Abstract
Childhood maltreatment is a risk factor for psychopathologies, and influences brain development at specific periods, particularly during early childhood and adolescence. This narrative review addresses phenotypic alterations in sensory systems associated with specific types of childhood maltreatment exposure, periods of vulnerability to the neurobiological effects of maltreatment, and the relationships between childhood maltreatment and brain structure, function, connectivity, and network architecture; psychopathology; and resilience. It also addresses neurobiological alterations associated with maternal communication and attachment disturbances, and uses laboratory-based measures during infancy and case–control studies to elucidate neurobiological alterations in reactive attachment disorders in children with maltreatment histories. Moreover, we review studies on the acute effects of oxytocin on reactive attachment disorder and maltreatment and methylation of oxytocin regulatory genes. Epigenetic changes may play a critical role in initiating or producing the atypical structural and functional brain alterations associated with childhood maltreatment. However, these changes could be reversed through psychological and pharmacological interventions, and by anticipating or preventing the emergence of brain alterations and subsequent psychopathological risks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Childhood maltreatment (CM), per the World Health Organization [1], is the abuse and neglect of children aged < 18 years. It includes all types of physical, sexual, and psychological/emotional violence, and the neglect of infants, children, and adolescents by parents, caregivers, and other authority figures, at home and in settings such as schools and orphanages. CM markedly increases the risks for adverse psychiatric outcomes. A recent comprehensive meta-analysis showed that CM is a cross-diagnostic risk factor for a variety of psychiatric diagnoses [2]; CM is a risk factor for (1) psychiatric diagnoses such as anxiety, depression, psychosis, complex post-traumatic stress disorder (PTSD), substance use, and (2) syndromes, symptoms, and personality traits such as sleep disruption, antisocial behavior, suicide and non-suicidal self-injury, and it impairs cognitive abilities [3,4,5,6,7,8,9].
CM is a potent risk factor for atypical brain development with marked effects on morphology, function, and circuitry [10,11,12,13,14]. It exerts a more significant impact on trajectories of brain development than categorical psychiatric disorders [15,16,17]. The nature and intensity of the effects greatly depend on the type and timing of CM during sensitive developmental periods. Moreover, CM appears to be a critically important etiological factor as maltreated and non-maltreated individuals with the same primary psychiatric diagnosis appear to be clinically and neurobiologically distinct, with the maltreated ecophenotype showing an earlier onset, more comorbidities, a more pernicious clinical course, and an array of alterations in the structure, function, and connectivity of stress-susceptible brain regions [18].
Hundreds of studies have reported associations between CM and alterations in brain structure, function, connectivity, and network architecture in children or adults. A recent meta-analysis focused on gray matter (GM) abnormalities linked to CM, incorporating 30 Voxel-Based Morphometry (VBM) studies with 1264 individuals having a history of CM and 1201 non-maltreated controls [19]. The meta-analysis revealed distinctive GM changes in young individuals exposed to maltreatment, including reduced GM in the cerebellum and increased GM in the bilateral middle cingulate/paracingulate gyri and bilateral visual cortex when compared to maltreated adults. Notably, opposing GM changes were observed in the bilateral middle cingulate/paracingulate gyri between maltreated adults (decreased) and maltreated children/adolescents (increased). These differences suggest that the impact of CM on GM varies based on the proximity to maltreatment events, potentially influencing the developmental trajectory of brain structure. This vast field cannot be covered in a single review article.
Maturation of the human brain is regionally specific, non-linear, and heterogenous between individuals [20,21,22]. Pediatric brain maturation is characterized by increased connectivity among disparate brain regions, environmentally driven specialization of brain circuitry, and a changing balance between earlier maturing limbic regions and later maturing prefrontal regions that continues to undergo substantial changes well into the third decade [21, 23]. Brain regions and fiber tracts have age-dependent developmental windows and may have sensitive periods when they are maximally susceptible to environmental factors [24,25,26,27]. Overall, trajectories of brain development are likely affected by CM during periods of rapid developmental changes, which predominantly occur during early postnatal development and adolescence [25, 28]. Healthy brain development requires exposure to an array of expected species-specific stimuli and protection from threats or stressors that may overwhelm the developing brain [29]. During childhood, caregivers play a pivotal role in providing the necessary stimulation and protection against the effects of stress; this role is best exercised through a secure attachment. Hence, early neglect, deprivation, and threats to the attachment bond may have particularly deleterious effects on brain development [30]. While early childhood is characterized by the overproduction of axons, dendrites, synapses, and receptors, adolescence is characterized by rapid pruning [31, 32]. In addition, adolescence is a peak period of vulnerability to the emergence of psychiatric disorders [33, 34] and susceptibility to maltreatment and peer bullying [35].
Sensory systems develop early as myelination of sensory pathways begins in utero and takes place at a rapid rate during the early infancy. Further, the development of sensory systems is experience dependent and highly plastic during childhood [36]. Hence, sensory systems are not only vulnerable to the effects of CM but may be affected in important ways by the quality of a child’s attachment to their primary caregivers. Indeed, attachment theory suggests that the quality of early relationships and attachment bonds with caregivers can significantly impact the development of sensory processing abilities [37]. Children who have experienced maltreatment, such as neglect, abuse, or inconsistent caregiving, may develop insecure or disorganized attachment styles [38] that can disrupt the normal development of sensory systems, leading to problems in perceiving, processing, and integrating sensory information. For example, children with insecure attachment patterns may exhibit heightened sensitivity or hypervigilance to sensory stimuli, resulting in sensory over-responsivity. They may struggle regulating their emotional responses to sensory input or filtering and organizing sensory information. In contrast, children with disorganized attachment patterns may display inconsistent or unpredictable responses to sensory input, causing challenges in effectively integrating sensory information and forming coherent perceptual experiences [38,39,40].
These sensory system problems can manifest in various ways, such as sensory seeking or avoidance behaviors, sensory sensitivities or aversions, poor modulation of sensory input, and difficulties with attention, self-regulation, and social interactions [37]. Understanding the relationship between CM, disturbed attachment and sensory system problems is crucial for interventions and support services to promote healthy sensory development and address sensory challenges in individuals who have experienced early adversity.
Delineating the effects of early adversity on trajectories of brain development is a complex challenge that researchers have studied in myriad ways. One approach has been to compare adults with different histories of CM to identify associations that may be reflective of the enduring neurobiological consequences of CM in participants who may have experienced the full range of adverse experiences across each year of childhood. Another approach has been to look more specifically at infants or children to identify early neurobiological differences related to CM or disrupted attachment during particular phases of development. Our aim in this review is to highlight some of the key findings that have emerged from these two different approaches.
This narrative review is divided into four chapters. “Diversity of studies on maltreatment-associated neurobiology” delves into the neurobiology associated with maltreatment. “Neurobiology and type and timing of maltreatment” focuses on the atypical consequences of various types of CM on the young adult brain morphology, particularly in the areas regulating sensory systems, which act as our primary filter to the outside world and are modified by experience during sensitive or critical periods [41]. The review emphasizes the significant impact of CM on brain structure, function, connectivity, and network architecture. In “Studies on maltreatment and attachment”, the discussion centers around attachment, including a prospective cohort study on neurobiological correlates of impaired maternal communication and infant attachment and case–control studies on neurobiological alterations in Reactive attachment disorder (RAD), which emerges in some children as a consequence of extreme early social deprivation. Finally, “Effects of CM on genetics and hormones” explores the clinical and neurobiological effects of oxytocin on children, along with studies focusing on the methylation of glucocorticoid-regulated and oxytocinergic genes in maltreated children.
Thus, the central questions that this review answers are: (1) How does CM affect the sensory system and result in phenotypic changes? (2) Which sensitive periods in childhood and adolescence are most vulnerable to the neurobiological effects of abuse? (3) What is the relationship between CM and brain structure, function, connectivity, and network building? (4) How does CM affect psychopathology and resilience? The aim is to comprehensively understand the complex relationships between CM, brain development, psychopathology, and resilience by addressing the abovementioned questions and synthesizing the existing literature. We also conducted a targeted literature review in PubMed on the specific set of findings we bring in as evidence (e.g., CM, sensory systems, neurobiology of attachment, and related terms) to assess if there are more recent findings that either support what we have written or challenge it. This knowledge can contribute to developing effective interventions and support systems for abuse survivors.
Diversity of studies on maltreatment-associated neurobiology
Initially, imaging studies on the potential neurobiological effects of CM focused on children with verified exposure and psychopathology, notably PTSD [42,43,44], and adults with psychopathology and retrospectively recalled sexual abuse [45,46,47]. Subsequently, interest has explosively increased. This includes assessing the potential consequences of exposure to various types of CM [48,49,50,51,52,53,54], and influential studies on the neurobiological consequences of severe early neglect, such as the Romanian orphan studies [55,56,57,58,59,60,61,62]. Functional imaging studies have identified prominent effects of CM on systems involved in threat detection and response [63,64,65,66,67,68], reward processing [68,69,70,71,72,73], and autobiographical memory [74,75,76] in children and adults. Structural and functional connectivity studies have tested hypotheses regarding mechanisms responsible for these alterations in threat and reward response [77,78,79,80,81]. Studies have also revealed associations between CM and abnormalities in functional brain networks [82,83,84,85,86,87] and the structural and functional network architecture of the brain [11, 15, 88, 89]. Multiple studies have explored the link between CM, neurobiology, and risk of psychopathology [77, 90,91,92,93,94,95], including those assessing ecophenotypic differences between maltreated and non-maltreated individuals with the same psychiatric diagnosis [16, 73, 96,97,98]. Many individuals reporting exposure to CM are relatively resilient (e.g., have better than expected psychiatric outcomes), and studies have identified brain differences specifically associated with resilience [89, 99,100,101,102,103,104,105,106]. Resilience is not driven by a single biomarker, but by complex processes and influences across multiple levels. Few studies, however, have assessed the effects of treatment on CM-associated brain differences [107,108,109,110].
Despite the array of relevant studies, many essential gaps still exist. In most studies, there is a considerable and often uncertain time lapse between exposure to CM and measures of brain structure, function, and connectivity. Hence, little is known about factors mediating these brain changes (e.g., epigenetic modifications, inflammation, trophic factors, stress hormones) and how CM interacts with normal developmental processes [20, 111,112,113] to affect trajectories of brain development. Mandated reporting requirements are a challenge, prompting important longitudinal investigations, such as the Adolescent Brain and Cognitive Development study, to refrain from systematically collecting information on exposure to the types of early adversity that would need to be reported to child protective services.
Most studies examining the potential neurobiological effects of CM are cohort studies in which comparisons are made between exposed and unexposed cohorts to identify differences in brain measures and clinical outcomes. The alternative observational strategy is the case–control study in which participants are selected based on the presence or absence of specific clinical criteria (e.g., depression, PTSD) [114]. These groups can be compared for differences in exposure rates to CM and other factors. A vital advantage of the case–control study is that it can be effectively used to study risk factors for rare disorders, such as RAD.
Neurobiology and type and timing of maltreatment
CM alters brain structures, impacting the hippocampus, anterior cingulate, ventromedial and dorsomedial cortices, and fiber tracts. Teicher et al. reviewed consistent findings of altered amygdala response, reduced ventral striatal response, disrupted prefrontal–amygdala connectivity, and increased precuneus volume in maltreated individuals [115]. Despite similar psychiatric diagnoses, maltreated and non-maltreated individuals exhibit distinct clinical, neurobiological, and genetic differences. The impact of maltreatment on resilient individuals, without psychopathological symptoms, suggests underlying compensatory mechanisms for stress-related neurobiological changes.
Ohashi et al. used diffusion tensor imaging (DTI) MRI and tractography on 262 unmedicated, healthy 18–25-year-old individuals. Those with moderate-to-high maltreatment exposure (n = 140) displayed lower global network metrics, including degree, strength, and efficiency, indicating potential links to psychopathology. Major depression, anxiety, and ADHD (attention-deficit hyperactivity disorder) history did not independently influence global network measures, emphasizing the pivotal role of maltreatment in understanding network differences and psychopathology [15]. Another study examined corpus callosum (CC) abnormalities in psychiatric disorders and their association with maltreatment [116]. In 345 healthy 18–25-year-olds, AI predictive analytics identified maltreatment types and timings. Males showed CC alterations in axial diffusivity linked to emotional abuse or neglect, while females had alterations in radial diffusivity and fractional anisotropy associated with early physical neglect. Sex differences in CC were limited to the genu, emphasizing the need to consider maltreatment and sex in understanding the role of CC in psychiatric disorders.
Further, abnormal amygdala responses to threat-related stimuli are linked to various disorders. CM, a significant risk factor, correlates with atypical amygdala function. Zhu et al. investigated impact of maltreatment timing on amygdala response, finding prepubertal differences in associations. Understanding such nuances aids targeted interventions for at-risk youth [64]. Thus, neurobiological distinctions between maltreated and non-maltreated individuals underscore the need for DSM-5 updates, proposing a Developmental Trauma Disorder and modified diagnostic categories to enhance precision and treatment outcomes [117].
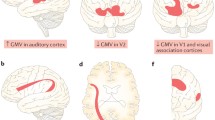
Previously, Tomoda and Teicher et al. investigated structural brain abnormalities with volumetric magnetic resonance imaging (MRI) in young adults with CM identifying CM-specific structural changes supporting the hypothesis that the nature of maltreatment matters [48,49,50,51,52,53, 118] (Fig. 1). Screenings were conducted on 1455 volunteers to select specific subsets of participants with moderate-to-severe exposure to one particular type of CM and minimal exposure to other types of CM or adversity for T1-weighted and diffusion tensor MRI.
