Abstract
Background
Leucine-rich α-2 glycoprotein 1 (LRG-1) is a secreted glycoprotein that is mainly produced in the liver. Elevated levels of LRG-1 are found in a multitude of pathological conditions including eye diseases, diabetes, infections, autoimmune diseases, and cancer. In patients with early breast cancer (BC), high intratumoral LRG-1 protein expression levels are associated with reduced survival. In this study, we assessed serum levels of LRG-1 in patients with early BC and investigated its correlation with the presence of disseminated tumor cells (DTCs) in the bone marrow and survival outcomes.
Methods
Serum LRG-1 levels of 509 BC patients were determined using ELISA and DTCs were assessed by immunocytochemistry using the pan-cytokeratin antibody A45-B/B3. We stratified LRG-1 levels according to selected clinical parameters. Using the log-rank (Mantel–Cox) test and multivariate Cox regression analysis, Kaplan–Meier survival curves and prognostic relevance were assessed.
Results
Mean serum levels of LRG-1 were 29.70 ± 8.67 µg/ml. Age was positively correlated with LRG-1 expression (r = 0.19; p < 0.0001) and significantly higher LRG-1 levels were found in patients over 60 years compared to younger ones (30.49 ± 8.63 µg/ml vs. 28.85 ± 8.63 µg/ml; p = 0.011) and in postmenopausal patients compared to premenopausal patients (30.15 ± 8.34 µg/ml vs. 26.936.94 µg/ml; p = 0.002). Patients with no DTCs showed significantly elevated LRG-1 levels compared to the DTC-positive group (30.51 ± 8.69 µg/ml vs. 28.51 ± 8.54 µg/ml; p = 0.004). Overall and BC-specific survival was significantly lower in patients with high serum LRG-1 levels (above a cut-off of 33.63 µg/ml) compared to patients with lower LRG-1 levels during a mean follow-up of 8.5 years (24.8% vs. 11.1% BC-specific death; p = 0.0003; odds ratio 2.63, 95%CI: 1.56—4.36). Multivariate analyses revealed that LRG-1 is an independent prognostic marker for BC-specific survival (p = 0.001; hazard ratio 2.61).
Conclusions
This study highlights the potential of LRG-1 as an independent prognostic biomarker in patients with early BC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Our study reveals that serum leucine-rich α-2 glycoprotein 1 (LRG-1) is an independent prognostic marker for breast cancer-specific survival. This finding might translate into novel diagnostic approaches especially as the analysis of serum samples in patients with cancer are more readily available, cost-effective and can be easily implemented into routine clinical diagnostics. |
Background
Leucine-rich α-2 glycoprotein 1 (LRG-1) is a secreted glycoprotein that contains the evolutionary conserved leucine-rich repeat (LRR) motif [1]. Members of the LRR protein family exist in a broad range of organisms including plants, bacteria, animals, and fungi where they hold essential functions in innate immunity and protein–protein interactions, among others [2, 3]. LRG-1 is mainly expressed by hepatocytes and differentiating neutrophils and can be stimulated by a spectrum of inflammatory factors such as lipopolysaccharide, interleukin (IL)-1β, IL-6, and tumor necrosis factor alpha (TNF-α), initially pointing towards a potential role as an important mediator of acute phase reactions [4,5,6]. The physiological role of LRG-1 is not fully understood yet, especially as LRG-1 knockout mice are viable and present only with a mild phenotype [7]. LRG-1 is described to be locally and systemically upregulated in disease [2]. In murine models of retinal vascular pathology, LRG-1 is overexpressed in the vasculature of the retina and contributes to pathogenic angiogenesis by modulating transforming growth factor beta signaling [7]. In patients with age-related macular degeneration and diabetic retinopathy, LRG-1 levels are increased in the serum, the endothelia of abnormally formed blood vessels in the retina, and in the aqueous humor [8, 9]. By interfering with either the communication between endothelial cells and pericytes or by direct activation of endothelial cells, LRG-1 facilitates the formation of highly permeable, disorganized new blood vessels [10]. Similar mechanisms of pathological neovascularization by upregulated LRG-1 are seen in diabetic and chronic kidney disease [11, 12].
