volcanic gases, black smokers, and the great oxidation event. Geochim Cosmochim Acta 66(21):3811–3826. https://doi.org/10.1016/S0016-7037(02)00950-X
Holland TJB, Powell R (1998) An internally consistent thermodynamic data set for phases of petrological interest. J Metamorph Geol 16(3):309–343. https://doi.org/10.1111/j.1525-1314.1998.00140.x
Holland TJB, Green ECR, Powell R (2018) Melting of peridotites through to granites: a simple thermodynamic model in the system KNCFMASHTOCr. J Petrol 59(5):881–900. https://doi.org/10.1093/petrology/egy048
Hughes EC, Saper L, Liggins P et al (2023) The sulfur solubility minimum and maximum in silicate melt. J Geol Soc. https://doi.org/10.1144/jgs2021-125
Jennings ES, Holland TJB (2015) A simple thermodynamic model for melting of peridotite in the system NCFMASOCr. J Petrol. https://doi.org/10.1093/petrology/egv020
Jochum KP, Willbold M, Raczek I et al (2005) Chemical characterisation of the USGS reference glasses GSA-1G, GSC-1G, GSD-1G, GSE-1G, BCR-2G, BHVO-2G and BIR-1G using EPMA, ID-TIMS, ID-ICP-MS and LA-ICP-MS. Geostand Geoanal Res 29:285–302
Jugo PJ (2009) Sulfur content at sulfide saturation in oxidized magmas. Geology 37(5):415–418. https://doi.org/10.1130/G25527A.1
Kelley KA, Cottrell E (2009) Water and the oxidation state of subduction zone Magmas. Science 325(5940):605–607. https://doi.org/10.1126/science.1174156
Kouchi A, Sugawara Y, Kashima K et al (1983) Laboratory growth of sector zones clinopyroxenes in the system CaMgSi2O6 - CaTiAl\(_{{\rm 2}}\)O\(_{{\rm 6}}\). Contrib Miner Petrol 83:177–184
Kress VC, Carmichael ISE (1991) The compressibility of silicate liquids containing Fe2O3 and the effect of composition, temperature, oxygen fugacity and pressure on their redox states. Contrib Miner Petrol 108(1–2):82–92. https://doi.org/10.1007/BF00307328
Larsen LM (1976) Clinopyroxenes and Coexisting Mafic Minerals from the Alkaline Ilimaussaq Intrusion, South Greenland. J Petrol 17(2):258–290. https://doi.org/10.1093/petrology/17.2.258
Lee CTA, Leeman WP, Canil D et al (2005) Similar V/Sc systematics in MORB and arc basalts: Implications for the oxygen fugacities of their mantle source regions. J Petrol 46(11):2313–2336. https://doi.org/10.1093/petrology/egi056
Lee CTA, Luffi P, Chin EJ et al (2012) Copper systematics in Arc magmas and implications for Crust-Mantle differentiation. Science 336(6077):64–68. https://doi.org/10.1126/science.1217313
Leung IS (1974) Sector-zoned Titanaugites: morphology, crystal chemistry, and growth. Am Miner 59:127–138
Lindsley DH (1983) Pyroxene thermometry. Am Miner 68(5–6):477–493. https://doi.org/10.1007/BF00372872
Lindsley DH, Andersen DJ (1983) A two-pyroxene thermometer. J Geophys Res 88 Supplem:A887–A906. https://doi.org/10.1029/JB088iS02p0A887
Loughlin SC (1995) The evolution of the Eyjafjöll volcanic system, southern Iceland. PhD, Durham University, Durham
Luth RW, Canil D (1993) Ferric iron in mantle-derived pyroxenes and a new oxybarometer for the mantle. Contrib Miner Petrol 113(2):236–248. https://doi.org/10.1007/BF00283231
MacDonald A, Ubide T, Mollo S, Pontesilli A, Masotta M (2023) The influence of undercooling and sector zoning on Clinopyroxene-Melt equilibrium and thermobarometry. J Petrol 64(10):egad074. https://doi.org/10.1093/petrology/egad074
Mallmann G, O’Neill HSC (2009) The crystal/melt partitioning of V during mantle melting as a function of oxygen fugacity compared with some other elements (Al, P, Ca, Sc, Ti, Cr, Fe, Ga, Y, Zr and Nb). J Petrol 50(9):1765–1794. https://doi.org/10.1093/petrology/egp053
McCammon C (2021) Mössbauer spectroscopy with high spatial resolution: spotlight on geoscience. In: Yoshida Y, Langouche G (eds) Modern Mössbauer spectroscopy, vol 137. Springer Singapore, Singapore, pp 221–266. https://doi.org/10.1007/978-981-15-9422-9_5
