Abstract
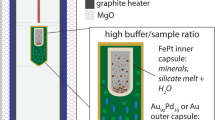
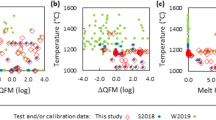
Vanadium has multiple oxidation states in silicate melts and minerals, a property that also promotes fractionation of its isotopes. As a result, vanadium isotopes vary during magmatic differentiation, and can be powerful indicators of redox processes at high temperatures if their partitioning behaviour can be determined. To quantify the partitioning and isotope fractionation factor of V between magnetite and melt, piston cylinder experiments were performed in which magnetite and a hydrous, haplogranitic melt were equilibrated at 800 °C and 0.5 GPa over a range of oxygen fugacities (\({f_{{{\text{O}}_{\text{2}}}}}\)), bracketing those of terrestrial magmas. Magnetite is isotopically light with respect to the coexisting melt, a tendency ascribed to the VI-fold V3+ and V4+ in magnetite, and a mixture of IV- and VI-fold V5+ and V4+ in the melt. The magnitude of the fractionation factor systematically increases with increasing log\({f_{{{\text{O}}_{\text{2}}}}}\) relative to the Fayalite–Magnetite–Quartz buffer (FMQ), from ∆51Vmag-gl = − 0.63 ± 0.09‰ at FMQ − 1 to − 0.92 ± 0.11‰ (SD) at ≈ FMQ + 5, reflecting constant V3+/V4+ in magnetite but increasing V5+/V4+ in the melt with increasing log\({f_{{{\text{O}}_{\text{2}}}}}\). These first mineral-melt measurements of V isotope fractionation factors underline the importance of both oxidation state and co-ordination environment in controlling isotopic fractionation. The fractionation factors determined experimentally are in excellent agreement with those needed to explain natural isotope variations in magmatic suites. Furthermore, these experiments provide a useful framework in which to interpret vanadium isotope variations in natural rocks and magnetites, and may be used as a potential fingerprint the redox state of the magma from which they crystallise.







Similar content being viewed by others
References
Anbar AD, Roe JE, Barling J, Nealson KH (2000) Nonbiological fractionation of iron isotopes. Science 288:126–128
Arató R, Audétat A (2017) Experimental calibration of a new oxybarometer for silicic magmas based on vanadium partitioning between magnetite and silicate melt. Geochim Cosmochim Acta 209:284–295
Ardia P, Giordano D, Schmidt MW (2008) A model for the viscosity of rhyolite as a function of H2O-content and pressure: a calibration based on centrifuge piston cylinder experiments. Geochim Cosmochim Acta 72:6103–6123
Balan E, De Villiers JPR, Eeckhout SG, Glatzel P, Toplis MJ, Fritsch E, Allard T, Galoisy L, Calas G (2006) The oxidation state of vanadium in titanomagnetite from layered basic intrusions. Am Mineral 91:953–956
Baldridge WS, McGetchin TR, Frey FA, Jarosewich E (1973) Magmatic evolution of Hekla, Iceland. Contrib Mineral Petrol 42(3):245–258
Bédard JH (2010) Parameterization of the Fe–Mg exchange coefficient (K D) between clinopyroxene and silicate melts. Chem Geol 274(3–4):169–176
Behrens H, Jantos N (2001) The effect of anhydrous composition on water solubility in granitic melts. Am Mineral 86(1–2):14–20
Berry AJ, Stewart GA, O’Neill HSC, Mallmann G, Mosselmans JFW (2018) A re-assessment of the oxidation state of iron in MORB glasses. Earth Planet Sci Lett 483:114–123
Bigeleisen J, Mayer MG (1947) Calculation of equilibrium constants for isotopic exchange reactions. J Chem Phys 15(5):261–267
Boettcher AL, Mysen BO, Allen JC (1973) Techniques for the control of water fugacity and oxygen fugacity for experimentation in solid-media high-pressure apparatus. J Geophys Res 78:5898–5901