Studies from inflammatory diseases have elucidated that LRG-1 supports pro-inflammatory immune reactions as well as the differentiation and recruitment of neutrophils [2]. In rheumatoid arthritis, Crohn’s disease and ulcerative colitis, serum LRG-1 levels are increased and correlate with disease activity [13, 14]. Other pathologies in which LRG-1 is reported to be involved include infectious, metabolic, cardiovascular, nervous system-related diseases and chronic wound formation and fibrosis [2, 12, 15,16,17].
LRG-1 plays a role in inflammation, fibrosis, metabolism, and angiogenesis, all of which contribute to tumor initiation and progression. Consequently, an increasing number of studies have reported an aberrant activation and functional role of LRG-1 in cancer. Here, LRG-1 from different cellular sources promotes pathological vessel formation, immunosuppression, epithelial-to-mesenchymal transition, metastasis and tumor cell proliferation [18,19,20,21,22,23]. In addition, tumor tissue and serum levels of LRG-1 are increased and associated with worse prognosis in numerous malignancies such as melanoma, colorectal, pancreatic, prostate, lung, hepatocellular, and ovarian cancer [18, 20, 21, 24,25,26,27,28,29,30,31,32,33].
In breast cancer (BC), LRG-1 has anti-apoptotic potential and its increased tumor tissue expression is associated with worse prognosis and lymph node metastasis [34, 35]. However, the prognostic potential of serum LRG-1 in patients with early BC remains unknown. In this study, we aimed at addressing this question by using a well-defined patient cohort, comparing LRG-1 with clinicopathological markers as well as with the presence of disseminated tumor cells (DTCs). DTCs are a micrometastatic tumor spread to the bone marrow (BM), surviving in a state of dormancy [36], called minimal residual disease [37, 38]. DTCs are an independent prognostic marker for BC overall survival (OS), disease-free survival and distant disease-free survival [39]. We therefore also assessed potential correlations between serum LRG-1 levels and the presence of DTCs in patients with early BC.
Patients and methods
Patient population and study design
We used a cohort that has been previously described in published biomarker studies [40, 41] and report on this cohort again according to the REMARK guidelines [42]. The cohort consists of 509 patients with primary, early BC diagnosed between 2004 and 2009. Using protocols approved by the clinical ethics committee of the University Hospital Essen (05/2856), serum samples were collected before systemic therapy/at the beginning of surgery after written informed consent was obtained from all patients. The eligibility criteria for the inclusion were: histologically proven BC, no severe uncontrolled co-morbidities or medical conditions, BM aspiration at the time of primary diagnosis, no neoadjuvant chemotherapy and no present or previous other malignancies and metastasis. Therapeutic treatment followed current national guidelines [43] including adjuvant chemotherapy (anthracyclines, 5-fluorouracil, taxanes, and cyclophosphamide), anti-hormonal therapy in the case of hormone-responsive tumors (tamoxifen or an aromatase inhibitor), trastuzumab in the case of HER2-positivity (after FDA approval in November 2006) and radiotherapy, if indicated. Grading, TNM-staging and assessment of tumor type were performed at the Institute of Pathology of the University Hospital Essen as part of the West German Comprehensive Cancer Center.
Selection and detection of DTCs
DTC status was determined in all patients included in this study as previously described [41]. Briefly, BM aspirates (10–20 ml) were obtained from the anterior iliac crests of all patients at the beginning of surgery of the primary tumor and processed within 24 h. DTC isolation and detection was performed based on the recommendations for standardized tumor cell detection that have been published by the German Consensus group of Senology [44]. For the staining, cells were isolated from heparinized bone marrow (5000 U/ml BM) by Ficoll-Hypaque density gradient centrifugation (density 1.077 g/mol; Pharmacia, Freiburg, Germany) at 400 × g for 30 min. Using immunocytochemical staining with the pan-cytokeratin antibody A45-B/B3, slides were assessed for the presence of DTCs. Microscopic evaluation of the slides was performed according to the ISHAGE evaluation criteria using the ARIOL system (Applied Imaging).
Sampling of serum
Prior to surgery of each patient, nine milliliters of blood were collected using an S-Monovette (Sarstedt AG & Co). Samples were immediately stored at 4 °C. To avoid blood cell lysis, samples were processed within 4 h. The fractionation of the blood was performed by centrifugation for ten minutes at 2,500 × g. Afterwards, 3 to 4 ml of blood serum (upper phase of the fractions) were removed. Serum samples were frozen at −80 °C until performing the ELISAs.