McCanta MC, Dyar MD, Rutherford MJ et al (2004) Iron partitioning between basaltic melts and clinopyroxene as a function of oxygen fugacity. Am Miner 89(11–12):1685–1693. https://doi.org/10.2138/am-2004-11-1214
McGuire AV, Dyar MD, Ward KA (1989) Neglected Fe\(^{3+}\)/Fe\(^{2+}\) ratios - a study of Fe\(^{3+}\) content of megacrysts from alkali basalts. Geology 17(8):687–690. https://doi.org/10.1130/0091-7613(1989)017%3C0687:NFFRAS%3E2.3.CO;2
Mollo S, Del Gaudio P, Ventura G et al (2010) Dependence of clinopyroxene composition on cooling rate in basaltic magmas: implications for thermobarometry. Lithos 118(3–4):302–312. https://doi.org/10.1016/j.lithos.2010.05.006
Mollo S, Putirka KD, Misiti V et al (2013) A new test for equilibrium based on clinopyroxene-melt pairs: clues on the solidification temperatures of Etnean alkaline melts at post-eruptive conditions. Chem Geol 352:92–100. https://doi.org/10.1016/j.chemgeo.2013.05.026
Mollo S, Blundy JD, Scarlato P et al (2018) An integrated P-T-H2O-lattice strain model to quantify the role of clinopyroxene fractionation on REE+Y/HFSE patterns of mafic alkaline magmas: application to eruptions at Mt. Etna. Earth-Sci Rev 185:32–56. https://doi.org/10.1016/j.earscirev.2018.05.014
Mollo S, Moschini P, Ubide T, MacDonald A, Vetere F, Nazzari M, Misiti V, Miyajima N, Melai C, Di Genova D, Vona A, Di Fiore F, Romano C (2023) Kinetic partitioning of trace cations between zoned clinopyroxene and a variably cooled-decompressed alkali basalt: thermodynamic considerations on lattice strain and electrostatic energies of substitution. Geochim Cosmochim Acta 361:40–66. https://doi.org/10.1016/j.gca.2023.10.012
Morimoto N, Fabries J, Ferguson AK et al (1988) Nomenclature of pyroxenes. Am Miner 73:1123–1133. https://doi.org/10.1007/BF01226262
Moussallam Y, Oppenheimer C, Scaillet B et al (2014) Tracking the changing oxidation state of Erebus magmas, from mantle to surface, driven by magma ascent and degassing. Earth Planet Sci Lett 393:200–209. https://doi.org/10.1016/j.epsl.2014.02.055
Moussallam Y, Edmonds M, Scaillet B et al (2016) The impact of degassing on the oxidation state of basaltic magmas: a case study of Kilauea volcano. Earth Planet Sci Lett 450:317–325. https://doi.org/10.1016/j.epsl.2016.06.031
Moussallam Y, Longpré MA, McCammon CA et al (2019) Mantle plumes are oxidised. Earth Planet Sci Lett 527:115798. https://doi.org/10.1016/j.epsl.2019.115798
Musu A, Corsaro RA, Higgins O et al (2023) The magmatic evolution of South-East Crater (Mt Etna) during the February-April 2021 sequence of lava fountains from a mineral chemistry perspective. Bull Volcanol 85(5):33. https://doi.org/10.1007/s00445-023-01643-2
Muth MJ, Cottrell E (2023) No detectable redox exchange between sulfur and iron during rapid cooling of basalts. Earth Planet Sci Lett 616:118210. https://doi.org/10.1016/j.epsl.2023.118210
Neave DA, Maclennan J (2020) Clinopyroxene dissolution records rapid Magma ascent. Front Earth Sci 8:188. https://doi.org/10.3389/feart.2020.00188
Neave DA, Putirka KD (2017) A new clinopyroxene-liquid barometer, and implications for magma storage pressures under Icelandic rift zones. Am Miner 102:777–794. https://doi.org/10.2138/am-2017-5968
Neave DA, Passmore E, Maclennan J et al (2013) Crystal-melt relationships and the record of deep mixing and crystallization in the AD 1783 Laki eruption, Iceland. J Petrol 54(8):1661–1690. https://doi.org/10.1093/petrology/egt027
Neave DA, Maclennan J, Hartley ME et al (2014) Crystal storage and transfer in basaltic systems: the Skuggafjöll eruption, Iceland. J Petrol 55(12):2311–2346. https://doi.org/10.1093/petrology/egu058