Borisov A, Behrens H, Holtz F (2017) Effects of strong network modifiers on Fe3+/Fe2+ in silicate melts: an experimental study. Contrib Mineral Petrol 172(5):34
Bosi F, Halenius U, Skogby H (2009) Crystal chemistry of the magnetite-ulvospinel series. Am Mineral 94:181–189
Boutroy E, Dare SAS, Beaudoin G, Barnes S-J, Lightfoot PC (2014) Magnetite composition in Ni–Cu–PGE deposits worldwide: application to mineral exploration. J Geochem Exp 145:64–81
Bowen NL, Schairer JF, Willems HMV (1930) The ternary system Na2SiO3–Fe2O3–SiO2. Am J Sci 20:405–455
Brounce MN, Kelley KA, Cottrell E (2014) Variations in Fe3+/∑Fe of mariana arc basalts and mantle wedge \({f_{{{\text{O}}_{\text{2}}}}}\). J Petrol 55(12):2513–2536
Canil D (1997) Vanadium partitioning and the oxidation state of Archaean komatiite magmas. Nature 389:842–845
Canil D (1999) Vanadium partitioning between orthopyroxene, spinel and silicate melt and the redox states of mantle source regions for primary magmas. Geochim Cosmochim Acta 63:557–572
Canil D, Fedortchouk Y (2001) Olivine-liquid partitioning of vanadium and other trace elements, with applications to modern and ancient picrites. Can Mineral 39(2):319–330
Canil D, Grondahl C, Lacourse T, Pisiak LK (2016) Trace elements in magnetite from porphyry Cu–Mo–Au deposits in British Columbia, Canada. Ore Geol Rev 72:1116–1128
Carmichael ISE (1991) The redox states of basic and silicic magmas: a reflection of their source regions? Contrib Mineral Petrol 106:129–141
Chapman JB, Mason TFD, Weiss DJ, Coles BJ, Wilkinson JJ (2006) Chemical Separation and Isotopic Variations of Cu and Zn From Five Geological Reference Materials. Geostand Geoanal Res 30(1):5–16
Chappell BW, White AJR (1974) Two contrasting granite types. Pac Geol 8:173–174
Chou I-M (1986) Permeability of precious metals to hydrogen at 2 kb total pressure and elevated temperatures. Am J Sci 286:638–658
Dare SAS, Barnes S-J, Beaudoin G (2012) Variation in trace element content of magnetite crystallized from a fractionating sulfide liquid, Sudbury, Canada: implications for provenance discrimination. Geochim Cosmochim Acta 88:27–50
Dare SAS, Barnes S-J, Beaudoin G, Meric J, Boutroy E, Potvin-Doucet C (2014) Trace elements in magnetite as petrogenetic indicators. Mineral Deposita 49:785–796
Dauphas N, Roskosz M, Alp EE, Neuville DR, Hu MY, Sio CK, Zhao J, Tissandier L, Médard E, Cordier C (2014) Magma redox and structural controls on iron isotope variations in Earth’s mantle and crust. Earth Planet Sci Lett 398:127–140
Dickenson MP, Hess PC (1986) The structural role and homogeneous redox equilibria of iron in peraluminous, metaluminous and peralkaline silicate melts. Contrib Mineral Petrol 92(2):207–217
Dieckmann R (1982) Defects and cation diffusion in magnetite (IV): nonstoichiometry and point defect structure of magnetite (Fe3–δO4). Ber Bunsenges Phys Chem 86:112–118
Eugster HP (1957) Heterogeneous reactions involving oxidation and reduction at high pressures and temperatures. J Chem Phys 26:1760–1761
Feig ST, Koepke J, Snow JE (2010) Effect of oxygen fugacity and water on phase equilibria of a hydrous tholeiitic basalt. Contrib Mineral Petrol 160(4):551–568
Fleet ME (1981) The structure of magnetite. Acta Crystallogr B37:917–920
Foden J, Sossi PA, Wawryk CM (2015) Fe isotopes and the contrasting petrogenesis of A-, I-and S-type granite. Lithos 212:32–44
Frost CD, Frost BR (2010) On ferroan (A-type) granitoids: their compositional variability and modes of origin. J Petrol 52(1):39–53
Fuchs JN (1825) Ueber ein neues Product aus Kieselerde und Kali. Arch Für die gesammte Naturlehre 5:385–412