Structural changes depending on the type of childhood maltreatment (CM) to see what brain structures are affected by exposure to CM and if the type of maltreatment matters [51,52,53, 118]. (Top from the left) Prefrontal cortex gray matter volume (GMV) reduction in participants who experienced harsh corporal punishment [118], visual cortex GMV reduction in participants who experienced repeated exposure to childhood sexual abuse [118], superior temporal gyrus GMV increase in participants who experienced parental verbal abuse [52], lingual gyrus GMV reduction in participants who witnessed interparental violence during childhood [53], and corpus callosum volume reduction in participants who experienced neglect [236]. (Middle) Right lateral and superior views of the dynamic sequence of GM maturation over the cortical surface. Copyright (2004) National Academy of Sciences, USA [23]. (Bottom from the left) Illustrations of physical abuse, sexual abuse, emotional abuse, and neglect
Childhood sexual abuse (CSA)
First, Tomoda et al. investigated brain structures in young adults with repeated exposure to CSA [118] unaccompanied by exposure to physical abuse, neglect, or witnessing domestic violence (WDV). Seventy-five percent of CSA victims experienced CSA by individuals outside their families. Participants with repeated exposure to CSA (n = 23, 20.2 ± 1.3 years) had a 12.6 and 18.1% GMV reduction in their right and left primary visual and visual association cortices, respectively, compared with healthy control participants (n = 14, 19.0 ± 1.1 years) [118]. This reduction was directly related to the duration of sexual abuse before 12 years of age. Moreover, cortical surface-based analysis indicated that the GMV of CSA participants was reduced in the left fusiform (face recognition), left middle occipital, and right lingual gyri (global aspects of figure recognition) regions. Heim et al. [119] reported thinning of the specific portion of the somatosensory cortex that is critical to the perception of touch in the clitoris and surrounding genital area of women who had experienced penetrative sexual abuse (n = 51, 27.0 ± 7.0 years). Bremner et al. [120] reported decreased blood flow in the visual association cortex of women with CSA and PTSD (n = 10 with PTSD, 35.0 ± 6.0 years; n = 12 without PTSD, 32.0 ± 8.0 years). Berman et al. [121] reported that GMV in the lingual gyrus and intracalcarine cortex of women who had experienced sexual assault was inversely correlated with their degree of assault-related self-blame (n = 68, 19–37 years).
Hubel and Wiesel [122] identified environmentally sensitive periods of brain development, particularly in the organization of the visual cortex. Tomoda et al. tested the hypothesis that there are regionally specific developmentally sensitive periods when brain regions are maximally affected by exposure to CM. In association with CSA, Tomoda et al. found reductions in hippocampal volume at ages 3–5 and 11–13 years, corpus callosum volume at ages 9–10 years, and frontal cortex volume at ages 14–16 years [26]. Tomoda et al. also reported that the degree of reduction in visual cortex GMV was directly related to the duration of sexual abuse before 12 years of age [118], which is consistent with Hubel and Wiesel’s observation that the mammalian visual cortex is highly plastic until puberty [122]. Subsequent studies have also provided evidence for regionally specific age-dependent windows of vulnerability to CM [10, 63, 64, 116, 123,124,125,126].
Parental verbal abuse (PVA)
Similarly, Tomoda et al. assessed regionally specific effects of PVA among individuals with moderate-to-severe exposure to PVA and minimal exposure to other forms of adversity from a healthy community sample [52]. Participants with PVA (n = 21, 21.2 ± 2.4 years) had a 14.1% GMV increase in the left superior temporal gyrus (STG) auditory cortex compared to healthy controls (n = 19, 21.9 ± 2.4 years) [52]. GMV in this cluster was associated most strongly with levels of maternal and paternal verbal aggression and inversely associated with parental education.
Further, diffusion tensor imaging tractography revealed that participants with PVA (n = 16, 21.9–37 years) had reduced integrity in three fiber pathways [49]. The most significant difference was in the arcuate fasciculus, which is believed to be a critical substrate for speech, language, and communication. Interestingly, some brain regions respond to stress with a reduction in dendritic arborization (e.g., hippocampus, prefrontal cortex) and others by an increase (e.g., amygdala) [127, 128], which may be related to changes in brain-derived neurotrophic factor levels [129]. Hypertrophy of the STG has been reported in youths with CM and PTSD [130] and in women with comorbid borderline personality disorder (BPD) and PTSD [131]; thus, hypertrophy may be a phenotypic adaptive response to stress in this structure.
Witnessing domestic violence (WDV)
Tomoda et al. investigated participants who experienced WDV during childhood [53] in a manner similar to that used in the PVA study [52]. Using VBM, Tomoda et al. found that participants (n = 22, 21.8 ± 2.4 years) who had visually witnessed multiple episodes of interparental violence during childhood had a 6.1% GMV reduction in the right lingual gyrus (Brodmann area [BA] 18) compared with healthy age-equivalent controls (n = 30, 21.6 ± 2.1 years) [53]. Thickness in this region, the secondary visual cortex, and left occipital pole was reduced bilaterally. Regional reductions in GMV and thickness were observed in both susceptible and resilient WDV subjects. WDV also affected the integrity of the left inferior longitudinal fasciculus (ILF) [49], which interconnects the visual and limbic systems and provides emotional and memory responses to what is seen. Olson et al. [132] confirmed that CM was associated with alterations in the integrity of the ILF and was sensitive to exposure to both physical/sexual and emotional forms of abuse (n = 32, 20–50 years). Further, Howell et al. [133], in a longitudinal cross-fostering study in primates, provided direct evidence that the ILF was affected explicitly by parental maltreatment. Brain regions involved in that process and conveying the adverse sensory input of the maltreatment may be specifically modified by this experience, particularly in participants exposed to a single type of maltreatment. Exposure to multiple types of maltreatment is more commonly associated with morphological alterations in corticolimbic regions [134]. These findings correlate with preclinical studies showing that the visual cortex is a highly plastic structure [122].
Harsh corporal punishment (HCP)
Tomoda et al. also investigated the regional effect of HCP during childhood [51]. The criteria for inclusion was spanking of the buttocks by hand with occasional (or more often) use of objects (e.g., strap, hairbrush) beginning before the child’s 12th birthday and lasting for at least 3 years, with a frequency of about 12 episodes or more per year. Participants who experienced HCP (n = 23, 21.7 ± 2.2 years) had a 19.1% GMV reduction in the right medial frontal gyrus (medial prefrontal cortex, BA10), 14.5% reduction in the left medial frontal gyrus (dorsolateral prefrontal cortex, BA9), and 16.9% reduction in the right anterior cingulate gyrus (BA24) compared with healthy age-equivalent controls (n = 22, 21.7 ± 1.8 years) [51]. The GMV in these identified regions showed significantly positively correlated with performance IQ. Further, HCP was associated with reduced blood flow in dopamine-rich regions, including the caudate, putamen, nucleus accumbens, dorsolateral prefrontal cortex, and substantia nigra [50].
Hence, CM alters the trajectories of brain development to affect sensory systems and circuits involved in threat detection, emotional regulation, and reward anticipation [115, 117, 135], but there is a need to elucidate how CM increases the risk of psychiatric disorders. Several studies have been conducted in the broad area of CM and its neurobiological effects. One suggests that alterations in amygdala volume vary in direction (smaller/larger volumes) and depend on the timing of traumatization [136]. The right amygdala GMV negatively correlated with retrospectively assessed maltreatment severity at 10–11 years of age [10]. Further, the effects on hippocampal volume in individuals who have experienced CM vary by sex during the sensitive period [126].
Studies on maltreatment and attachment
Neurobiological consequences of disrupted attachment
An increasing number of studies have assessed the potential impact of alterations in attachment behavior and maternal–infant communication on brain development. These studies include detailed evaluation of attachment behaviors in infancy, followed by longitudinal evaluation of clinical and neurobiological consequences of disorganized attachment. One series of neuroimaging studies was based on a 30-year follow-up of infants assessed for attachment behaviors [137]. The early-life stress cohort consisted of families at or below 200% of the federal poverty line. The quality of mother–infant interaction was directly observed and videotaped in the well-validated Strange Situation Procedure [138] at 18 months of age and reliably coded using the Atypical Maternal Behavior Instrument for Assessment and Classification [139]. Disorganized attachment and impaired maternal communication at 18 months were associated with enlarged left amygdala volume at around 30 years of age [140]. Contrastingly, right amygdala volume negatively correlated with retrospectively assessed maltreatment severity at 10–11 years of age [10]. The amygdala plays a critical role in threat detection and response, leading to the hypothesis that the left amygdala may be specialized for detecting threats to the attachment bond or abandonment. Conversely, the right amygdala may be specialized for detecting physical, sexual, or emotional threats [140]. This is appealing as the left hemisphere is generally more involved in motivating approach behaviors, which may be most helpful when the attachment bond is threatened, while the right hemisphere is more involved in motivating avoidance behaviors [141]. Khoury et al. [142] reported left hippocampal volume in adulthood was associated with maternal withdrawal during infancy, but not with other components of disrupted parenting. Left hippocampal volume was also linked to increased BPD features and tendencies towards suicidality and self-injury. In addition, left hippocampal volume partially mediated the connection between early maternal withdrawal and later suicidality/self-injury [142].
More recently, Lyons-Ruth et al. [143] assessed attachment behaviors in 57 mother–infant dyads—the infants underwent MRI during natural sleep at a mean age of 12.3 months. The aim was to explore the neural correlates of maternal withdrawal and negative/inappropriate interactions with infants during infancy, as these behaviors may represent different aspects of early deprivation and threat, respectively. Maternal withdrawal and negative/inappropriate behaviors were observed and coded from the Still-Face Paradigm when infants were four months old. Maternal withdrawal was associated with lower infant GMV, while negative/inappropriate interaction was linked to lower overall WMV. These effects were not influenced by age. Maternal withdrawal was also connected to reduced right hippocampal volume at older ages. Exploratory analyses of white matter tracts revealed that negative/inappropriate maternal behavior specifically correlated with reduced volume in the ventral language network.
Reactive attachment disorder (RAD)
The previous study explored the potential neurobiological consequences of impaired or withdrawn maternal behaviors leading to alterations within the typical constellation of attachment behaviors (i.e., secure, insecure, disorganized). However, more severe neglect/deprivation can cause profound alterations in attachment-related behaviors, as manifested in Reactive attachment disorder (RAD). RAD is characterized by persistent difficulties in social and emotional relationships and emotionally withdrawn/inhibited behaviors toward caregivers. The belief that CM (specifically social–emotional neglect) is of critical importance in the genesis of this disorder is apparent as a DSM-5 diagnosis requires either social neglect or deprivation in the form of a persistent lack of having the basic emotional needs for comfort, stimulation, and affection met by caregiving adults or rearing in unusual circumstances that severely limit opportunities to form selective attachments [144]. Children with RAD display difficulties with cognition and behavior, and they manifest social and emotional disturbances [145, 146]. They will likely experience disturbed and developmentally inappropriate social interactions throughout adulthood [147, 148]. The prevalence of RAD is approximately 1% in the general population [149] and 19–40% in children in foster care [150, 151]. Hence, case–control studies play an important role in delineating the underlying neurobiological alterations associated with profound alterations in attachment behaviors. Our laboratory is one of the few research institutions that have investigated RAD using brain imaging [123, 152,153,154]. Due to severe maltreatment, the reward and sensory system pathways mediating emotion regulation are altered in children with RAD compared to those in healthy controls [123, 125, 152,153,154]. In RAD, the brain appears less likely to experience a sense of accomplishment. RAD might be associated with altered brain function and pathways affecting emotion regulation, leading to core RAD symptoms such as the absence of focused attachment behavior toward a preferred caregiver and emotion regulation disturbances [155, 156]. Contrarily, Leblanc et al. [157] examined attachment stability to the mother in 15-month-old infants and subsequently measured their regional brain GMV at 10 and 11 years of age. More stable attachments were associated with more significant GMV in the superior temporal sulcus, temporal–parietal junction, and precentral gyrus. Thus, attachment stability may influence the development of brain regions involved in social cognition and emotion.
Neurobiology of attachment and role of oxytocin (OXT)
Attachment is the deep emotional bond that forms between an infant and their primary caregiver, typically the mother [37]. It is a fundamental aspect of human development that is essential in shaping an individual’s relationships and overall well-being. The quality of attachment bonds formed during infancy and childhood has long-term effects on a person’s social, emotional, and cognitive development. Attachment relationships formed during this period serve as models for future relationships and influence a person’s ability to form and maintain healthy social connections throughout life. Attachment bonds are believed to be established through infant–caregiver interactions that are characterized by responsiveness, sensitivity, and consistent care. A secure attachment is formed when the caregiver is consistently available and provides a responsive, safe, nurturing environment. This secure foundation allows the infant to explore their surroundings, knowing that they can seek comfort and support from the caregiver as needed.