Neave DA, Bali E, Guðfinnsson GH et al (2019) Clinopyroxene-liquid equilibria and geothermobarometry in natural and experimental tholeiites: the 2014–2015 Holuhraun eruption, Iceland. J Petrol 60:1653–1680. https://doi.org/10.1093/petrology/egz042
Neave D, Stewart A, Hartley M et al (2024) Re-evaluating stoichiometric estimates of iron valence in magmatic clinopyroxene crystals. Contrib Miner Petrol 179(5):17. https://doi.org/10.1007/s00410-023-02080-2
Nicklas R, Hahn R, Day J (2022) Oxidation of La Réunion lavas with MORB-like fO2 by assimilation. Geochem Perspect Lett 20:32–36. https://doi.org/10.7185/geochemlet.2205
Nicklas RW, Hahn RK, Willhite LN et al (2022) Oxidized mantle sources of HIMU- and EM-type Ocean Island Basalts. Chem Geol 602:120901. https://doi.org/10.1016/j.chemgeo.2022.120901
Nikkola P, Bali E, Kahl M et al (2019) Mid-crustal storage and crystallization of Eyjafjallajökull. Jökull 69:77–96
Novella D, Maclennan J, Shorttle O, Prytulak J, Murton BJ (2020) A multi-proxy investigation of mantle oxygen fugacity along the Reykjanes Ridge. Earth Planet Sci Lett 531:115973. https://doi.org/10.1016/j.epsl.2019.115973
O’Neill HSC (2021) The thermodynamic controls on sulfide saturation in silicate melts with application to ocean floor basalts. In: Neuville DR, Moretti R (eds) Magma Redox Geochemistry, Geophysical Monograph 266. American Geophysical Union, Washington D.C., pp 177–213. https://doi.org/10.1002/9781119473206.ch10
O’Neill HSC, Berry AJ, Mallmann G (2018) The oxidation state of iron in Mid-Ocean Ridge Basaltic (MORB) glasses: implications for their petrogenesis and oxygen fugacities. Earth Planet Sci Lett 504:152–162. https://doi.org/10.1016/j.epsl.2018.10.002
Oppenheimer C, Moretti R, Kyle PR et al (2011) Mantle to surface degassing of alkalic magmas at Erebus volcano. Antarctica. Earth and Planetary Science Letters 306(3–4):261–271. https://doi.org/10.1016/j.epsl.2011.04.005
Passmore E, Maclennan J, Fitton JG et al (2012) Mush disaggregation in basaltic magma chambers: Evidence from the AD 1783 Laki eruption. J Petrol 53(12):2593–2623. https://doi.org/10.1093/petrology/egs061
Pilet S, Ulmer P, Villiger S (2010) Liquid line of descent of a basanitic liquid at 1.5 GPa: constraints on the formation of metasomatic veins. Contrib Miner Petrol 159(5):621–643. https://doi.org/10.1007/s00410-009-0445-y
Powell R, Holland TJB, Worley N (1998) Calculating phase diagrams involving solid solutions via non-linear equations, with examples using THERMOCALC. J Metamorph Geol 16:577–588. https://doi.org/10.1111/j.1525-1314.1998.00157.x
Putirka KD (2008) Thermometers and barometers for volcanic systems. Rev Mineral Geochem 69(1):61–120. https://doi.org/10.2138/rmg.2008.69.3
Putirka KD, Johnson M, Kinzler RJ et al (1996) Thermobarometry of mafic igneous rocks based on clinopyroxene-liquid equilibria, 0–30 kbar. Contrib Miner Petrol 123(1):92–108. https://doi.org/10.1007/s004100050145
Reay A, Johnstone R, Kawachi Y (1989) Kaersutite, a possible international microprobe standard. Geostand Geoanal Res 13(1):187–190. https://doi.org/10.1111/j.1751-908X.1989.tb00471.x
Riel N, Kaus BJP, Green ECR et al (2022) MAGEMin, an efficient Gibbs energy minimizer: application to igneous systems. Geochem Geophys Geosyst 23(7):1–27. https://doi.org/10.1029/2022GC010427
Robinson P (1980) The composition space of terrestrial pyroxenes -internal and external limits. Rev Mineral Geochem 7:419–494. https://doi.org/10.1515/9781501508257-013
Roeder PL, Emslie RF (1970) Olivine-liquid equilibrium. Contrib Miner Petrol 29(4):275–289. https://doi.org/10.1007/BF00371276