Detection of LRG-1 by ELISA
LRG-1 serum levels were detected by ELISA (Biomedica, Vienna, Austria) according to the manufacturer’s instructions. Briefly, samples were diluted one to 4,000 with assay buffer and subsequently added to the wells. Following 2 h of incubation at room temperature, wells were washed five times using washing buffer. Now, 100 µl of antibody conjugate were added to each well and the preparation incubated for 1 h. Substrate was added after another washing step for five times and the plate incubated for 30 min at room temperature in the dark. Fifty µl of stop solution were added and the absorbance was measured immediately at 450 nm with reference at 630 nm using FLUOstar Omega (BMG Labtech, Ortenberg, Germany).
Statistical analysis
The statistical analysis was conducted with R, Version 4.0.2 and GraphPad Prism version 9.0.0 (GraphPad Software, La Jolla, CA, USA) as described previously [40, 41], and listed in each figure legend. Results are presented as the mean ± 95% confidence interval (CI), unless otherwise stated. Comparison of two groups was assessed using the non-parametric, two‐sided Mann–Whitney test. Groups of three were assessed by ANOVA. The correlation was assessed by non-parametric Spearman correlation. Cut-off analysis was performed using maximally selected rank statistics (maxstat package). Kaplan–Meier analyses were performed with significance levels indicated by log-rank (Mantel–Cox) analysis and hazard ratios (HRs; Mantel–Haenszel) are shown with 95%CI. BC-specific survival was defined as time between diagnosis of the primary tumor and death directly related to the disease. Uni‐ and multivariate Cox proportional hazards model regression analyses were performed and HRs are indicated with 95%CI. P values < 0.05 were considered statistically significant.
Results
Cohort
Table 1 lists the main clinical characteristics of the patients at the time of initial diagnosis. The median age of patients included was 60.8 years and ranged from 27 to 86 years. Seventy-two patients (14.1%) were premenopausal, 374 were postmenopausal (73.5%) and 63 were considered perimenopausal (12.4%). Ductal breast carcinomas were found to be the predominant histological subtype (385/509; 75.6%). Of the different tumor stages ranging from pT1 to pT4, most women presented with pT1 tumors (321/509; 63.1%). Half of the cohort had a moderately differentiated tumor (270/509, 53%) and the majority of the cohort (340/509, 66.8%) were lymph-node negative. Stratification according to immunohistochemical subtype revealed that the majority of the patients was ER- and/or PR-positive and HER2-negative patients (364/509; 71.5%), 11.2% were triple-positive (57/509), 65 patients were triple-negative (12.8%) and 4.5% only showed HER2 overexpression (23/509). Two hundred and seven patients presented with a positive DTC status (207/509; 40.7%). Survival data were available for 504/509 patients (5 patients lost to follow-up) and the median follow-up was 8.5 years (range 0.16–13.64). Of the 76 patients who died, 74 (97.4%) specifically died from breast cancer.
High LRG-1 correlated with clinicopathological parameters
We quantified serum levels of LRG-1 in all patients. Valid LRG-1 levels were recorded in 99.4% (506/509) of the samples. Mean serum levels of LRG-1 were 29.70 ± 8.67 µg/ml in this patient cohort. Table 2 shows LRG-1 levels at baseline when stratifying the cohort according to age, menopausal status, histology, tumor stage, nodal status, grading, hormone receptor status, and DTCs status. In patients older than 60 years, LRG-1 levels were significantly increased compared to younger patients (30.49 ± 8.63 µg/ml vs. 28.85 ± 8.63 µg/ml; p = 0.011; Fig. 1a). In addition, age was positively correlated with serum LRG-1 levels (r = 0.19; 95% CI: 0.10 – 0.28; p < 0.0001; Fig. 2b). Moreover, LRG-1 serum levels in postmenopausal patients were significantly increased compared to premenopausal patients (30.15 ± 8.34 µg/ml vs. 26.93 ± 6.94 µg/ml; p = 0.002; Fig. 1c). Of note, an increased LRG-1 concentration was found in patients with no detectable DTCs compared to those present with DTCs positivity (30.51 ± 8.69 µg/ml vs. 28.51 ± 8.54 µg/ml; p = 0.004; Fig. 1d). Neither tumor histology and stage, grading, nodal status, nor hormone receptor expression status did affect circulating LRG-1 levels.