Giuli G, Paris E, Mungall JE, Romano C, Dingwell DB (2004) V oxidation state and coordination number in silicate glasses by XAS. Am Mineral 89:1640–1646
Grigsby JD (1990) Detrital magnetite as a provenance indicator. J Sedim Res 60:940–951
Holtz F, Johannes W, Tamic N, Behrens H (2001) Maximum and minimum water contents of granitic melts generated in the crust: a reevaluation and implications. Lithos 56:1–14
Jackson WE, Farges F, Yeager M, Mabrouk PA, Rossano S, Waychunas GA, Solomon EI, Brown GE Jr (2005) Multi-spectroscopic study of Fe (II) in silicate glasses: Implications for the coordination environment of Fe (II) in silicate melts. Geochim Cosmochim Acta 69(17):4315–4332
Jarosewich E, Nelen JA, Norberg JA (1980) Reference samples for electron microprobe analysis. Geostand Geoanalytical Res 4:43–47
Jenner FE, O’Neill HSC, Arculus RJ, Mavrogenes JA (2010) The magnetite crisis in the evolution of arc-related magmas and the initial concentration of Au, Ag and Cu. J Petrol 51(12):2445–2464
Karner J, Papike JJ, Shearer CK (2006) Comparative planetary mineralogy: pyroxene major-and minor-element chemistry and partitioning of vanadium between pyroxene and melt in planetary basalts. Am Mineral 91(10):1574–1582
Katsura T, Wakihara M, Hara S-I, Sugihara T (1975) Some thermodynamic properties in spinel solid solutions with the Fe3O4 component. J Sol State Chem 13(1–2):107–113
Knipping JL, Behrens H, Wilke M, Goettlicher J, Stabile P (2015) Effect of oxygen fugacity on the coordination and oxidation state of iron in alkali bearing silicate melts. Chem Geol 411:143–154
Lee C-TA, Leeman WP, Canil D, Li ZXA (2005) Similar V/Sc systematics in MORB and arc basalts: implications for the oxygen fugacities of their mantle source regions. J Petrol 46:2313–2336
Mallmann G, O’Neill HSC (2009) The crystal/melt partitioning of V during mantle melting as a function of oxygen fugacity compared with some other elements (Al, P, Ca, Sc, Ti, Cr, Fe, Ga, Y, Zr and Nb). J Petrol 50:1765–1794
Mallmann G, O’Neill HSC (2013) Calibration of an empirical thermometer and oxybarometer based on the partitioning of Sc, Y and V between olivine and silicate melt. J Petrol 54(5):933–949
Massalski TB (1990) Binary alloy phase diagrams, 2nd edn. ASM International, Materials Park
Matjuschkin V, Brooker RA, Tattitch B, Blundy JD, Stamper CC (2015) Control and monitoring of oxygen fugacity in piston cylinder experiments. Contrib Mineral Petrol 169:1–16
Medard E, McCammon CA, Barr JA, Grove TL (2008) Oxygen fugacity, temperature reproducibility, and H2O contents of nominally anhydrous piston-cylinder experiments using graphite capsules. Am Mineral 93:1838–1844
Muan A (1963) Silver-palladium alloys As crucible material in studies of low-melting iron silicates. Ceram Bull 42:344–347
Mungall JE (2002) Empirical models relating viscosity and tracer diffusion in magmatic silicate melts. Geochim Cosmochim Acta 66(1):125–143
Nadoll P, Angerer T, Mauk JL, French D, Walshe J (2014) The chemistry of hydrothermal magnetite: a review. Ore Geol Rev 16:1–32
Nielsen RL, Forsythe LM, Gallahan WE, Fisk MR (1994) Major- and trace-element magnetite-melt equilibria. Chem Geol 117:167–191
Nielsen SG, Prytulak J, Halliday AN (2011) Determination of precise and accurate 51V/50V isotope ratios by MC-ICP-MS, part 1: chemical separation of vanadium and mass spectrometric protocols. Geostand Geoanal Res 35:293–306
Nielsen SG, Prytulak J, Wood BJ, Halliday AN (2014) Vanadium isotopic difference between the silicate Earth and meteorites. Earth Planet Sci Lett 389:167–175