Rudra A, Hirschmann MM (2022) Fe3+ partitioning between clinopyroxene and silicate melt at 1–2.5 GPa: implications for Fe3+ content of MORB and OIB source mantle. Geochim Cosmochim Acta 328:258–279. https://doi.org/10.1016/j.gca.2022.04.023. (Retracted)
Rudra A, Cottrell E, Hirschmann MM (2021) Experimental determination of ferric iron partitioning between pyroxene and melt at 100 kPa. Chem Geol 584:120532. https://doi.org/10.1016/j.chemgeo.2021.120532. (Retracted)
Sack RO, Ghiorso MS (1994) Thermodynamics of multicomponent pyroxenes: III. Calibration of Fe\(^{\text{2+ }}\)(Mg)\(_{\text{-1 }}\), TiAl\(_{\text{2 }}\)(MgSi\(_{\text{2 }}\))\(_{\text{-1 }}\), TiFe\(^{\text{3+ }}_{\text{2 }}\)(MgSi\(_{\text{2 }}\))\(_{\text{-1 }}\), AlFe\(^{\text{3+ }}\)(MgSi)\(_{\text{-1 }}\), NaAl(CaMg)\(_{\text{-1 }}\), Al\(_{\text{2 }}\)(MgSi)\(_{\text{-1 }}\) and Ca(Mg)\(_{\text{-1 }}\) exchange reactions between pyroxenes and silicate melts. Contrib Miner Petrol 118(3):271–296. https://doi.org/10.1007/BF00306648
Salazar-Naranjo AF, Vlach SRF (2023) New experimental constraints for the evolution and thermobarometry of alkali ultrabasic to intermediate igneous rocks. J Petrol 64(11):egad078. https://doi.org/10.1093/petrology/egad078
Saper LM, Stolper MB, Baker EM (2022) Fe2+-Mg partitioning between olivine and liquid at low oxygen fugacity: an experimental and thermodynamic framework. Contrib Miner Petrol 177:94. https://doi.org/10.1007/s00410-022-01955-0
Shorttle O (2015) Geochemical variability in MORB controlled by concurrent mixing and crystallisation. Earth Planet Sci Lett 424:1–14. https://doi.org/10.1016/j.epsl.2015.04.035
Sisson TW, Grove TL (1993) Experimental investigations of the role of H2O in calc-alkaline differentiation and subduction zone magmatism. Contrib Miner Petrol 113(2):143–166. https://doi.org/10.1007/BF00283225
Steven CJ, Dyar MD, McCanta M et al (2022) The absorption indicatrix as an empirical model to describe anisotropy in X-ray absorption spectra of pyroxenes. Am Miner 107(4):654–663. https://doi.org/10.2138/am-2021-7950
Stracke A (2012) Earth’s heterogeneous mantle: a product of convection-driven interaction between crust and mantle. Chem Geol 330–331:274–299. https://doi.org/10.1016/j.chemgeo.2012.08.007
Taracsák Z, Longpré MA, Tartèse R et al (2022) Highly oxidising conditions in volatile-rich El Hierro magmas: implications for ocean island magmatism. J Petrol 63:1–21. https://doi.org/10.1093/petrology/egac011
Thomson A, Maclennan J (2013) The distribution of olivine compositions in Icelandic basalts and picrites. J Petrol 54(4):745–768. https://doi.org/10.1093/petrology/egs083
Toplis MJ, Carroll MR (1995) An experimental study of the influence of oxygen Fugacity on Fe-Ti Oxide stability, phase relations, and mineral-melt equilibria in Ferro-Basaltic systems. J Petrol 36(5):1137–1170 (http://petrology.oxfordjournals.org/content/36/5/1137.abstract)
Ubide T, Mollo S, Zhao JX et al (2019) Sector-zoned clinopyroxene as a recorder of magma history, eruption triggers, and ascent rates. Geochim Cosmochim Acta 251:265–283. https://doi.org/10.1016/j.gca.2019.02.021
Ulmer P, Kägi R, Müntener O (2018) Experimentally derived intermediate to silica-rich arc magmas by fractional and equilibrium crystallization at 1.0 GPa: An evaluation of phase relationships, compositions, liquid lines of descent and oxygen fugacity. J Petrol 59:11–58. https://doi.org/10.1093/petrology/egy017/4866144
van Gerve TD, Neave DA, Wieser P et al (2024) The origin and differentiation of CO2-rich primary melts in Ocean Island volcanoes: integrating 3D X-ray tomography with chemical microanalysis of olivine-hosted melt inclusions from Pico (Azores). J Petrol. https://doi.org/10.1093/petrology/egae006