High LRG-1 correlated with clinicopathological parameters. a Scatter plots comparing LRG-1 levels in breast cancer patients by age with 60 years as cut-off. b The correlation of LRG-1 and age, using non-parametric Spearman correlation (n = 506) with simple linear regression (red line). c Scatter plots comparing LRG-1 levels in pre-, post and perimenopausal breast cancer patients. d Scatter plots comparing LRG-1 levels in patients with DTC and without DTC (DTC positive and DTC negative). The black horizontal lines indicate median LRG-1 levels in each group, with error bars showing the 95%CI. P-value according to the non-parametric, two‐sided Mann–Whitney test
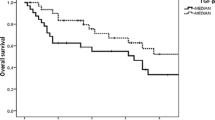
High LRG-1 levels are associated with a poor prognosis and is an independent prognostic marker. Kaplan–Meier analysis comparing a cumulative breast cancer-specific survival and b cumulative overall survival of LRG-1 high (n = 133) versus LRG-1 low (n = 368) breast cancer patients. HR and 95% CI according to Mantel Haenszel and p-value according to log-rank (Mantel–Cox) are indicated. c The univariate and multivariate analysis is shown for patients with high LRG-1 versus patients with low LRG-1 serum levels. HR, 95%CI and p-values are shown. The cut-off (33.64 µg/ml) is determined as described in the methods section
LRG-1 is an independent prognostic marker in patients with early breast cancer
Next, we assessed the potential of serum LRG-1 as a prognostic biomarker in patients with early BC. Using maximally rank statistics, the cohort was divided at the optimal cut-off into a LRG-1high and a LRG-1low group (cut-off of 33.64 µg/ml). The mean levels of LRG-1 was the following: LRG-1high group: 40.63 µg/ml (range 33.73–80.70 µg/ml) and LRG-1low group: 25.76 µg/ml (range 9.92–33.63 µg/ml). During the follow-up, 33/133 (24.8%) cases of breast cancer-specific deaths were reported within the LRG-1high group, whereas only 41/368 (11.1%) cases were documented in the LRG-1low group (p = 0.0003; odds ratio 2.63; 95%CI: 1.56–4.36). BC-specific survival was significantly reduced for patients within the LRG-1high group compared with the LRG-1low group (HR 3.25; 95%CI: 1.90–5.58; p < 0.0001; Fig. 2a). In addition, OS was significantly reduced for patients within the LRG-1high group compared with the LRG-1low group (HR 3.17; 95%CI: 1.86–5.23; p < 0.0001; Fig. 2b). Multivariate Cox analyses identified LRG-1 as an independent prognostic marker for breast cancer-specific survival (p = 0.001; hazard ratio 2.61; 95%CI: 1.63–4.18; Fig. 2c).
Discussion
Our investigation identified serum LRG-1 as an independent prognostic marker in patients with early BC at time of diagnosis. These findings are in line with a previous study that assessed tumor tissue expression levels of LRG-1 in patients with early BC. Here, high LRG-1 levels were associated with a decreased disease-free survival (HR 2.090, 95%CI: 1.205–3.625; p = 0.009) as well as lymph node metastasis and histological grade [34]. Although LRG-1 did not correlate with tumor grade or lymph node involvement, our findings on circulating levels of LRG-1 are underpinning its relevance as a marker of poor survival in patients with early BC. Similar correlations of high LRG-1 levels in tumor tissue with reduced patient survival were also shown for melanoma, pancreatic and colorectal cancer [20, 22, 24, 26]. The advantage of having identified serum LRG-1 as a prognostic marker is based on the comparatively easy sampling, its incorporation into routine clinical diagnostics and the reduced costs and time.