The neuropeptide OXT has been implicated in mediating numerous prosocial effects ranging from approach/avoidance behavior [158] to interpersonal trust [159]. Oxytocinergic neurons project to brain regions involved in social and maternal behavior [30]. Animal and human studies showed that OXT is critical for mother–infant bonding and pair-bonding (i.e., secure attachment formation with a primary caregiver) [160, 161]. Bonds are based on OXT and dopamine action within the nucleus accumbens that promote the synaptic plasticity required for bonding with the infant. Attachment between a parent and their infant induces the release of OXT, which may mediate anti-stress effects [162,163,164]. Notably, early-life experiences appear to have a long-lasting effect on the OXT system.
A paucity of research concerns paternal nurturing behavior. For a considerable time, parenting has primarily been a maternal responsibility [37]. Therefore, researchers and academia have devoted more attention and resources to studying the parenting behavior of mothers [165, 166]. Research on the parenting behavior of fathers is relatively recent and has been influenced by traditional gender role biases. Further, social expectations and role images of fathers have constrained research on paternal behavior. Traditionally, fathers have been viewed as providers of financial support, while mothers are expected to provide emotional care and parenting [167]. Therefore, there has been a decreased interest in research on paternal child-rearing behaviors. Moreover, research on the parenting behaviors of fathers involves accessibility constraints; in some cultures and social contexts, the involvement of the father may be more limited than that of the mother. Work environments or institutional issues may also limit the ability of fathers to devote time to child-rearing. These challenges may prevent researchers from obtaining sufficient data and participants.
Contrastingly, CM dysregulates the brain’s oxytocinergic system, causing anxiety and dysfunctional attachment patterns [168, 169]. Evidence for long-term consequences of negative child–caregiver relationships include differences in OXT that are centrally involved in social relationships. For example, lower-than-average levels of OXT are found in the cerebrospinal fluid (CSF) of adult women with a history of CM [170] and in the urine of socially deprived children interacting with their mothers [171]. Furthermore, there is an association between a history of CM and abnormal OXT levels in the saliva and blood, specifically in adults: increased salivary OXT levels are seen even with a history of less severe forms of CM [172] and lower OXT receptor (OXTR) protein expression is found in peripheral blood mononuclear cells [168]. Children exposed to CM show lowered salivary OXT levels with visual attention to social cues [173], presumably hyper-regulated diurnal OXT secretion to cope with the environment, survive, and thrive [174]. There is a relationship between maladaptive mother–child interaction and OXT secretion, as evidenced by reports of decreased blood OXT levels in mothers with BPD who experience disrupted interaction with their child [175].
Further, harsh parenting experiences moderate the OXT effect on the use of excessive force while listening to infant crying sounds [176]. Thus, the current concepts of endogenous OXT effects emphasize the vital role of neuropeptide OXT in regulating mother–infant bonding and attachment in children subjected to maltreatment and lacking attachment or bonding with a primary caregiver [169, 173, 174, 177,178,179].
The proportion of children exposed to severe CM who can recover from adversity and demonstrate high competency levels across multiple domains (e.g., behavioral, emotional, and educational) is 10–25% [180]. The exogenous intranasal administration of OXT also alters attachment representations later in life, with less anxiously attached individuals remembering their mother as more caring after intranasal OXT administration [160]. Conversely, more anxiously attached individuals remember their mother as less caring [181]. Thus, early intervention and treatment combined with supplementary OXT administration may be one approach to foster the formation of a stable attachment in maltreated children with impaired attachments [182]. In a preliminary placebo-controlled study, intranasal OXT enhanced the activity of the bilateral caudate in children and adolescents with RAD, albeit at a relatively lenient statistical threshold [154]. Although OXT is ineffective for treating autism spectrum disorder symptoms [183], given its central role in attachment formation and prosociality, it may strengthen the neural basis of the reward system for attachment change in individuals with RAD. Further investigations are needed to elucidate the molecular and neural mechanisms of intranasal OXT and identify novel targets for maltreated children with trauma-related disorders such as RAD.
Effects of CM on genetics and hormones
Exposure to CM profoundly affects the developing brain [28, 135]. Neurological changes due to unfortunate early-life stress can cause lifelong psychiatric sequelae [184]. Hence, there is a great interest in understanding how CM gets “under the skin” to affect brain development. A fundamental mechanism may include early-life stress-induced epigenetic modifications. The human epigenome changes throughout life, and of particular relevance to brain development and risk for psychopathology are epigenetic modifications to genes involved in glucocorticoid regulation, leading to a blunted or enhanced hypothalamic–pituitary–adrenal (HPA) axis response to stress [185,186,187,188]. Frequent and chronic exposure of the developing brain to excessive glucocorticoids can affect gene expression, myelination, neural morphology, neurogenesis, synaptogenesis [28, 189, 190], and immune and inflammatory processes (for review, see Andersen [191]), leading to alterations in trajectories of brain development. The HPA axis is exceptionally responsive to stress during adolescence [192, 193]. Shirtcliff et al. introduced a novel hypothesis that specific stressors can produce different developmental effects during adolescence when the HPA and hypothalamic–pituitary–gonadal axes are jointly regulated (for review, see Shirtcliff et al. [194]). Sex hormones exert important organizing effects on brain development during the peripubertal period and work with stress hormones to foster biological and social adaption to stressful and novel events.
Epigenetic modification to OXT-related genes
Glucocorticoids regulate OXT synthesis and secretion in response to chronic stress [195] (Fig. 2). The possibility of epigenetic regulation of OXTR gene methylation and attachment formation has recently been assessed in several studies (for review, see Darling Rasmussen and Storebø [196]); however, conclusive evidence in humans is lacking. OXTR gene methylation density was associated with perinatal depression, attachment style, and trauma history, but attachment insecurity was not a significant mediator [197]. Per Ein-Dor et al. [198], attachment avoidance in young adults was specifically associated with increased OXTR and NR3C1 promoter methylation. We investigated how CM alters OXTR gene promoter methylation in children who have experienced CM compared with TD children (CM: n = 44 vs. TD: n = 41, 6–20 years). A specific region of the OXTR gene (CpG 5,6) was highly methylated in maltreated children and a neuroimaged subset (n = 24, 31) was associated with reduced orbitofrontal cortex GMV [199]. This makes sense, given that OXTR localizes in reward-associated circuits.
An overview of the possible role of epigenetic modification due to childhood maltreatment (CM). The long-term effects of CM on hypothalamic–pituitary–adrenal axis function, including dysregulation of cortisol secretion, are presumed to result in suppressed oxytocin (OXT) secretion and altered methylation of OXT-related genes and other genes. Thus, exposure to CM during sensitive periods may lead to altered brain developmental trajectories and an increased risk of psychopathology after childhood and adolescence. Epigenetic modifications, particularly DNA methylation is considered critical events in this process and can be targeted by pharmacological or psychotherapeutic interventions. Part of the figure was designed using resources from Biorender.com and http://www.istockphoto.com
Although most oxytocinergic system studies focus on the receptor gene, the involvement of the OXT gene that synthesizes the OXT peptide itself cannot be ignored. Haas et al. [200] reported that individuals with lower levels of OXT methylation (presumably linked to higher OXT expression) displayed more secure attachments, an improved ability to recognize emotional facial expressions, and greater superior temporal sulcus activity during social–cognitive functional MRI (fMRI) tasks than participants with higher OXT DNA methylation. Consistently, we found that the OXT gene promoter region was more widely methylated in children with CM than in TD controls. In addition, whole-brain VBM analysis showed that higher methylation was associated with reduced left superior parietal lobe GMV. This region is critically involved in visual spatial attention control [201], which may provide a neurobiological mechanism for our finding that maltreated children had deficient gaze fixation for the eye area of the human face and reduced salivary OXT [173]. We also found that OXT methylation was associated with reduced right putamen activation during a monetary reward task. The putamen is part of the reward system, and reduced reward system activation is one of the most consistently observed fMRI abnormalities in individuals with CM. The finding that the reward system response is greatly regulated by the degree of OXT gene methylation supports the hypothesis that maltreatment affects brain development through epigenetic alterations. Moreover, promoters of the OXT and OXTR genes may play a significant role in this process.
Conclusions and future directions
This narrative review discusses phenotypic alterations in sensory systems related to specific types of CM, vulnerable periods to its neurobiological effects, and the connections between maltreatment and brain structure, function, connectivity, and network architecture. It also covers psychopathology and resilience in this context. Future studies should embrace a complexity theory approach, investigating various biological levels and their temporal dynamics, which requires longitudinal studies on neurobiological mechanisms considering genetics, endocrine and immune systems, brain structure and function, cognition, and environmental factors. Embracing complexity can foster collaboration and guide efforts to promote resilience in those affected by CM (for review, see study by Ioannidis et al. [202]).
The review delves into neurobiological changes linked to disturbances in maternal communication and attachment, using laboratory measures and case–control studies to examine such alterations in children with maltreatment histories, including reactive attachment disorders. In addition, the acute effects of oxytocin on reactive attachment disorder, maltreatment, and the methylation of oxytocin regulatory genes are discussed. The passage highlights the potential role of epigenetic changes in initiating structural and functional brain alterations due to CM, suggesting the possibility of reversal through psychological and pharmacological interventions.
Understanding these variations is crucial for tailoring therapeutic interventions and support systems. Clinically, the identified brain structural and functional changes associated with CM underscore the need for a comprehensive approach to mental health care [18]. Healthcare professionals should consider the potential neurological impact of CM when assessing and designing treatment plans for individuals with such histories. Early detection of these brain alterations may inform targeted interventions aimed at mitigating the long-term psychological consequences of CM, promoting more effective therapeutic strategies, and improving overall clinical outcomes. Meanwhile, trauma-focused interventions such as Cognitive Behavior Therapy (CBT) trauma-focused strategies and Eye Movement Desensitization and Reprocessing (EMDR) and preventive measures should be implemented in clinical care to reduce CM and, ultimately, the prevalence of mental illness [203].
Further, we need to establish longitudinal assessments after CM to develop more effective diagnostic and treatment strategies, such as those based on regional brain structural and functional differences and epigenetic targets. Specifically, if the inhibition of OXT synthesis caused by promoter methylation results in adverse symptoms, early intervention and treatment combined with interventional supplementation of oxytocinergic function through intranasal OXT administration may be effective for improving clinical outcomes [154, 182, 204]. As most mental disorders result from deviations from the path of typical development, with different illnesses deviating at other times or in different ways (i.e., the neurodevelopmental hypothesis) [205, 206], a greater understanding of these specifics is the key to more effective preventative and interventional measures to ameliorate their effects. The comprehensive goal of elucidating the mechanisms and influences on brain development during adolescence is to improve the lives of children and their families. It is also crucial for optimizing healthy development [207]. To this end, we aim to conceive a synergy of genomic, neurobiological, psychosocial, and developmental components with technical advances in artificial intelligence and tele-present robotic care. Although ambitious, a strategy of this scope is needed to address these fundamental questions about the influence of early adversity on brain development and mental health, and to improve outcomes [117, 208,209,210].
This review has some limitations. First, there are several shortcomings, particularly “confirmation biases,” inherent to narrative reviews being less methodologically rigorous than are systematic reviews [211]. These biases can reduce the reliability and scientific validity of narrative reviews.
Second, the main limitation includes the small sample sizes and modest number of replication studies. However, while it may take thousands of participants to reliably identify brain structures associated with complex cognitive or mental health phenotypes [212], translational studies have shown that early experience exerts a prepotent effect on brain development, which can be reliably observed with small sample sizes [122, 213,214,215,216]. Moreover, the study designs employed in the works presented in the first part of this review—which focus on individuals who have suffered one type of CM (exceptions) and exclude those who have suffered multiple types (typical cases)—pose another risk of bias.
Third, it is challenging to delineate the neurobiological effects of exposure to specific types of CM and identify sensitive exposure periods, as many individuals were exposed to multiple types of maltreatment over many years. We have addressed this problem through selective recruitment [51,52,53, 118] and machine learning strategies to identify the most important predictor variables in highly collinear data sets [63, 64, 116, 123, 125, 126]. Nevertheless, there is a need to replicate studies using large sample sizes and prospective longitudinal designs.
Fourth, DNA samples such as blood, buccal mucosa, and saliva may not reflect brain conditions due to tissue specificity of methylation patterns. However, correlations between peripheral and brain methylation levels have recently been confirmed in independent databases constructed with Caucasian (https://han-lab.org/methylation/default/imageCpG) [217] and Japanese samples (https://snishit-amaze-cpg.web.app) [218]. Fifth, this review has focused on a few target genes, but a data-driven exploration at the genome-wide level is needed.
Lastly, the results of previous studies should be interpreted carefully, as prospective and retrospective evaluations of CM differ in identified population groups [219]. Among the 101 studies, 13 were prospective and 88 retrospective evaluation studies. In total, 38 used the Childhood Trauma Questionnaire (CTQ), 14 used the Maltreatment and Abuse Chronology of Exposure Scale (MACE), 13 used the Adverse Childhood Experiences Questionnaire (ACEs), and 5 used the Early Trauma Inventory [220] as self-report measures.
