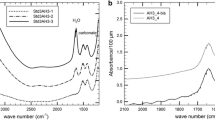
Abstract
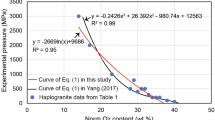
The H2O solubility in peralkaline haplogranitic melts has been experimentally determined as a function of pressure (27–200 MPa) and temperature (1123–1523 K). The compositions were based on Ab38Or34Qz28 (AOQ) with 4 and 8 wt% Na2O in excess. H2O solubility experiments were performed in an internally heated pressure vessel and quenched to glasses for analysis. For quantification of H2O contents in the glasses using FTIR analysis, the linear molar absorption coefficients as a function of Na2O excess with respect to AOQ composition were determined, as well as the glass densities as a function of H2O concentration. The H2O solubility increases with increasing pressure, decreasing temperature, and with increasing peralkalinity. A linear dependence between Na2O excess (wt%) and H2O solubility (wt%) was found. It has been previously shown that on a molar basis the different alkalis contribute similarly to the H2O solubility increase so that H2O solubility increases linearly with excess alkali (difference between mole fractions of alkalis and that of alumina). Thus, the dependence of H2O solubility on pressure, temperature and excess alkali obtained from the new data of this study allow a simple prediction of H2O solubility for peralkaline rhyolitic melts based on the excess alkali content. This new empirical model was tested with H2O solubility data from literature for peralkaline haplogranitic and natural peralkaline rhyolitic melt compositions, yielding good agreement (< 10% deviation) between predicted and observed H2O solubility, which is an improvement compared to previous models. The model can be applied to natural peralkaline rhyolitic melts that occur, e.g. on Pantelleria, Gran Canaria, or the East African Rift.










Similar content being viewed by others
Data availability statement
Tables, supplementary data and information can be found in the electronic supplementary material.
References
Acocella J, Tomozowa M, Watson EB (1984) The nature of dissolved water in sodium silicate glasses and its effect on various properties. J Non Cryst Solids 65:355–372
Allabar A, Nowak M (2020) High spatial resolution analysis of H2O in silicate glass using attenuated total reflection FTIR spectroscopy coupled with a focal plane array detector. Chem Geol 556:119833
Allabar A, Dobson KJ, Bauer CC, Nowak M (2020) Vesicle shrinkage in hydrous phonolitic melt during cooling. Contrib Mineral Pet 175:21
Balzer R, Behrens H, Waurischk T, Reinsch S, Müller R, Kiefer P, Deubener J, Fechtelkord M (2020) Water in alkali aluminosilicate glasses. Front Mater. https://doi.org/10.3389/fmats.2020.00085
Behrens H (2005) Determination of water solubilities in high-viscosity melts: an experimental study on NaAlSi3O8 and KAlSi3O8 melts. Eur J Mineral 7:905–920
Behrens H, Jantos N (2001) The effect of anhydrous composition on water solubility in granitic melts. Am Mineral 86:14–20
Behrens H, Nowak B (2003) Quantification of H2O speciation in silicate glasses and melts by IR Spectroscopy – in situ versus quench techniques. Phase Transitions 76:45–61
Behrens H, Romano C, Nowak M, Holtz F, Dingwell DB (1996) Near-infrared spectroscopic determination of water species in glasses of the system MAlSi3O8(M=Li, Na, K): an interlaboratory study. Chem Geol 128:41–63
Behrens H, Misiti V, Freda C, Vetere F, Botcharnikov RE, Scarlato P (2009) Solubility of H2O and CO2 in ultrapotassic melts at 1200 and 1250 °C and pressure from 50 to 500 MPa. Am Mineral 94:105–120
Bottinga Y, Richet P (1995) Silicate melts: The “anomalous” pressure dependence of the viscosity. Geochim Cosmochim Acta 59:2725–2731
Burnham CW, Jahns RH (1962) A method for determining the solubility of water in silicate melts. Am J Sci 260:721–745
Clarke B, Calder ES, Dessalegn D, Fontijn K, Cortés JA, Naylor M, Hutchinson W, Yirgu G (2019) Fluidal pyroclasts reveal the intensity of peralkaline rhyolite pumice cone eruptions. Nat Commun 10:2010
Dingwell DB, Harris DM, Scarfe CM (1984) The solubility of H2O in melts in the system SiO2 – Al2O3 – Na2O – K2O – at 1 to 2 kbars. J Geol 92:387–395
Dingwell DB, Holtz F, Behrens H (1997) The solubility of H2O in peralkaline and peraluminous granitic melts. Am Mineral 82:434–437
Duan X (2014) A general model for predicting the solubility behavior of H2O-CO2 fluids in silicate melts over a wide range of pressure, temperature and compositions. Geochim Cosmochim Acta 125:582–609
Gaillard F, Scaillet B, Pichavant M, Bény JM (2001) The effect of water and fO2 on the ferric-ferrous ratio of silicic melts. Chem Geol 174:255–273
Ghiorso M, Gualda GAR (2015) An H2O-CO2 mixed fluid saturation model compatible with rhyolite-MELTS. Contrib Mineral Pet 169:53
Giordano D, Russell JK, Dingwell DB (2008) Viscosity of magmatic liquids: a model. Earth Planet Sci Lett 271:123–134
Goranson RW (1931) The solubility of water in granitic magmas. Am J Sci 22:481–502
Holtz F, Behrens H, Dingwell DB, Johannes W (1995) H2O solubility in haplogranitic melts: Compositional, pressure, and temperature dependence. Am Mineral 80:94–108