Weis FA, Skogby H, Troll VR et al (2015) Magmatic water contents determined through clinopyroxene: examples from the Western Canary Islands, Spain. Geochem Geophys Geosyst 16:2127–2146. https://doi.org/10.1002/2015GC005800
Weis D, Harpp KS, Harrison LN et al (2023) Earth’s mantle composition revealed by mantle plumes. Nat Rev Earth Environ. https://doi.org/10.1038/s43017-023-00467-0 (https://www.nature.com/articles/s43017-023-00467-0)
White JC, Ren M, Parker DF (2005) Variation in mineralogy, temperature, and oxygen fugacity in a suite of strongly peralkaline lavas and tuffs, Pantelleria, Italy. Canadian Mineralogist 43(4):1331–1347. https://doi.org/10.2113/gscanmin.43.4.1331
Wieser PE, Kent AJR, Till CB (2023) Barometers Behaving Badly II: a Critical Evaluation of Cpx-Only and Cpx-Liq Thermobarometry in Variably-Hydrous Arc Magmas. J Petrol 64(8):egad050. https://doi.org/10.1093/petrology/egad050
Wieser PE, Kent AJR, Till CB et al (2023) Barometers behaving badly I: assessing the influence of analytical and experimental uncertainty on clinopyroxene thermobarometry calculations at crustal conditions. J Petrol 64(2):1–27. https://doi.org/10.1093/petrology/egac126
Wood BJ, Blundy JD (1997) A predictive model for rare earth element partitioning between clinopyroxene and anhydrous silicate melt. Contrib Miner Petrol 129(2–3):166–181. https://doi.org/10.1007/s004100050330
Wood BJ, Bryndzia LT, Johnson KE (1990) Mantle oxidation state and its relationship to tectonic environment and fluid speciation. Science 248(4953):337
Zanon V, Pimentel A, Auxerre M et al (2020) Unravelling the magma feeding system of a young basaltic oceanic volcano. Lithos 352–353:105325. https://doi.org/10.1016/j.lithos.2019.105325
Zindler A, Hart SR (1986) Chemical geodynamics. Ann Rev Earth Planet Sci 14(1):493–571. https://doi.org/10.1146/annurev.earth.14.1.493
Acknowledgements
We thank Lee Paul, David Oliver and Lewis Hughes at the University of Manchester for their help with sample preparation, thin section production and scanning electron microscopy, respectively. We also thank Stuart Kearns and Ben Buse for their help with electron probe microanalysis at the University of Bristol. We thank Luca Caricchi, Elizabeth Cottrell, Alice MacDonald and one anonymous reviewer for their constructive comments on earlier versions of this manuscript, and Othmar Müntener for his editorial handling. We note that Rudra et al. (2021) and Rudra and Hirschmann (2022) recently presented highly relevant clinopyroxene Fe\(^{3+}\)/\(\Sigma\)Fe determinations by Fe-XANES. However, these articles have been retracted and can no longer form part of our discussion. This work was supported by a NERC Independent Research Fellowship (NE/T011106/1) and a Royal Society Research Grant (RGS\(\backslash\)R1\(\backslash\)201344) to DAN.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Othmar Müntener.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Appendix A:Scheme for assigning clinopyroxene components
Appendix A:Scheme for assigning clinopyroxene components
The following scheme for assigning clinopyroxene components is described in Part I and repeated here for reference:
-
1.
Calculate clinopyroxene cation fractions (\(X_{\mathrm{{cation}}}\)) from oxide concentrations on a six oxygen basis
-
2.
Determine clinopyroxene \(X_{{\textrm{Fe}}^{2+}}\) and \(X_{{\textrm{Fe}}^{3+}}\) contents following Droop (1987) encompassing the renormalisation of cations (including total Fe) by multiplying each cation by T/S as outlined in his point (iv); the total number of all original cations will now equal four and values of \(X_{{\textrm{Fe}}^{2+}}\) and \(X_{{\textrm{Fe}}^{3+}}\) can be used to convert FeO\(_{\mathrm{{T}}}\) into FeO and Fe\(_{2}\)O\(_{3}\)
-
3.