CM experiences can be assessed using various methods, including retrospective self-report measures and interviews. Self-report measures typically involve participants completing questionnaires that assess their history of maltreatment, such as the CTQ [221], MACE scale [222], or ACEs questionnaire [223]. These measures rely on participant recollection and the perception of their experiences. Interview-based assessments involve structured or semi-structured interviews conducted by trained professionals to obtain accurate information. These measures can include structured interviews, such as the Childhood Trauma Interview (CTI) [224] or Childhood Experience of Care and Abuse (CECA) interview [225], which involve detailed questioning about various forms of maltreatment. Ideally—using a combination of interview-based, self-report, and caregiver-report measures—a validated instrument designed to assess CM should be used to obtain accurate information. Further, earlier research concerning surveys conducted among children with legally verified CM, including children with CM verified by the Department of Social Services, is outdated, and this approach has as many disadvantages as advantages. These considerations suggest careful interpretation of the studies presented in the current review. Addressing the issue of heterogeneity in definitions of CM and data collection methods requires a comprehensive and standardized approach. By taking a multi-faceted and collaborative approach (1) establish clear definitions, (2) standardize assessment tools, (3) provide training and guidelines, (4) cross-disciplinary collaboration, and (5) longitudinal studies, it would be possible to mitigate the challenges associated with heterogeneity in definitions of CM and data collection methods, ultimately improving the comparability and reliability of research findings in this critical area.
Although methylation is considered the most stable form of epigenetic modification, there is evidence that DNA methylation is plastic from childhood to adulthood. Therapeutic interventions may alter methylation patterns and reduce the biological risks posed by CM [226,227,228]. Moreover, psychological and pharmacological interventions may reverse these epigenetic changes and prevent or delay the development of childhood-onset psychiatric disorders and psychopathology in adulthood caused by CM exposure [229].
Recent studies have focused on the role of CM in adolescent-onset psychiatric disorders [59, 230,231,232,233]. Exposure to stress during periods of rapid brain change, particularly during adolescence, may make youth exceptionally susceptible to developing mental illness [25]. Many cross-sectional and longitudinal cohort studies report potential sensitive period effects for psychopathology; however, for most studied outcomes, no consistent period of increased susceptibility has been identified (see review by Schaefer et al. [234]). One potential explanation is that studies reporting later ages of peek susceptibility [35, 64] include peer physical and emotional bullying as risk factors, whereas studies on earlier sensitive periods do not. Findings of distinct stages of vulnerability for regional brain development support the sensitive period hypothesis [235]. Although firm conclusions cannot be drawn now, identifying the most important type/time risk factors for various psychiatric disorders will be immensely valuable in developing strategies for prevention, preemption, and treatment. Further, we anticipate that sensitive exposure periods for developing specific psychiatric disorders will intersect with windows of vulnerability for brain systems critically involved in the genesis of these disorders and will shed new light on gene–development–environment interactions that lie at the heart of most psychiatric vulnerabilities [14].
References
World Health Organization (2006) Preventing child maltreatment: a guide to taking action and generating evidence. WHO Press
Hogg B, Gardoki-Souto I, Valiente-Gómez A, Rosa AR, Fortea L, Radua J, Amann BL, Moreno-Alcázar A (2023) Psychological trauma as a transdiagnostic risk factor for mental disorder: an umbrella meta-analysis. Eur Arch Psychiatry Clin Neurosci 273:397–410. https://doi.org/10.1007/s00406-022-01495-5
Degli Esposti M, Pinto Pereira SM, Humphreys DK, Sale RD, Bowes L (2020) Child maltreatment and the risk of antisocial behaviour: a population-based cohort study spanning 50 years. Child Abuse Negl 99:104281. https://doi.org/10.1016/j.chiabu.2019.104281
Dube SR, Felitti VJ, Dong M, Giles WH, Anda RF (2003) The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Pediatrics 37:268–277. https://doi.org/10.1016/s0091-7435(03)00123-3
Gardner MJ, Thomas HJ, Erskine HE (2019) The association between five forms of child maltreatment and depressive and anxiety disorders: a systematic review and meta-analysis. Child Abuse Negl 96:104082. https://doi.org/10.1016/j.chiabu.2019.104082
Knefel M, Lueger-Schuster B, Karatzias T, Shevlin M, Hyland P (2019) From child maltreatment to ICD-11 complex post-traumatic stress symptoms: the role of emotion regulation and re-victimisation. J Clin Psychol 75:392–403. https://doi.org/10.1002/jclp.22655
Teicher MH, Ohashi K, Khan A, Hernandez Garcia LC, Klengel T, Anderson CM, Silveri MM (2017) Does sleep disruption mediate the effects of childhood maltreatment on brain structure? Eur J Psychotraumatol 8:1450594. https://doi.org/10.1080/20008198.2018.1450594
Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, Read J, van Os J, Bentall RP (2012) Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull 38:661–671. https://doi.org/10.1093/schbul/sbs050
Wade M, Wright L, Finegold KE (2022) The effects of early life adversity on children’s mental health and cognitive functioning. Transl Psychiatry 12:244. https://doi.org/10.1038/s41398-022-02001-0
Pechtel P, Lyons-Ruth K, Anderson CM, Teicher MH (2014) Sensitive periods of amygdala development: the role of maltreatment in preadolescence. Neuroimage 97:236–244. https://doi.org/10.1016/j.neuroimage.2014.04.025
Teicher MH, Anderson CM, Ohashi K, Polcari A (2014) Childhood maltreatment: altered network centrality of cingulate, precuneus, temporal pole and insula. Biol Psychiatry 76:297–305. https://doi.org/10.1016/j.biopsych.2013.09.016
De Brito SA, Viding E, Sebastian CL, Kelly PA, Mechelli A, Maris H, McCrory EJ (2013) Reduced orbitofrontal and temporal grey matter in a community sample of maltreated children. J Child Psychol Psychiatry 54:105–112. https://doi.org/10.1111/j.1469-7610.2012.02597.x
Kelly PA, Viding E, Wallace GL, Schaer M, De Brito SA, Robustelli B, McCrory EJ (2013) Cortical thickness, surface area, and gyrification abnormalities in children exposed to maltreatment: neural markers of vulnerability. Biol Psychiatry 74:845–852. https://doi.org/10.1016/j.biopsych.2013.06.020
McCrory E, De Brito SA, Viding E (2010) Research review: the neurobiology and genetics of maltreatment and adversity. J Child Psychol Psychiatry 51:1079–1095. https://doi.org/10.1111/j.1469-7610.2010.02271.x
Ohashi K, Anderson CM, Bolger EA, Khan A, McGreenery CE, Teicher MH (2017) Childhood maltreatment is associated with alteration in global network fiber-tract architecture independent of history of depression and anxiety. Neuroimage 150:50–59. https://doi.org/10.1016/j.neuroimage.2017.02.037
Poletti S, Vai B, Smeraldi E, Cavallaro R, Colombo C, Benedetti F (2016) Adverse childhood experiences influence the detrimental effect of bipolar disorder and schizophrenia on cortico-limbic grey matter volumes. J Affect Disord 189:290–297. https://doi.org/10.1016/j.jad.2015.09.049
Teicher MH, Anderson CM, Polcari A (2012) Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci USA 109:E563–E572. https://doi.org/10.1073/pnas.1115396109
Teicher MH, Samson JA (2013) Childhood maltreatment and psychopathology: a case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am J Psychiatry 170:1114–1133. https://doi.org/10.1176/appi.ajp.2013.12070957
Li L, Jiang J, Wu B, Lin J, Roberts N, Sweeney JA, Gong Q, Jia Z (2023) Distinct gray matter abnormalities in children/adolescents and adults with history of childhood maltreatment. Neurosci Biobehav Rev. https://doi.org/10.1016/j.neubiorev.2023.105376
Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL (1999) Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 2:861–863. https://doi.org/10.1038/13158
Giedd JN (2004) Structural magnetic resonance imaging of the adolescent brain. Ann NY Acad Sci 1021:77–85. https://doi.org/10.1196/annals.1308.009
Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW (1999) In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci 2:859–861. https://doi.org/10.1038/13154
Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM (2004) Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 101:8174–8179. https://doi.org/10.1073/pnas.0402680101
Andersen SL (2003) Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev 27:3–18. https://doi.org/10.1016/s0149-7634(03)00005-8
Andersen SL, Teicher MH (2008) Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci 31:183–191. https://doi.org/10.1016/j.tins.2008.01.004
Andersen SL, Tomoda A, Vincow ES, Valente E, Polcari A, Teicher MH (2008) Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci 20:292–301. https://doi.org/10.1176/jnp.2008.20.3.292
Knudsen EI (2004) Sensitive periods in the development of the brain and behavior. J Cogn Neurosci 16:1412–1425. https://doi.org/10.1162/0898929042304796
Malave L, van Dijk MT, Anacker C (2022) Early life adversity shapes neural circuit function during sensitive postnatal developmental periods. Transl Psychiatry 12:306. https://doi.org/10.1038/s41398-022-02092-9
McLaughlin KA, Sheridan MA, Lambert HK (2014) Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev 47:578–591. https://doi.org/10.1016/j.neubiorev.2014.10.012
Strathearn L (2011) Maternal neglect: oxytocin, dopamine and the neurobiology of attachment. J Neuroendocrinol 23:1054–1065. https://doi.org/10.1111/j.1365-2826.2011.02228.x
Mallya AP, Wang HD, Lee HNR, Deutch AY (2019) Microglial pruning of synapses in the prefrontal cortex during adolescence. Cereb Cortex 29:1634–1643. https://doi.org/10.1093/cercor/bhy061
Teicher MH, Andersen SL, Hostetter JC Jr (1995) Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res 89:167–172. https://doi.org/10.1016/0165-3806(95)00109-q
Paus T, Keshavan M, Giedd JN (2008) Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 9:947–957. https://doi.org/10.1038/nrn2513
Solmi M, Radua J, Olivola M, Croce E, Soardo L, Salazar de Pablo G, Il Shin J, Kirkbride JB, Jones P, Kim JH, Kim JY, Carvalho AF, Seeman MV, Correll CU, Fusar-Poli P (2022) Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Mol Psychiatry 27:281–295. https://doi.org/10.1038/s41380-021-01161-7
Khan A, McCormack HC, Bolger EA, McGreenery CE, Vitaliano G, Polcari A, Teicher MH (2015) Childhood maltreatment, depression, and suicidal ideation: critical importance of parental and peer emotional abuse during developmental sensitive periods in males and females. Front Psychiatry 6:42. https://doi.org/10.3389/fpsyt.2015.00042
Hooks BM, Chen C (2007) Critical periods in the visual system: changing views for a model of experience-dependent plasticity. Neuron 56:312–326. https://doi.org/10.1016/j.neuron.2007.10.003
Keller H, Bard KA (2017) The cultural nature of attachment: contextualizing relationships and development. The MIT Press, London. https://doi.org/10.7551/mitpress/11425.001.0001
Arancibia M, Lutz M, Ardiles Á, Fuentes C (2023) Neurobiology of disorganized attachment: a review of primary studies on human beings. Neurosci Insights 18:26331055221145680. https://doi.org/10.1177/26331055221145681
Le TL, Geist R, Hunter J, Maunder RG (2020) Relationship between insecure attachment and physical symptom severity is mediated by sensory sensitivity. Brain Behav 10:e01717. https://doi.org/10.1002/brb3.1717
Spitoni GF, Zingaretti P, Giovanardi G, Antonucci G, Galati G, Lingiardi V, Cruciani G, Titone G, Boccia M (2020) Disorganized attachment pattern affects the perception of affective touch. Sci Rep 10:9658. https://doi.org/10.1038/s41598-020-66606-5
Takesian AE, Hensch TK (2013) Balancing plasticity/stability across brain development. Prog Brain Res 207:3–34. https://doi.org/10.1016/B978-0-444-63327-9.00001-1
Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, Reiss AL (2001) Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry 50:943–951. https://doi.org/10.1016/s0006-3223(01)01218-5