Iacono-Marziano G, Morizet Y, Le Trong E, Gaillard F (2012) New experimental data and semi-empirical parameterization of H2O-CO2 solubility in mafic melts. Geochim Coscmochim Acta 97:1–23
Iacovino et al (2021) VESIcal Part I: An open-source thermodynamic model engine for mixed volatile (H2O-CO2) solubility in silicate melts. Earth Space Sci 8:e2020EA001584
Linnen R, Pichavant M, Holtz F (1996) The combined effects of fO2 and melt composition on SnO2 solubility and tin diffusivity in haplogranitic melts. Geochim Cosmochim Acta 60:4965–4976
Liu Y, Zhang Y, Behrens H (2005) Solubility of H2O in rhyolitic melts at low pressures and a new empirical model for mixed H2O–CO2 solubility in rhyolitic melts. J Volcanol Geotherm Res 143:219–235
Lowenstern J, Mahood GA (1991) New data on magmatic H2O contents of pantellerites, with implications for petrogenesis and eruptive dynamics at Pantelleria. Bull Volcanol 54:78–83
McDonald R, Sumita M, Schmincke HU, Baginski B, White JC, Ilnicki SS (2015) Peralkaline felsic magmatism at the Nemrut volcano, Turkey: impact of volcanism on the evolution of Lake Van (Anatolia) IV. Contrib Mineral Petrol 169:34
Moore G, Vennemann T, Carmichael ISE (1998) An empirical model for the solubility of H2O in magmas to 3 kbars. Am Mineral 83:36–42
Neave DA, Fabbro G, Herd RA, Petrone CM, Edmonds M (2012) Melting, differentiation and degassing at the Pantelleria Volcano, Italy. J Petrol 53:637–663
Newman S, Lowenstern JB (2002) VolatileCalc: a silicate melt–H2O–CO2 solution model written in Visual Basic for excel. Comput Geosci 28:597–604
Nowak M, Behrens H (1997) An experimental investigation on diffusion of water in haplogranitic melts. Contrib Mineral Petrol 126:365–376
Ohlhorst S, Behrens H, Holtz F (2001) Compositional dependence of molar absorptivities of near-infrared OH- and H2O bands in rhyolitic to basaltic glasses. Chem Geol 174:5–20
Papale P, Moretti R, Barbato D (2016) The compositional dependence of the saturation surface of H2O+CO2 fluids in silicate melts. Chem Geol 229:87–95
Preuss O, Marxer H, Ulmer S, Wolf J, Nowak M (2016) Degassing of hydrous trachytic Campi Flegrei and phonolitic Vesuvius melts: experimental limitations and chances to study homogeneous bubble nucleation. Am Mineral 101:859–875
Scaillet B, Macdonald R (2001) Phase relations of peralkaline silicic magmas and petrogenetic implications. J Petrol 42:825–845
Scaillet B, Macdonald R (2006) Experimental constraints on pre-eruption conditions of pantelleritic magmas: Evidence from the Eburru complex, Kenyan Rift. Lithos 91:95–108
Shishkina TA, Botcharnikov RE, Holtz F, Almeev RR, Jazwa AM, Jakubiak AA (2014) Compositional and pressure effects on the solubility of H2O and CO2 in mafic melts. Chem Geol 388:112–129
Sierralta M, Nowak M, Keppler H (2002) The influence of bulk composition on the diffusivity of carbon dioxide in sodium aluminosilicate melts. Am Mineral 87:1710–1716
Spickenbom K, Sierralta M, Nowak M (2010) Carbon dioxide and Argon diffusion in silicate melts: insights into the CO2 speciation in magmas. Geochim Cosmochim Acta 74:6541–6564
Stabile P, Radica F, Bello M, Behrens H, Carroll MR, Paris E, Giuli G (2018) H2O solubility in pantelleritic melts: pressure and alkali effects. J Min Geochem 195(1):1–9
Wieser PE, Iacovino K, Matthews S, Moore G, Allison CM (2022) VESical: 2. Critical approach to volatile solubility modeling using an open-source Python3 engine. Earth Space Sci 9:e2021EA001932
Yamashita S (1999) Experimental study of the effect of temperature on water solubility in natural rhyolite melt to 100 MPa. J Pet 40:1497–1507
Acknowledgements
This project was partially funded by the German Science Foundation (DFG NO378/12-2). We thank Penny Wieser and one anonymous reviewer for their very helpful comments on the manuscript. We thank Barbara Maier and Annette Flicker for technical support and maintenance of the IHPV and the FTIR spectrometer. We acknowledge the high-quality sample preparation by Simone Schafflick. We thank Harald Behrens at the Institute for Mineralogy at the Leibniz University of Hannover to enable us to use the Karl-Fischer-Titration apparatus. We thank Nikolaus Krumrein for performing four solubility experiments within the frame of his bachelor’s thesis.
Author information
Authors and Affiliations
Contributions
AA: conceptualization, glass synthesis, experiments and analysis of AOQ4 and AOQ8, computation, evaluation, and visualization; PP: glass synthesis, experiments, analysis and evaluation of AOQ2; DE: glass synthesis, computation and data collection; MN: conceptualization; AA wrote the original draft of the paper; all authors discussed the results and commented on the draft of the paper.
Corresponding author
Additional information
Communicated by Mark S Ghiorso.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Allabar, A., Petri, P.L., Eul, D. et al. An empirical H2O solubility model for peralkaline rhyolitic melts. Contrib Mineral Petrol 177, 52 (2022). https://doi.org/10.1007/s00410-022-01915-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00410-022-01915-8