Determine the relative proportions of tetrahedral and octahedral Al (\(\mathrm {^{IV}}\)Al and \(\mathrm {^{VI}}\)Al, respectively) such that \(X_{\mathrm{{^{IV}}Al}} = 2 - X_{{\textrm{Si}}}\) and \(X_{\mathrm{{^{VI}}Al}} = X_{{\textrm{Al}}} - X_{\mathrm{{^{IV}}Al}}\); if \(X_{{\textrm{Si}}}\) exceeds 2 there is no \(X_{\mathrm{{^{IV}}Al}}\)
-
4.
Form Jd (NaAlSi\(_{2}\)O\(_{6}\)) from whichever is less between Na and \(\mathrm {^{VI}}\)Al such that \(X_{{\textrm{Jd}}} = X_{{\textrm{Na}}}\) or \(X_{{\textrm{Jd}}}= X_{\mathrm{{^{VI}}Al}}\)
-
5.
Form Ae (NaFe\(^{3+}\)Si\(_{2}\)O\(_{6}\)) from whichever is less between Na remaining after Jd formation and Fe\(^{3+}\) such that \(X_{{\textrm{Ae}}} = X_{{\textrm{Na}}}-X_{{\textrm{Jd}}}\) or \(X_{{\textrm{Ae}}} = X_{\mathrm{{Fe^{3+}}}}\)
-
6.
Form Np(NaFe\(^{2+}_{0.5}\)Ti\(_{0.5}\)Si\(_{2}\)O\(_{6}\)) from any Na remaining after Ae formation such that \(X_{{\textrm{Np}}} = X_{{\textrm{Na}}}-X_{{\textrm{Jd}}}-X_{{\textrm{Ae}}}\); only relevant for alkali clinopyroxenes
-
7.
Form Es (CaFe\(^{3+}\)AlSiO\(_{6}\)) from any Fe\(^{3+}\) remaining after Ae formation such that \(X_{{\textrm{Es}}} = X_{\mathrm{{Fe^{3+}}}}-X_{{\textrm{Ae}}}\)
-
8.
Form CaTs (CaAlAlSiO\(_{6}\)) from any \(X_{\mathrm{{^{VI}}Al}}\) remaining after Jd formation such that \(X_{\mathrm{{CaTs}}} = X_{\mathrm{{^{VI}}Al}}-X_{{\textrm{Jd}}}\)
-
9.
Form CaTi (CaTiAl\(_{2}\)O\(_{6}\)) from any Ti remaining after Np formation such that \(X_{\mathrm{{CaTi}}} = X_{{\textrm{Ti}}}-X_{{\textrm{Np}}}/2\); Np is only present in alkali clinopyroxenes
-
10.
Form CrAlTs (CaCrAlSiO\(_{6}\)) from Cr such that \(X_{\mathrm{{CrAlTs}}} = X_{{\textrm{Cr}}}\)
-
11.
Form DiHd (Ca(Mg,Fe\(^{2+}\),Mn)Si\(_{2}\)O\(_{6}\)) from any Ca remaining after Es, CaTs, CaTi and CrAlTs formation such that \(X_{\mathrm{{DiHd}}} = X_{{\textrm{Ca}}}-X_{{\textrm{Es}}}-X_{\mathrm{{CaTs}}}-X_{\mathrm{{CaTi}}}-X_{\mathrm{{CrAlTs}}}\)
-
12.
Form EnFs ((Mg,Fe\(^{2+}\),Mn)\(_{2}\)Si\(_{2}\)O\(_{6}\)) from any Mg, Fe\(^{2+}\) and Mn remaining after DiHd formation such that \(X_{\mathrm{{EnFs}}} = (X_{{\textrm{Mg}}}+X_{\mathrm{{Fe^{2+}}}}+X_{{\textrm{Mn}}})-X_{\mathrm{{DiHd}}}/2\)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Neave, D.A., Stewart, A.G., Hartley, M.E. et al. Iron valence systematics in clinopyroxene crystals from ocean island basalts. Contrib Mineral Petrol 179, 67 (2024). https://doi.org/10.1007/s00410-024-02144-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00410-024-02144-x