De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, Frustaci K, Ryan ND (1999) Developmental traumatology. Part II: brain development. Biol Psychiatry 45:1271–1284. https://doi.org/10.1016/s0006-3223(99)00045-1
Teicher MH, Ito Y, Glod CA, Andersen SL, Dumont N, Ackerman E (1997) Preliminary evidence for abnormal cortical development in physically and sexually abused children using EEG coherence and MRI. Ann NY Acad Sci 821:160–175. https://doi.org/10.1111/j.1749-6632.1997.tb48277.x
Anderson CM, Teicher MH, Polcari A, Renshaw PF (2002) Abnormal T2 relaxation time in the cerebellar vermis of adults sexually abused in childhood: potential role of the vermis in stress-enhanced risk for drug abuse. Psychoneuroendocrinology 27:231–244. https://doi.org/10.1016/s0306-4530(01)00047-6
Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, Khan S, Vaccarino LV, Soufer R, Garg PK, Ng CK, Staib LH, Duncan JS, Charney DS (2003) MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry 160:924–932. https://doi.org/10.1176/appi.ajp.160.5.924
Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B (1997) Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med 27:951–959. https://doi.org/10.1017/s0033291797005242
Choi J, Jeong B, Rohan ML, Polcari AM, Teicher MH (2009) Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biol Psychiatry 65:227–234. https://doi.org/10.1016/j.biopsych.2008.06.022
Choi J, Jeong B, Polcari A, Rohan ML, Teicher MH (2012) Reduced fractional anisotropy in the visual limbic pathway of young adults witnessing domestic violence in childhood. Neuroimage 59:1071–1079. https://doi.org/10.1016/j.neuroimage.2011.09.033
Sheu YS, Polcari A, Anderson CM, Teicher MH (2010) Harsh corporal punishment is associated with increased T2 relaxation time in dopamine-rich regions. Neuroimage 53:412–419. https://doi.org/10.1016/j.neuroimage.2010.06.043
Tomoda A, Suzuki H, Rabi K, Sheu YS, Polcari A, Teicher MH (2009) Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. Neuroimage 47(Suppl 2):T66–T71. https://doi.org/10.1016/j.neuroimage.2009.03.005
Tomoda A, Sheu YS, Rabi K, Suzuki H, Navalta CP, Polcari A, Teicher MH (2011) Exposure to parental verbal abuse is associated with increased gray matter volume in superior temporal gyrus. Neuroimage 54(Suppl 1):S280–S286. https://doi.org/10.1016/j.neuroimage.2010.05.027
Tomoda A, Polcari A, Anderson CM, Teicher MH (2012) Reduced visual cortex gray matter volume and thickness in young adults who witnessed domestic violence during childhood. PLoS ONE 7:e52528. https://doi.org/10.1371/journal.pone.0052528
Zhang J, Zhao T, Zhang J, Zhang Z, Li H, Cheng B, Pang Y, Wu H, Wang J (2022) Prediction of childhood maltreatment and subtypes with personalized functional connectome of large-scale brain networks. Hum Brain Mapp 43:4710–4721. https://doi.org/10.1002/hbm.25985
Beckett C, Maughan B, Rutter M, Castle J, Colvert E, Groothues C, Kreppner J, Stevens S, O’Connor TG, Sonuga-Barke EJ (2006) Do the effects of early severe deprivation on cognition persist into early adolescence? Findings from the English and Romanian adoptees study. Child Dev 77:696–711. https://doi.org/10.1111/j.1467-8624.2006.00898.x
Bick J, Zhu T, Stamoulis C, Fox NA, Zeanah C, Nelson CA (2015) Effect of early institutionalization and foster care on long-term white matter development: a randomized clinical trial. JAMA Pediatr 169:211–219. https://doi.org/10.1001/jamapediatrics.2014.3212
Chugani HT, Behen ME, Muzik O, Juhász C, Nagy F, Chugani DC (2001) Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. Neuroimage 14:1290–1301. https://doi.org/10.1006/nimg.2001.0917
Eluvathingal TJ, Chugani HT, Behen ME, Juhász C, Muzik O, Maqbool M, Chugani DC, Makki M (2006) Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics 117:2093–2100. https://doi.org/10.1542/peds.2005-1727
McLaughlin KA, Sheridan MA, Winter W, Fox NA, Zeanah CH, Nelson CA (2014) Widespread reductions in cortical thickness following severe early-life deprivation: a neurodevelopmental pathway to attention-deficit/hyperactivity disorder. Biol Psychiatry 76:629–638. https://doi.org/10.1016/j.biopsych.2013.08.016
Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SCR, Rutter M, Sonuga-Barke EJS (2009) Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. J Child Psychol Psychiatry 50:943–951. https://doi.org/10.1111/j.1469-7610.2009.02084.x
Mehta MA, Gore-Langton E, Golembo N, Colvert E, Williams SC, Sonuga-Barke E (2010) Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. J Cogn Neurosci 22:2316–2325. https://doi.org/10.1162/jocn.2009.21394
Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, Nelson CA 3rd (2012) Variation in neural development as a result of exposure to institutionalization early in childhood. Proc Natl Acad Sci USA 109:12927–12932. https://doi.org/10.1073/pnas.1200041109
Zhu J, Anderson CM, Ohashi K, Khan A, Teicher MH (2023) Potential sensitive period effects of maltreatment on amygdala, hippocampal and cortical response to threat. Mol Psychiatry. https://doi.org/10.1038/s41380-023-02002-5
Zhu J, Lowen SB, Anderson CM, Ohashi K, Khan A, Teicher MH (2019) Association of prepubertal and postpubertal exposure to childhood maltreatment with adult amygdala function. JAMA Psychiat 76:843–853. https://doi.org/10.1001/jamapsychiatry.2019.0931
Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, Lindner C, Postert C, Konrad C, Arolt V, Heindel W, Suslow T, Kugel H (2012) Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry 71:286–293. https://doi.org/10.1016/j.biopsych.2011.10.021
Weissman DG, Jenness JL, Colich NL, Miller AB, Sambrook KA, Sheridan MA, McLaughlin KA (2020) Altered neural processing of threat-related information in children and adolescents exposed to violence: a transdiagnostic mechanism contributing to the emergence of psychopathology. J Am Acad Child Adolesc Psychiatry 59:1274–1284. https://doi.org/10.1016/j.jaac.2019.08.471
McLaughlin KA, Sheridan MA, Gold AL, Duys A, Lambert HK, Peverill M, Heleniak C, Shechner T, Wojcieszak Z, Pine DS (2016) Maltreatment exposure, brain structure, and fear conditioning in children and adolescents. Neuropsychopharmacology 41:1956–1964. https://doi.org/10.1038/npp.2015.365
Young KS, Ward C, Vinograd M, Chen K, Bookheimer SY, Nusslock R, Zinbarg RE, Craske MG (2022) Individual differences in threat and reward neural circuitry activation: testing dimensional models of early adversity, anxiety and depression. Eur J Neurosci 55:2739–2753. https://doi.org/10.1111/ejn.15592
Boecker R, Holz NE, Buchmann AF, Blomeyer D, Plichta MM, Wolf I, Baumeister S, Meyer-Lindenberg A, Banaschewski T, Brandeis D, Laucht M (2014) Impact of early life adversity on reward processing in young adults: EEG-fMRI results from a prospective study over 25 years. PLoS ONE 9:e104185. https://doi.org/10.1371/journal.pone.0104185
Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA (2009) Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biol Psychiatry 66:206–213. https://doi.org/10.1016/j.biopsych.2009.02.019
Kamkar NH, Lewis DJ, van den Bos W, Morton JB (2017) Ventral striatal activity links adversity and reward processing in children. Dev Cogn Neurosci 26:20–27. https://doi.org/10.1016/j.dcn.2017.04.002
Oltean LE, Șoflău R, Miu AC, Szentágotai-Tătar A (2023) Childhood adversity and impaired reward processing: a meta-analysis. Child Abuse Negl 142:105596. https://doi.org/10.1016/j.chiabu.2022.105596
Pechtel P, Harris J, Karl A, Clunies-Ross C, Bower S, Moberly NJ, Pizzagalli DA, Watkins ER (2022) Emerging ecophenotype: reward anticipation is linked to high-risk behaviours after sexual abuse. Soc Cogn Affect Neurosci 17:1035–1043. https://doi.org/10.1093/scan/nsac030
McCrory EJ, Puetz VB, Maguire EA, Mechelli A, Palmer A, Gerin MI, Kelly PA, Koutoufa I, Viding E (2017) Autobiographical memory: a candidate latent vulnerability mechanism for psychiatric disorder following childhood maltreatment. Br J Psychiatry 211:216–222. https://doi.org/10.1192/bjp.bp.117.201798
Puetz VB, Viding E, Hoffmann F, Gerin MI, Sharp M, Rankin G, Maguire EA, Mechelli A, McCrory EJ (2021) Autobiographical memory as a latent vulnerability mechanism following childhood maltreatment: association with future depression symptoms and prosocial behavior. Dev Psychopathol 33:1300–1307. https://doi.org/10.1017/S0954579420000504
Puetz VB, Viding E, Maguire EA, Mechelli A, Armbruster-Genç D, Sharp M, Rankin G, Gerin MI, McCrory EJ (2023) Functional brain plasticity following childhood maltreatment: a longitudinal fMRI investigation of autobiographical memory processing. Dev Psychopathol. https://doi.org/10.1017/S0954579421001292
Hanson JL, Knodt AR, Brigidi BD, Hariri AR (2015) Lower structural integrity of the uncinate fasciculus is associated with a history of child maltreatment and future psychological vulnerability to stress. Dev Psychopathol 27:1611–1619. https://doi.org/10.1017/S0954579415000978
Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, Essex MJ (2013) Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci USA 110:19119–19124. https://doi.org/10.1073/pnas.1310766110
Jedd K, Hunt RH, Cicchetti D, Hunt E, Cowell RA, Rogosch FA, Toth SL, Thomas KM (2015) Long-term consequences of childhood maltreatment: altered amygdala functional connectivity. Dev Psychopathol 27:1577–1589. https://doi.org/10.1017/S0954579415000954
Kaiser RH, Clegg R, Goer F, Pechtel P, Beltzer M, Vitaliano G, Olson DP, Teicher MH, Pizzagalli DA (2018) Childhood stress, grown-up brain networks: corticolimbic correlates of threat-related early life stress and adult stress response. Psychol Med 48:1157–1166. https://doi.org/10.1017/S0033291717002628
Wolf RC, Herringa RJ (2016) Prefrontal-amygdala dysregulation to threat in pediatric posttraumatic stress disorder. Neuropsychopharmacology 41:822–831. https://doi.org/10.1038/npp.2015.209
Boccadoro S, Siugzdaite R, Hudson AR, Maeyens L, Van Hamme C, Mueller SC (2019) Women with early maltreatment experience show increased resting-state functional connectivity in the theory of mind (ToM) network. Eur J Psychotraumatol 10:1647044. https://doi.org/10.1080/20008198.2019.1647044
Luo Q, Chen J, Li Y, Wu Z, Lin X, Yao J, Yu H, Wu H, Peng H (2022) Aberrant brain connectivity is associated with childhood maltreatment in individuals with major depressive disorder. Brain Imaging Behav 16:2021–2036. https://doi.org/10.1007/s11682-022-00672-3
McLaughlin KA, Peverill M, Gold AL, Alves S, Sheridan MA (2015) Child maltreatment and neural systems underlying emotion regulation. J Am Acad Child Adolesc Psychiatry 54:753–762. https://doi.org/10.1016/j.jaac.2015.06.010
Rakesh D, Kelly C, Vijayakumar N, Zalesky A, Allen NB, Whittle S (2021) Unraveling the consequences of childhood maltreatment: deviations from typical functional neurodevelopment mediate the relationship between maltreatment history and depressive symptoms. Biol Psychiatry Cogn Neurosci Neuroimaging 6:329–342. https://doi.org/10.1016/j.bpsc.2020.09.016
van der Werff SJ, Pannekoek JN, Veer IM, van Tol MJ, Aleman A, Veltman DJ, Zitman FG, Rombouts SA, Elzinga BM, van der Wee NJ (2013) Resting-state functional connectivity in adults with childhood emotional maltreatment. Psychol Med 43:1825–1836. https://doi.org/10.1017/S0033291712002942
Wang Q, He C, Fan D, Liu X, Zhang H, Zhang H, Zhang Z, Xie C (2022) Neural effects of childhood maltreatment on dynamic large-scale brain networks in major depressive disorder. Psychiatry Res 317:114870. https://doi.org/10.1016/j.psychres.2022.114870
Puetz VB, Parker D, Kohn N, Dahmen B, Verma R, Konrad K (2017) Altered brain network integrity after childhood maltreatment: a structural connectomic DTI-study. Hum Brain Mapp 38:855–868. https://doi.org/10.1002/hbm.23423
Sun D, Haswell CC, Morey RA, De Bellis MD (2019) Brain structural covariance network centrality in maltreated youth with PTSD and in maltreated youth resilient to PTSD. Dev Psychopathol 31:557–571. https://doi.org/10.1017/s0954579418000093
De Bellis MD (2002) Developmental traumatology: a contributory mechanism for alcohol and substance use disorders. Psychoneuroendocrinology 27:155–170. https://doi.org/10.1016/s0306-4530(01)00042-7
Huang H, Gundapuneedi T, Rao U (2012) White matter disruptions in adolescents exposed to childhood maltreatment and vulnerability to psychopathology. Neuropsychopharmacology 37:2693–2701. https://doi.org/10.1038/npp.2012.133