O’Neill HSC (1987) Quartz-fayalite-iron and quartz-fayalite-magnetite equilibria and the free energy of formation of fayalite (Fe2SiO4) and magnetite (Fe3O4). Am Mineral 72(1–2):67–75
O’Neill HSC, Navrotsky A (1984) Cation distributions and thermodynamic properties of binary spinel solid solutions. Am Mineral 69:733–753
Pauling L (1929) The principles determining the structure of complex ionic crystals. J Am Chem Soc 51:1010–1026
Petric A, Jacob KT (1982) Thermodynamic properties of Fe3O4–FeV2O4 and Fe3O4–FeCr2O4 spinel solid solutions. J Am Ceram Soc 65(2):117–123
Prytulak J, Nielsen SG, Halliday AN (2011) Determination of precise and accurate 51V/50V isotope ratios by multi-collector ICP-MS, part 2: isotopic composition of six reference materials plus the allende chondrite and verification tests. Geostand Geoanal Res 35:307–318
Prytulak J, Nielsen SG, Ionov DA, Halliday AN, Harvey J, Kelley KA, Niu Y, Peate DW, Shimizu K, Sims KWW (2013) The stable vanadium isotope composition of the mantle and mafic lavas. Earth Planet Sci Lett 365:177–189
Prytulak J, Sossi PA, Halliday AN, Plank T, Savage PS, Woodhead JD (2017) Stable vanadium isotopes as a redox proxy in magmatic systems? Geochem Persp Lett 3(1):75–84
Radtke AS (1962) Coulsonite, FeV2O4, a spinel-type mineral from Lovelock, Nevada. Am Mineral 47:1284–1291
Righter K, Sutton SR, Newville M, Le L, Schwandt C, Uchida H, Lavina B, Downs RT (2006a) An experimental study of the oxidation state of vanadium in spinel and basaltic melt with implications for the origin of planetary basalt. Am Mineral 91:1643–1656
Righter K, Leeman W, Hervig R (2006b) Partitioning of Ni, Co and V between spinel-structured oxides and silicate melts: Importance of spinel composition. Chem Geol 227:1–25
Schauble E (2004) Applying stable isotope fractionation theory to new systems. Rev Mineral Geochem 55:65–111
Schindler M, Hawthorne FC, Baur WH (2000) Crystal Chemical aspects of vanadium: polyhedral geometries, characteristic bond valences, and polymerization of (Von) polyhedra. Chem Mater 12:1248–1259
Schuth S, Horn I, Brüske A, Wolff PE, Weyer S (2017) First vanadium isotope analyses of V-rich minerals by femtosecond laser ablation and solution-nebulization MC-ICP-MS. Ore Geo Rev 81:1271–1286
Shannon RT (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystall A 32(5):751–767
Shellnutt JG, Zhou MF, Zellmer GF (2009) The role of Fe–Ti oxide crystallization in the formation of A-type granitoids with implications for the Daly gap: an example from the Permian Baima igneous complex, SW China. Chem Geol 259(3–4):204–217
Sievwright RH, Wilkinson JJ, O’Neill HSC, Berry AJ (2017) Thermodynamic controls on element partitioning between titanomagnetite and andesitic–dacitic silicate melts. Contrib Mineral Petrol 172(8):62
Sisson TW, Grove TL (1993) Experimental investigations of the role of H2O in calc-alkaline differentiation and subduction zone magmatism. Contrib Mineral Petrol 113(2):143–166
Smith JF (1989) Phase diagrams of binary vanadium alloys. In: Monograph series on alloy phase diagrams. ASM International, Materials Park, p 251
Sossi PA, O’Neill HSC (2017) The effect of bonding environment on iron isotope fractionation between minerals at high temperature. Geochim Cosmochim Acta 196:121–143
Sossi PA, Halverson GP, Nebel O, Eggins SM (2015) Combined separation of Cu, Fe and Zn from rock matrices and improved analytical protocols for stable isotope determination. Geostand Geoanal Res 39(2):129–149
Sossi PA, Moynier F, Chaussidon M, Villeneuve J, Kato C, Gounelle M (2017) Early solar system irradiation quantified by linked vanadium and beryllium isotope variations in meteorites. Nat Astron 1:0055