Nemeroff CB (2016) Paradise lost: the neurobiological and clinical consequences of child abuse and neglect. Neuron 89:892–909. https://doi.org/10.1016/j.neuron.2016.01.019
Opel N, Redlich R, Dohm K, Zaremba D, Goltermann J, Repple J, Kaehler C, Grotegerd D, Leehr EJ, Böhnlein J, Förster K, Meinert S, Enneking V, Sindermann L, Dzvonyar F, Emden D, Leenings R, Winter N, Hahn T, Kugel H, Heindel W, Buhlmann U, Baune BT, Arolt V, Dannlowski U (2019) Mediation of the influence of childhood maltreatment on depression relapse by cortical structure: a 2-year longitudinal observational study. Lancet Psychiatry 6:318–326. https://doi.org/10.1016/S2215-0366(19)30044-6
Skokauskas N, Carballedo A, Fagan A, Frodl T (2015) The role of sexual abuse on functional neuroimaging markers associated with major depressive disorder. World J Biol Psychiatry 16:513–520. https://doi.org/10.3109/15622975.2015.1048723
Weissman DG, Lambert HK, Rodman AM, Peverill M, Sheridan MA, McLaughlin KA (2020) Reduced hippocampal and amygdala volume as a mechanism underlying stress sensitization to depression following childhood trauma. Depress Anxiety 37:916–925. https://doi.org/10.1002/da.23062
Edalati H, Afzali MH, Conrod PJ (2018) Poor response inhibition and peer victimization: a neurocognitive ecophenotype of risk for adolescent interpersonal aggression. J Abnorm Psychol 127:830–839. https://doi.org/10.1037/abn0000380
Frodl T, Reinhold E, Koutsouleris N, Reiser M, Meisenzahl EM (2010) Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J Psychiatr Res 44:799–807. https://doi.org/10.1016/j.jpsychires.2010.01.006
Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, Brummer M, Staib L, Vermetten E, Charney DS, Nemeroff CB, Bremner JD (2002) Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry 159:2072–2080. https://doi.org/10.1176/appi.ajp.159.12.2072
Burt KB, Whelan R, Conrod PJ, Banaschewski T, Barker GJ, Bokde AL, Bromberg U, Büchel C, Fauth-Bühler M, Flor H, Galinowski A, Gallinat J, Gowland P, Heinz A, Ittermann B, Mann K, Nees F, Papadopoulos-Orfanos D, Paus T, Pausova Z, Poustka L, Rietschel M, Robbins TW, Smolka MN, Ströhle A, Schumann G, Garavan H (2016) Structural brain correlates of adolescent resilience. J Child Psychol Psychiatry 57:1287–1296. https://doi.org/10.1111/jcpp.12552
Cisler JM, James GA, Tripathi S, Mletzko T, Heim C, Hu XP, Mayberg HS, Nemeroff CB, Kilts CD (2013) Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress. Psychol Med 43:507–518. https://doi.org/10.1017/S0033291712001390
Dennison MJ, Sheridan MA, Busso DS, Jenness JL, Peverill M, Rosen ML, McLaughlin KA (2016) Neurobehavioral markers of resilience to depression amongst adolescents exposed to child abuse. J Abnorm Psychol 125:1201–1212. https://doi.org/10.1037/abn0000215
Moreno-López L, Ioannidis K, Askelund AD, Smith AJ, Schueler K, van Harmelen AL (2020) The resilient emotional brain: a scoping review of the medial prefrontal cortex and limbic structure and function in resilient adults with a history of childhood maltreatment. Biol Psychiatry Cogn Neurosci Neuroimaging 5:392–402. https://doi.org/10.1016/j.bpsc.2019.12.008
Morey RA, Haswell CC, Hooper SR, De Bellis MD (2016) Amygdala, hippocampus, and ventral medial prefrontal cortex volumes differ in maltreated youth with and without chronic posttraumatic stress disorder. Neuropsychopharmacology 41:791–801. https://doi.org/10.1038/npp.2015.205
Ohashi K, Anderson CM, Bolger EA, Khan A, McGreenery CE, Teicher MH (2019) Susceptibility or resilience to maltreatment can be explained by specific differences in brain network architecture. Biol Psychiatry 85:690–702. https://doi.org/10.1016/j.biopsych.2018.10.016
Rodman AM, Jenness JL, Weissman DG, Pine DS, McLaughlin KA (2019) Neurobiological markers of resilience to depression following childhood maltreatment: the role of neural circuits supporting the cognitive control of emotion. Biol Psychiatry 86:464–473. https://doi.org/10.1016/j.biopsych.2019.04.033
Teicher MH, Ohashi K, Khan A (2020) Additional insights into the relationship between brain network architecture and susceptibility and resilience to the psychiatric sequelae of childhood maltreatment. Advers Resil Sci 1:49–64. https://doi.org/10.1007/s42844-020-00002-w
Garrett AS, Abazid L, Cohen JA, van der Kooij A, Carrion V, Zhang W, Jo B, Franklin C, Blader J, Zack S, Reiss AL, Agras WS (2021) Changes in brain volume associated with trauma-focused cognitive behavioral therapy among youth with posttraumatic stress disorder. J Trauma Stress 34:744–756. https://doi.org/10.1002/jts.22678
Joss D, Lazar SW, Teicher MH (2020) Effects of a mindfulness based behavioral intervention for young adults with childhood maltreatment history on hippocampal morphometry: a pilot MRI study with voxel-based morphometry. Psychiatry Res Neuroimaging 301:111087. https://doi.org/10.1016/j.pscychresns.2020.111087
Joss D, Khan A, Lazar SW, Teicher MH (2021) A pilot study on amygdala volumetric changes among young adults with childhood maltreatment histories after a mindfulness intervention. Behav Brain Res 399:113023. https://doi.org/10.1016/j.bbr.2020.113023
Lu XW, Guo H, Sun JR, Dong QL, Zhao FT, Liao XH, Zhang L, Zhang Y, Li WH, Li ZX, Liu TB, He Y, Xia MR, Li LJ (2018) A shared effect of paroxetine treatment on gray matter volume in depressive patients with and without childhood maltreatment: a voxel-based morphometry study. CNS Neurosci Ther 24:1073–1083. https://doi.org/10.1111/cns.13055
Group BDC (2012) Total and regional brain volumes in a population-based normative sample from 4 to 18 years: the NIH MRI study of normal brain development. Cereb Cortex 22:1–12. https://doi.org/10.1093/cercor/bhr018
Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J (2006) Intellectual ability and cortical development in children and adolescents. Nature 440:676–679. https://doi.org/10.1038/nature04513
Tanaka C, Matsui M, Uematsu A, Noguchi K, Miyawaki T (2012) Developmental trajectories of the fronto-temporal lobes from infancy to early adulthood in healthy individuals. Dev Neurosci 34:477–487. https://doi.org/10.1159/000345152
Song JW, Chung KC (2010) Observational studies: cohort and case-control studies. Plast Reconstr Surg 126:2234–2242. https://doi.org/10.1097/PRS.0b013e3181f44abc
Teicher MH, Samson JA, Anderson CM, Ohashi K (2016) The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci 17:652–666. https://doi.org/10.1038/nrn.2016.111
Ohashi K, Anderson CM, Khan A, Rohan ML, Bolger EA, McGreenery CE, Teicher MH (2022) Sex and sensitive period differences in potential effects of maltreatment on axial versus radial diffusivity in the corpus callosum. Neuropsychopharmacology 47:953–964. https://doi.org/10.1038/s41386-021-01260-7
Teicher MH, Gordon JB, Nemeroff CB (2022) Recognizing the importance of childhood maltreatment as a critical factor in psychiatric diagnoses, treatment, research, prevention, and education. Mol Psychiatry 27:1331–1338. https://doi.org/10.1038/s41380-021-01367-9
Tomoda A, Navalta CP, Polcari A, Sadato N, Teicher MH (2009) Childhood sexual abuse is associated with reduced gray matter volume in visual cortex of young women. Biol Psychiatry 66:642–648. https://doi.org/10.1016/j.biopsych.2009.04.021
Heim CM, Mayberg HS, Mletzko T, Nemeroff CB, Pruessner JC (2013) Decreased cortical representation of genital somatosensory field after childhood sexual abuse. Am J Psychiatry 170:616–623. https://doi.org/10.1176/appi.ajp.2013.12070950
Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS (1999) Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry 156:1787–1795. https://doi.org/10.1176/ajp.156.11.1787
Berman Z, Assaf Y, Tarrasch R, Joel D (2018) Assault-related self-blame and its association with PTSD in sexually assaulted women: an MRI inquiry. Soc Cogn Affect Neurosci 13:775–784. https://doi.org/10.1093/scan/nsy044
Hubel DH, Wiesel TN (1998) Early exploration of the visual cortex. Neuron 20:401–412. https://doi.org/10.1016/s0896-6273(00)80984-8
Fujisawa TX, Shimada K, Takiguchi S, Mizushima S, Kosaka H, Teicher MH, Tomoda A (2018) Type and timing of childhood maltreatment and reduced visual cortex volume in children and adolescents with reactive attachment disorder. Neuroimage Clin 20:216–221. https://doi.org/10.1016/j.nicl.2018.07.018
Jensen SK, Dickie EW, Schwartz DH, Evans CJ, Dumontheil I, Paus T, Barker ED (2015) Effect of early adversity and childhood internalizing symptoms on brain structure in young men. JAMA Pediatr 169:938–946. https://doi.org/10.1001/jamapediatrics.2015.1486
Nishitani S, Fujisawa TX, Hiraoka D, Makita K, Takiguchi S, Hamamura S, Yao A, Shimada K, Smith AK, Tomoda A (2021) A multi-modal MRI analysis of brain structure and function in relation to OXT methylation in maltreated children and adolescents. Transl Psychiatry 11:589. https://doi.org/10.1038/s41398-021-01714-y
Teicher MH, Anderson CM, Ohashi K, Khan A, McGreenery CE, Bolger EA, Rohan ML, Vitaliano GD (2018) Differential effects of childhood neglect and abuse during sensitive exposure periods on male and female hippocampus. Neuroimage 169:443–452. https://doi.org/10.1016/j.neuroimage.2017.12.055
Fuchs E, Flugge G, Czeh B (2006) Remodeling of neuronal networks by stress. Front Biosci 11:2746–2758. https://doi.org/10.2741/2004
Radley JJ, Morrison JH (2005) Repeated stress and structural plasticity in the brain. Ageing Res Rev 4:271–287. https://doi.org/10.1016/j.arr.2005.03.004
Lakshminarasimhan H, Chattarji S (2012) Stress leads to contrasting effects on the levels of brain derived neurotrophic factor in the hippocampus and amygdala. PLoS ONE 7:e30481. https://doi.org/10.1371/journal.pone.0030481
De Bellis MD, Keshavan MS, Frustaci K, Shifflett H, Iyengar S, Beers SR, Hall J (2002) Superior temporal gyrus volumes in maltreated children and adolescents with PTSD. Biol Psychiatry 51:544–552. https://doi.org/10.1016/s0006-3223(01)01374-9
Niedtfeld I, Schulze L, Krause-Utz A, Demirakca T, Bohus M, Schmahl C (2013) Voxel-based morphometry in women with borderline personality disorder with and without comorbid posttraumatic stress disorder. PLoS ONE 8:e65824. https://doi.org/10.1371/journal.pone.0065824
Olson EA, Overbey TA, Ostrand CG, Pizzagalli DA, Rauch SL, Rosso IM (2020) Childhood maltreatment experiences are associated with altered diffusion in occipito-temporal white matter pathways. Brain Behav 10:e01485. https://doi.org/10.1002/brb3.1485
Howell BR, Ahn M, Shi Y, Godfrey JR, Hu X, Zhu H, Styner M, Sanchez MM (2019) Disentangling the effects of early caregiving experience and heritable factors on brain white matter development in rhesus monkeys. Neuroimage 197:625–642. https://doi.org/10.1016/j.neuroimage.2019.04.013
Edmiston EE, Wang F, Mazure CM, Guiney J, Sinha R, Mayes LC, Blumberg HP (2011) Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Arch Pediatr Adolesc Med 165:1069–1077. https://doi.org/10.1001/archpediatrics.2011.565
Teicher MH, Khan A (2019) Childhood maltreatment, cortical and amygdala morphometry, functional connectivity, laterality, and psychopathology. Child Maltreat 24:458–465. https://doi.org/10.1177/1077559519870845
Siehl S, Sicorello M, Herzog J, Nees F, Kleindienst N, Bohus M, Müller-Engelmann M, Steil R, Priebe K, Schmahl C, Flor H (2022) Neurostructural associations with traumatic experiences during child- and adulthood. Transl Psychiatry 12:515. https://doi.org/10.1038/s41398-022-02262-9
Lyons-Ruth K, Connell DB, Grunebaum HU, Botein S (1990) Infants at social risk: maternal depression and family support services as mediators of infant development and security of attachment. Child Dev 61:85–98. https://doi.org/10.1111/j.1467-8624.1990.tb02762.x
Ainsworth MDS, Blehar MC, Wall S, Waters E (1978) Patterns of attachment: a psychological study of the strange situation. Lawrence Erlbaum, Oxford
Lyons-Ruth K, Bronfman E, Parsons E (1999) Atypical attachment in infancy and early childhood among children at developmental risk. IV. Maternal frightened, frightening, or atypical behavior and disorganized infant attachment patterns. Monogr Soc Res Child Dev 64:67–96. https://doi.org/10.1111/1540-5834.00034
Lyons-Ruth K, Pechtel P, Yoon SA, Anderson CM, Teicher MH (2016) Disorganized attachment in infancy predicts greater amygdala volume in adulthood. Behav Brain Res 308:83–93. https://doi.org/10.1016/j.bbr.2016.03.050
Fetterman AK, Ode S, Robinson MD (2013) For which side the bell tolls: the laterality of approach-avoidance associative networks. Motiv Emot 37:33–38. https://doi.org/10.1007/s11031-012-9306-5
Khoury JE, Pechtel P, Andersen CM, Teicher MH, Lyons-Ruth K (2019) Relations among maternal withdrawal in infancy, borderline features, suicidality/self-injury, and adult hippocampal volume: a 30-year longitudinal study. Behav Brain Res 374:112139. https://doi.org/10.1016/j.bbr.2019.112139
Lyons-Ruth K, Ahtam B, Li FH, Dickerman S, Khoury JE, Sisitsky M, Ou Y, Bosquet Enlow M, Teicher MH, Grant PE (2023) Negative versus withdrawn maternal behavior: differential associations with infant gray and white matter during the first 2 years of life. Hum Brain Mapp 44:4572–4589. https://doi.org/10.1002/hbm.26401
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Association, Washington, DC
Guttmann-Steinmetz S, Crowell JA (2006) Attachment and externalizing disorders: a developmental psychopathology perspective. J Am Acad Child Adolesc Psychiatry 45:440–451. https://doi.org/10.1097/01.chi.0000196422.42599.63
Kay C, Green J (2013) Reactive attachment disorder following early maltreatment: systematic evidence beyond the institution. J Abnorm Child Psychol 41:571–581. https://doi.org/10.1007/s10802-012-9705-9
Kocovska E, Puckering C, Follan M, Smillie M, Gorski C, Barnes J, Wilson P, Young D, Lidstone E, Pritchett R, Hockaday H, Minnis H (2012) Neurodevelopmental problems in maltreated children referred with indiscriminate friendliness. Res Dev Disabil 33:1560–1565. https://doi.org/10.1016/j.ridd.2012.02.016
van der Vegt EJ, van der Ende J, Kirschbaum C, Verhulst FC, Tiemeier H (2009) Early neglect and abuse predict diurnal cortisol patterns in adults: a study of international adoptees. Psychoneuroendocrinology 34:660–669. https://doi.org/10.1016/j.psyneuen.2008.11.004
Minnis H, Macmillan S, Pritchett R, Young D, Wallace B, Butcher J, Sim F, Baynham K, Davidson C, Gillberg C (2013) Prevalence of reactive attachment disorder in a deprived population. Br J Psychiatry 202:342–346. https://doi.org/10.1192/bjp.bp.112.114074
Lehmann S, Havik O, Havik T, Heiervang E (2013) Mental disorders in foster children: a study of prevalence, comorbidity and risk factors. Child Adolesc Psychiatry Ment Health 7:39. https://doi.org/10.1186/1753-2000-7-39
Zeanah CH, Scheeringa M, Boris NW, Heller SS, Smyke AT, Trapani J (2004) Reactive attachment disorder in maltreated toddlers. Child Abuse Negl 28:877–888. https://doi.org/10.1016/j.chiabu.2004.01.010
Jung M, Takiguchi S, Hamamura S, Mizuno Y, Kosaka H, Tomoda A (2020) Thalamic volume is related to increased anterior thalamic radiations in children with reactive attachment disorder. Cereb Cortex 30:4238–4245. https://doi.org/10.1093/cercor/bhaa051
Makita K, Takiguchi S, Naruse H, Shimada K, Morioka S, Fujisawa TX, Shimoji K, Tomoda A (2020) White matter changes in children and adolescents with reactive attachment disorder: a diffusion tensor imaging study. Psychiatry Res Neuroimaging 303:111129. https://doi.org/10.1016/j.pscychresns.2020.111129
Takiguchi S, Makita K, Fujisawa TX, Nishitani S, Tomoda A (2023) Effects of intranasal oxytocin on neural reward processing in children and adolescents with reactive attachment disorder: a randomized controlled trial. Front Child Adolesc Psychiatry 1:1056115. https://doi.org/10.3389/frcha.2022.1056115
Green J, Goldwyn R (2002) Annotation: attachment disorganisation and psychopathology: new findings in attachment research and their potential implications for developmental psychopathology in childhood. J Child Psychol Psychiatry 43:835–846. https://doi.org/10.1111/1469-7610.00102
O’Connor TG, Rutter M, English and Romanian Adoptees Study Team (2000) Attachment disorder behavior following early severe deprivation: extension and longitudinal follow-up. J Am Acad Child Adolesc Psychiatry 39:703–712. https://doi.org/10.1097/00004583-200006000-00008
Leblanc E, Degeilh F, Daneault V, Beauchamp MH, Bernier A (2017) Attachment security in infancy: a preliminary study of prospective links to brain morphometry in late childhood. Front Psychol 8:2141. https://doi.org/10.3389/fpsyg.2017.02141
Scheele D, Striepens N, Güntürkün O, Deutschländer S, Maier W, Kendrick KM, Hurlemann R (2012) Oxytocin modulates social distance between males and females. J Neurosci 32:16074–16079. https://doi.org/10.1523/JNEUROSCI.2755-12.2012
Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E (2005) Oxytocin increases trust in humans. Nature 435:673–676. https://doi.org/10.1038/nature03701
Buchheim A, Heinrichs M, George C, Pokorny D, Koops E, Henningsen P, O’Connor MF, Gündel H (2009) Oxytocin enhances the experience of attachment security. Psychoneuroendocrinology 34:1417–1422. https://doi.org/10.1016/j.psyneuen.2009.04.002
Marlin BJ, Mitre M, D’Amour JA, Chao MV, Froemke RC (2015) Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 520:499–504. https://doi.org/10.1038/nature14402
Cong X, Ludington-Hoe SM, Hussain N, Cusson RM, Walsh S, Vazquez V, Briere CE, Vittner D (2015) Parental oxytocin responses during skin-to-skin contact in pre-term infants. Early Hum Dev 91:401–406. https://doi.org/10.1016/j.earlhumdev.2015.04.012
Uvnäs-Moberg K, Handlin L, Petersson M (2014) Self-soothing behaviors with particular reference to oxytocin release induced by non-noxious sensory stimulation. Front Psychol 5:1529. https://doi.org/10.3389/fpsyg.2014.01529
Verhage ML, Tharner A, Duschinsky R, Bosmans G, Fearon RMP (2023) Editorial perspective: on the need for clarity about attachment terminology. J Child Psychol Psychiatry 64:839–843. https://doi.org/10.1111/jcpp.13675
Davison KK, Charles JN, Khandpur N, Nelson TJ (2017) Fathers’ perceived reasons for their underrepresentation in child health research and strategies to increase their involvement. Matern Child Health J 21:267–274. https://doi.org/10.1007/s10995-016-2157-z
Deneault AA, Bakermans-Kranenburg MJ, Groh AM, Fearon PRM, Madigan S (2021) Child-father attachment in early childhood and behavior problems: a meta-analysis. New Dir Child Adolesc Dev 2021:43–66. https://doi.org/10.1002/cad.20434
Sarkadi A, Kristiansson R, Oberklaid F, Bremberg S (2008) Fathers’ involvement and children’s developmental outcomes: a systematic review of longitudinal studies. Acta Paediatr 97:153–158. https://doi.org/10.1111/j.1651-2227.2007.00572.x
Krause S, Boeck C, Gumpp AM, Rottler E, Schury K, Karabatsiakis A, Buchheim A, Gündel H, Kolassa IT, Waller C (2018) Child maltreatment is associated with a reduction of the oxytocin receptor in peripheral blood mononuclear cells. Front Psychol 9:173. https://doi.org/10.3389/fpsyg.2018.00173
Seltzer LJ, Ziegler T, Connolly MJ, Prososki AR, Pollak SD (2014) Stress-induced elevation of oxytocin in maltreated children: evolution, neurodevelopment, and social behavior. Child Dev 85:501–512. https://doi.org/10.1111/cdev.12136
Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB (2009) Lower CSF oxytocin concentrations in women with a history of childhood abuse. Mol Psychiatry 14:954–958. https://doi.org/10.1038/mp.2008.112
Wismer Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD (2005) Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proc Natl Acad Sci USA 102:17237–17240. https://doi.org/10.1073/pnas.0504767102
Mizuki R, Fujiwara T (2015) Association of oxytocin level and less severe forms of childhood maltreatment history among healthy Japanese adults involved with child care. Front Behav Neurosci 9:138. https://doi.org/10.3389/fnbeh.2015.00138
Suzuki S, Fujisawa TX, Sakakibara N, Fujioka T, Takiguchi S, Tomoda A (2020) Development of social attention and oxytocin levels in maltreated children. Sci Rep 10:7407. https://doi.org/10.1038/s41598-020-64297-6
Mizushima SG, Fujisawa TX, Takiguchi S, Kumazaki H, Tanaka S, Tomoda A (2015) Effect of the nature of subsequent environment on oxytocin and cortisol secretion in maltreated children. Front Psychiatry 6:173. https://doi.org/10.3389/fpsyt.2015.00173
Bonfig J, Herpertz SC, Schneider I (2022) Altered hormonal patterns in borderline personality disorder mother-child interactions. Psychoneuroendocrinology 143:105822. https://doi.org/10.1016/j.psyneuen.2022.105822
Bakermans-Kranenburg MJ, van Ijzendoorn MH, Riem MM, Tops M, Alink LR (2012) Oxytocin decreases handgrip force in reaction to infant crying in females without harsh parenting experiences. Soc Cogn Affect Neurosci 7:951–957. https://doi.org/10.1093/scan/nsr067
Donaldson ZR, Young LJ (2008) Oxytocin, vasopressin, and the neurogenetics of sociality. Science 322:900–904. https://doi.org/10.1126/science.1158668
Feldman R (2017) The neurobiology of human attachments. Trends Cogn Sci 21:80–99. https://doi.org/10.1016/j.tics.2016.11.007
Opacka-Juffry J, Mohiyeddini C (2012) Experience of stress in childhood negatively correlates with plasma oxytocin concentration in adult men. Stress 15:1–10. https://doi.org/10.3109/10253890.2011.560309
Walsh WA, Dawson J, Mattingly MJ (2010) How are we measuring resilience following childhood maltreatment? Is the research adequate and consistent? What is the impact on research, practice, and policy? Trauma Violence Abuse 11:27–41. https://doi.org/10.1177/1524838009358892
Bartz JA, Zaki J, Ochsner KN, Bolger N, Kolevzon A, Ludwig N, Lydon JE (2010) Effects of oxytocin on recollections of maternal care and closeness. Proc Natl Acad Sci USA 107:21371–21375. https://doi.org/10.1073/pnas.1012669107
Olff M, Langeland W, Witteveen A, Denys D (2010) A psychobiological rationale for oxytocin in the treatment of posttraumatic stress disorder. CNS Spectr 15:522–530. https://doi.org/10.1017/s109285290000047x
Sikich L, Kolevzon A, King BH, McDougle CJ, Sanders KB, Kim S-J, Spanos M, Chandrasekhar T, Trelles MDP, Rockhill CM, Palumbo ML, Witters Cundiff A, Montgomery A, Siper P, Minjarez M, Nowinski LA, Marler S, Shuffrey LC, Alderman C, Weissman J, Zappone B, Mullett JE, Crosson H, Hong N, Siecinski SK, Giamberardino SN, Luo S, She L, Bhapkar M, Dean R, Scheer A, Johnson JL, Gregory SG, Veenstra-VanderWeele J (2021) Intranasal oxytocin in children and adolescents with autism spectrum disorder. N Engl J Med 385:1462–1473. https://doi.org/10.1056/NEJMoa2103583
Felitti VJ (2002) The relation between adverse childhood experiences and adult health: turning gold into lead. Perm J 6:44–47. https://doi.org/10.7812/TPP/02.994
Cicchetti D, Rogosch FA (2001) The impact of child maltreatment and psychopathology on neuroendocrine functioning. Dev Psychopathol 13:783–804. https://doi.org/10.1017/S0954579401004035
Heim C, Owens MJ, Plotsky PM, Nemeroff CB (1997) Persistent changes in corticotropin-releasing factor systems due to early life stress: relationship to the pathophysiology of major depression and post-traumatic stress disorder. Psychopharmacol Bull 33:185–192
Tarullo AR, Gunnar MR (2006) Child maltreatment and the developing HPA axis. Horm Behav 50:632–639. https://doi.org/10.1016/j.yhbeh.2006.06.010
Weaver IC (2009) Epigenetic effects of glucocorticoids. Semin Fetal Neonatal Med 14:143–150. https://doi.org/10.1016/j.siny.2008.12.002
Andersen SL, Teicher MH (2004) Delayed effects of early stress on hippocampal development. Neuropsychopharmacology 29:1988–1993. https://doi.org/10.1038/sj.npp.1300528
Teicher MH, Tomoda A, Andersen SL (2006) Neurobiological consequences of early stress and childhood maltreatment: are results from human and animal studies comparable? Ann NY Acad Sci 1071:313–323. https://doi.org/10.1196/annals.1364.024
Andersen SL (2022) Neuroinflammation, early-life adversity, and brain development. Harv Rev Psychiatry 30:24–39. https://doi.org/10.1097/hrp.0000000000000325
Dahl RE, Gunnar MR (2009) Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Dev Psychopathol 21:1–6. https://doi.org/10.1017/s0954579409000017
Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C (2009) Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: normative changes and associations with puberty. Dev Psychopathol 21:69–85. https://doi.org/10.1017/S0954579409000054
Shirtcliff EA, Dismukes AR, Marceau K, Ruttle PL, Simmons JG, Han G (2015) A dual-axis approach to understanding neuroendocrine development. Dev Psychobiol 57:643–653. https://doi.org/10.1002/dev.21337
Liberzon I, Young EA (1997) Effects of stress and glucocorticoids on CNS oxytocin receptor binding. Psychoneuroendocrinology 22:411–422. https://doi.org/10.1016/s0306-4530(97)00045-0
Darling Rasmussen P, Storebø OJ (2021) Attachment and epigenetics: a scoping review of recent research and current knowledge. Psychol Rep 124:479–501. https://doi.org/10.1177/0033294120901846