Sutton SR, Karner J, Papike J, Delaney JS, Shearer C, Newville M, Eng P, Rivers M, Dyar MD (2005) Vanadium K edge XANES of synthetic and natural basaltic glasses and application to microscale oxygen barometry. Geochim Cosmochim Acta 69:2333–2348
Thornber CR, Roeder PL, Foster JR (1980) The effect of composition on the ferric-ferrous ratio in basaltic liquids at atmospheric pressure. Geochim Cosmochim Acta 44(3):525–532
Toplis MJ (2005) The thermodynamics of iron and magnesium partitioning between olivine and liquid: criteria for assessing and predicting equilibrium in natural and experimental systems. Contrib Mineral Petrol 149(1):22–39
Toplis MJ, Carroll MR (1995) An experimental study of the influence of oxygen fugacity on Fe–Ti oxide stability, phase relations, and mineral—melt equilibria in ferro-basaltic systems. J Petrol 36(5):1137–1170
Toplis MJ, Corgne A (2002) An experimental study of element partitioning between magnetite, clinopyroxene and iron-bearing silicate liquids with particular emphasis on vanadium. Contrib Mineral Petrol 144:22–37
Turner SP, Foden JD, Morrison RS (1992) Derivation of some A-type magmas by fractionation of basaltic magma: an example from the Padthaway Ridge, South Australia. Lithos 28(2):151–179
Urey HC (1947) The thermodynamic properties of isotopic substances. J Chem Soc 562–581
Wakihara M, Katsura T (1971) The phase equilibria in the FeO–Fe2O3–V2O3 system at 1500 °K. Bull Chem Soc Jpn 44(11):3043–3046
Woodland AB, O’Neill HSC (1997) Thermodynamic data for Fe-bearing phases obtained using noble metal alloys as redox sensors. Geochim Cosmochim Acta 61:4359–4366
Wu C, Mason TO (1981) Thermopower measurement of cation distributio in magnetite. J Am Ceram Soc 64(9):520–522
Wu F, Qin T, Li X, Liu Y, Huang JH, Wu Z, Huang F (2015) First-principles investigation of vanadium isotope fractionation in solution and during adsorption. Earth Planet Sci Lett 426:216–224
Wu F, Qi Y, Yu H, Tain S, Hou Z, Huang F (2016) Vanadium isotope measurement by MC-ICP-MS. Chem Geol 421:17–25
Young ED, Tonui E, Manning CE, Schauble EA, Macris CA (2009) Spinel–olivine magnesium isotope thermometry in the mantle and implications for the Mg isotopic composition of Earth. Earth Planet Sci Lett 288:524–533
Acknowledgements
PAS was funded by an Australian Postgraduate Award and an ANU Vice-Chancellor’s Scholarship. Analytical costs and JPs visit to ANU were funded by Australian Research Council Discovery Grant DP130101355 to H. St. C. O’Neill and J. Prytulak. Many thanks to Jeremy Wykes, Dave Clark and Dean Scott for discussions regarding the piston cylinder experiments, Graham Mortimer for facilitating the set-up of V isotopes at the RSES clean lab, and Les Kinsley for assistance and feedback on running V isotopes on the Neptune Plus. We greatly appreciate two thoughtful and comprehensive reviews by Alan Woodland and Dante Canil that helped improve many aspects of this study, and acknowledge perceptive comments from Adrian Fiege on an earlier version of this work. We are grateful to Othmar Müntener for his exemplary editorial handling.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Othmar Müntener.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sossi, P.A., Prytulak, J. & O’Neill, H.S.C. Experimental calibration of vanadium partitioning and stable isotope fractionation between hydrous granitic melt and magnetite at 800 °C and 0.5 GPa. Contrib Mineral Petrol 173, 27 (2018). https://doi.org/10.1007/s00410-018-1451-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00410-018-1451-8