Robakis TK, Zhang S, Rasgon NL, Li T, Wang T, Roth MC, Humphreys KL, Gotlib IH, Ho M, Khechaduri A, Watson K, Roat-Shumway S, Budhan VV, Davis KN, Crowe SD, Ellie Williams K, Urban AE (2020) Epigenetic signatures of attachment insecurity and childhood adversity provide evidence for role transition in the pathogenesis of perinatal depression. Transl Psychiatry 10:48. https://doi.org/10.1038/s41398-020-0703-3
Ein-Dor T, Verbeke WJMI, Mokry M, Vrtička P (2018) Epigenetic modification of the oxytocin and glucocorticoid receptor genes is linked to attachment avoidance in young adults. Attach Hum Dev 20:439–454. https://doi.org/10.1080/14616734.2018.1446451
Fujisawa TX, Nishitani S, Takiguchi S, Shimada K, Smith AK, Tomoda A (2019) Oxytocin receptor DNA methylation and alterations of brain volumes in maltreated children. Neuropsychopharmacology 44:2045–2053. https://doi.org/10.1038/s41386-019-0414-8
Haas BW, Filkowski MM, Cochran RN, Denison L, Ishak A, Nishitani S, Smith AK (2016) Epigenetic modification of OXT and human sociability. Proc Natl Acad Sci USA 113:E3816–E3823. https://doi.org/10.1073/pnas.1602809113
Wu Y, Wang J, Zhang Y, Zheng D, Zhang J, Rong M, Wu H, Wang Y, Zhou K, Jiang T (2016) The neuroanatomical basis for posterior superior parietal lobule control lateralization of visuospatial attention. Front Neuroanat 10:32. https://doi.org/10.3389/fnana.2016.00032
Ioannidis K, Askelund AD, Kievit RA, van Harmelen AL (2020) The complex neurobiology of resilient functioning after childhood maltreatment. BMC Med 18:32. https://doi.org/10.1186/s12916-020-1490-7
de Haan A, Meiser-Stedman R, Landolt MA, Kuhn I, Black MJ, Klaus K, Patel SD, Fisher DJ, Haag C, Ukoumunne OC, Jones BG, Flaiyah AM, Catani C, Dawson K, Bryant RA, de Roos C, Ertl V, Foa EB, Ford JD, Gilboa-Schechtman E, Tutus D, Hermenau K, Hecker T, Hultmann O, Axberg U, Jaberghaderi N, Jensen TK, Ormhaug SM, Kenardy J, Lindauer RJL, Diehle J, Murray LK, Kane JC, Peltonen K, Kangaslampi S, Robjant K, Koebach A, Rosner R, Rossouw J, Smith P, Tonge BJ, Hitchcock C, Dalgleish T (2023) Efficacy and moderators of efficacy of cognitive behavioural therapies with a trauma focus in children and adolescents: an individual participant data meta-analysis of randomised trials. Lancet Child Adolesc Health. https://doi.org/10.1016/S2352-4642(23)00253-5
Grinevich V, Neumann ID (2021) Brain oxytocin: how puzzle stones from animal studies translate into psychiatry. Mol Psychiatry 26:265–279. https://doi.org/10.1038/s41380-020-0802-9
Nour MM, Howes OD (2015) Interpreting the neurodevelopmental hypothesis of schizophrenia in the context of normal brain development and ageing. Proc Natl Acad Sci USA 112:E2745. https://doi.org/10.1073/pnas.1502170112
Owen MJ, O’Donovan MC, Thapar A, Craddock N (2011) Neurodevelopmental hypothesis of schizophrenia. Br J Psychiatry 198:173–175. https://doi.org/10.1192/bjp.bp.110.084384
Denckla CA, Cicchetti D, Kubzansky LD, Seedat S, Teicher MH, Williams DR, Koenen KC (2020) Psychological resilience: an update on definitions, a critical appraisal, and research recommendations. Eur J Psychotraumatol 11:1822064. https://doi.org/10.1080/20008198.2020.1822064
Abe K, Shiomi M, Pei Y, Zhang T, Ikeda N, Nagai T (2018) ChiCaRo: tele-presence robot for interacting with babies and toddlers. Adv Robot 32:176–190. https://doi.org/10.1080/01691864.2018.1434014
Saunders V, Beck M, McKechnie J, Lincoln M, Phillips C, Herbert J, Davey R (2022) A Good start in life: effectiveness of integrated multicomponent multisector support on early child development—study protocol. PLoS ONE 17:e0267666. https://doi.org/10.1371/journal.pone.0267666
Wakusawa K, Sugiyama T, Hotta H, Wada K, Suzuki F, Morimoto T, Shiino T, Tomoda A (2023) Triadic therapy based on somatic eye movement desensitization and reprocessing for posttraumatic stress disorder: a pilot randomized controlled study. J EMDR Pract Res. https://doi.org/10.1891/EMDR-2023-0014
Hallihan GM, Shu LH (2013) Considering confirmation bias in design and design research. J Integr Des Process Sci 17:19–35
Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, Donohue MR, Foran W, Miller RL, Hendrickson TJ, Malone SM, Kandala S, Feczko E, Miranda-Dominguez O, Graham AM, Earl EA, Perrone AJ, Cordova M, Doyle O, Moore LA, Conan GM, Uriarte J, Snider K, Lynch BJ, Wilgenbusch JC, Pengo T, Tam A, Chen J, Newbold DJ, Zheng A, Seider NA, Van AN, Metoki A, Chauvin RJ, Laumann TO, Greene DJ, Petersen SE, Garavan H, Thompson WK, Nichols TE, Yeo BTT, Barch DM, Luna B, Fair DA, Dosenbach NUF (2022) Reproducible brain-wide association studies require thousands of individuals. Nature 603:654–660. https://doi.org/10.1038/s41586-022-04492-9
Bock J, Gruss M, Becker S, Braun K (2005) Experience-induced changes of dendritic spine densities in the prefrontal and sensory cortex: correlation with developmental time windows. Cereb Cortex 15:802–808. https://doi.org/10.1093/cercor/bhh181
Greenough WT, Black JE, Wallace CS (1987) Experience and brain development. Child Dev 58:539–559. https://doi.org/10.2307/1130197
Hensch TK (2005) Critical period plasticity in local cortical circuits. Nat Rev Neurosci 6:877–888
Meaney MJ, Szyf M (2005) Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialog Clin Neurosci 7:103–123. https://doi.org/10.31887/DCNS.2005.7.2/mmeaney
Braun PR, Han S, Hing B, Nagahama Y, Gaul LN, Heinzman JT, Grossbach AJ, Close L, Dlouhy BJ, Howard MA 3rd, Kawasaki H, Potash JB, Shinozaki G (2019) Genome-wide DNA methylation comparison between live human brain and peripheral tissues within individuals. Transl Psychiatry 9:47. https://doi.org/10.1038/s41398-019-0376-y
Nishitani S, Isozaki M, Yao A, Higashino Y, Yamauchi T, Kidoguchi M, Kawajiri S, Tsunetoshi K, Neish H, Imoto H, Arishima H, Kodera T, Fujisawa TX, Nomura S, Kikuta K, Shinozaki G, Tomoda A (2023) Cross-tissue correlations of genome-wide DNA methylation in Japanese live human brain and blood, saliva, and buccal epithelial tissues. Transl Psychiatry 13:72. https://doi.org/10.1038/s41398-023-02370-0
Baldwin JR, Reuben A, Newbury JB, Danese A (2019) Agreement between prospective and retrospective measures of childhood maltreatment: a systematic review and meta-analysis. JAMA Psychiat 76:584–593. https://doi.org/10.1001/jamapsychiatry.2019.0097
Bremner JD, Bolus R, Mayer EA (2007) Psychometric properties of the Early Trauma Inventory-Self Report. J Nerv Ment Dis 195:211–218. https://doi.org/10.1097/01.nmd.0000243824.84651.6c
Bernstein DP, Fink L (1998) Childhood Trauma Questionnaire: a retrospective self-report manual. The Psychological Corporation, San Antonio
Teicher MH, Parigger A (2015) The “Maltreatment and Abuse Chronology of Exposure” (MACE) scale for the retrospective assessment of abuse and neglect during development. PLoS ONE 10:e0117423. https://doi.org/10.1371/journal.pone.0117423
Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS (1998) Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 14:245–258. https://doi.org/10.1016/s0749-3797(98)00017-8
Fink LA, Bernstein D, Handelsman L, Foote J, Lovejoy M (1995) Initial reliability and validity of the childhood trauma interview: a new multidimensional measure of childhood interpersonal trauma. Am J Psychiatry 152:1329–1335. https://doi.org/10.1176/ajp.152.9.1329
Bifulco A, Brown GW, Harris TO (1994) Childhood Experience of Care and Abuse (CECA): a retrospective interview measure. J Child Psychol Psychiatry 35:1419–1435. https://doi.org/10.1111/j.1469-7610.1994.tb01284.x
Szyf M (2015) Epigenetics, a key for unlocking complex CNS disorders? Therapeutic implications. Eur Neuropsychopharmacol 25:682–702. https://doi.org/10.1016/j.euroneuro.2014.01.009
Heerboth S, Lapinska K, Snyder N, Leary M, Rollinson S, Sarkar S (2014) Use of epigenetic drugs in disease: an overview. Genet Epigenet 6:9–19. https://doi.org/10.4137/geg.S12270
Vinkers CH, Geuze E, van Rooij SJH, Kennis M, Schür RR, Nispeling DM, Smith AK, Nievergelt CM, Uddin M, Rutten BPF, Vermetten E, Boks MP (2021) Successful treatment of post-traumatic stress disorder reverses DNA methylation marks. Mol Psychiatry 26:1264–1271. https://doi.org/10.1038/s41380-019-0549-3
Yehuda R, Daskalakis NP, Desarnaud F, Makotkine I, Lehrner AL, Koch E, Flory JD, Buxbaum JD, Meaney MJ, Bierer LM (2013) Epigenetic biomarkers as predictors and correlates of symptom improvement following psychotherapy in combat veterans with PTSD. Front Psychiatry 4:118. https://doi.org/10.3389/fpsyt.2013.00118
Breslau J, Miller E, Joanie Chung WJ, Schweitzer JB (2011) Childhood and adolescent onset psychiatric disorders, substance use, and failure to graduate high school on time. J Psychiatr Res 45:295–301. https://doi.org/10.1016/j.jpsychires.2010.06.014
Calhoun BH, Ridenour TA, Fishbein DH (2019) Associations between child maltreatment, harsh parenting, and sleep with adolescent mental health. J Child Fam Stud 28:116–130. https://doi.org/10.1007/s10826-018-1261-7
Dunn EC, Wang Y, Tse J, McLaughlin KA, Fitzmaurice G, Gilman SE, Susser ES (2017) Sensitive periods for the effect of childhood interpersonal violence on psychiatric disorder onset among adolescents. Br J Psychiatry 211:365–372. https://doi.org/10.1192/bjp.bp.117.208397
Tubman JG, Oshri A, Duprey EB, Sutton TE (2021) Childhood maltreatment, psychiatric symptoms, and suicidal thoughts among adolescents receiving substance use treatment services. J Adolesc 89:18–27. https://doi.org/10.1016/j.adolescence.2021.03.002
Schaefer JD, Cheng TW, Dunn EC (2022) Sensitive periods in development and risk for psychiatric disorders and related endpoints: a systematic review of child maltreatment findings. Lancet Psychiatry 9:978–991. https://doi.org/10.1016/s2215-0366(22)00362-5
Gomez SH, Tse J, Wang Y, Turner B, Millner AJ, Nock MK, Dunn EC (2017) Are there sensitive periods when child maltreatment substantially elevates suicide risk? Results from a nationally representative sample of adolescents. Depress Anxiety 34:734–741. https://doi.org/10.1002/da.22650
Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, Andersen SL (2004) Childhood neglect is associated with reduced corpus callosum area. Biol Psychiatry 56:80–85. https://doi.org/10.1016/j.biopsych.2004.03.016
Acknowledgements
We thank all the staff at the Research Center for Child Mental Development, the Department of Child and Adolescent Psychological Medicine, University of Fukui Hospital, and Ms Satomi Omotani for their clerical support. We would also like to thank our collaborators who are listed as the co-authors of our papers in the reference list.
Funding
Open Access funding provided by University of Fukui. All phases of this study were supported by Japan Agency for Medical Research and Development (grant number JP20gk0110052 (AT and SN)); JSPS KAKENHI Scientific Research (A) (19H00617 to AT, ST, TXF), JSPS KAKENHI Scientific Research (C) (21K02352 to AT), Challenging Exploratory Research (Houga) (21K18499 to AT), and Scientific Research (C) (20K02700 to SN; 18K13109 to ST; 18K02480 to TXF); a Grant-in-Aid for “Creating a Safe and Secure Living Environment in the Changing Public and Private Spheres” from the Japan Science and Technology Corporation (JST)/Research Institute of Science and Technology for Society (RISTEX); a research grant from Japan–United States Brain Research Cooperative Program and the Strategic Budget to Realize University Missions (AT). The effects of childhood maltreatment on sensory systems and longitudinal studies of attachment were supported by the NIH (grants DA-016934, MH-66222, DA-017846, UL1 RR 025758, and HD-079484 to MHT), Japan-United States Friendship Commission (Grant no. Group 2017-19).
Author information
Authors and Affiliations
Contributions
AT and MHT performed the reference search and wrote the manuscript. SN and AT prepared Figs. 1 and 2, respectively. SN, ST, TS, and TXF conducted a critical review of this draft of the review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This is a review article. The manuscript does not contain clinical studies or patient data.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tomoda, A., Nishitani, S., Takiguchi, S. et al. The neurobiological effects of childhood maltreatment on brain structure, function, and attachment. Eur Arch Psychiatry Clin Neurosci (2024). https://doi.org/10.1007/s00406-024-01779-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00406-024-01779-y