Our observations may suggest a tumor-promoting role of LRG-1 in early BC. Although the underlying mechanism is not fully understood, studies in leukemia, pancreatic and colorectal cancer cells describe an anti-apoptotic role of LRG-1 by modulating cell cycle factors and activating tumor-promoting signaling pathways [20, 30, 35, 45]. LRG-1 overexpression protects estrogen receptor-positive MCF-7 BC cells from apoptosis while its knockdown sensitizes them to pro-apoptotic stimuli [35]. Another tumor-promoting mechanism of LRG-1 is linked to tumor-related neoangiogenesis. Here, LRG-1 stimulates an aberrant vessel growth by modulating pericyte–endothelial cell interactions; a process that fuels the establishment of a tumor-supporting microenvironment [46]. In patients with gastric cancer, elevated LRG-1 levels positively correlate with local tumor tissue angiogenesis and tumor cell-conditioned medium promotes migration, as well as tube formation of endothelial cells in vitro [18]. LRG-1 was shown to stimulate vascular endothelial growth factor expression in colorectal cancer cells, as well as to activate migration and invasion, both of which are hallmarks of metastasizing cancer cells [22, 24]. In addition, the systemic increase of LRG-1 levels in endothelial cells adjacent to the primary tumor has been recently identified as a driver event for priming the premetastatic niche. This has been linked to the upregulation of prometastatic perivascular cells, which promote the local formation of highly permeable and disorganized capillaries [2, 21].
In summary, pleiotropic roles of LRG-1 in several steps of tumorigenesis have been described in numerous malignancies [2]. LRG-1 is likely to play a role in early BC biology, too, making it a potential novel therapeutic target. To the best of our knowledge, in vivo models of early or advanced BC have not yet been described in LRG-1 knockout mice. Similarly, studies are lacking using genetically manipulated host- or tumor-derived LRG-1 in BC-bearing animal models. One recent study was investigating the effect of a LRG-1-neutralizing antibody in the MMTV-PyMT BC metastasis model. Here, the postsurgical antibody therapy significantly enhanced the median survival of the mice after resection of the primary tumor [21]. Such investigations are highly warranted to understand the role of LRG-1 and its potential molecular and cellular interaction partners in different stages of tumorigenesis, i.e., ranging from precancerous lesions to overt BC metastases in clinically relevant organs, including bone, lungs, or the brain. In murine models of melanoma and lung cancer, genetic silencing of LRG-1 or pharmacological inhibition have shown to both improve tumor vessel function, to slowdown tumor growth and metastasis, and to improve the delivery and efficacy of cytotoxic drugs and immunotherapies including immune check point inhibitors [21, 26, 46, 47].
The cellular source of LRG-1 in our patients remains unclear but it might be possible that several tissue and cell types are able to secrete LRG-1, especially upon the presence of pathological stimuli [46]. Of note, tumor cells of varying malignancies are able to express and secrete LRG-1 [18, 23]. Although not significantly increased, patients who were diagnosed with pT3/pT4 tumors had elevated levels of LRG-1 compared to those with smaller tumors (pT1/pT2), which could be a result of LRG-1 secretion into systemic circulation by growing tumors. Here, tumor cells and the local tumor microenvironment, such as infiltrating immune cells and cancer-associated fibroblasts are potential sources of LRG-1 [2, 19]. Moreover, LRG-1 can be produced within bone tissue [48] and by endothelial cells [7]. However, it remains to be shown whether cellular LRG-1 expression changes at different stages of the primary tumor, by metastatic spread or by local changes of the immune signature in BC. The clinical or molecular significance of the reduced levels of LRG-1 in patients with micrometastasis evidenced by the presence of DTCs remains unclear. Nonetheless, local LRG-1 tissue levels within BC metastases do not necessarily reflect systemic levels. Combining serum analyses with local primary and secondary tumor tissue immunohistochemistry would help to shed more light on these crucial aspects. Likewise, post-translational modifications of LRG-1 might be produced by different cell and tissue types, potentially resulting in distinct prognostic relevance in BC [49]. In addition, in our cohort, few patients developed bone metastasis during the follow-up period. To further clarify the potential role of serum LRG-1 in predicting bone and/or other metastases, future investigations involving high risk populations with BC are needed.
Notably, we observed higher serum LRG-1 levels in older patients (≥ 60 years). This could be attributed to the increasing levels of inflammation in older adults, particularly considering that LRG-1 can be activated by inflammatory cytokines [2, 50]. Furthermore, findings from additional studies not only show a correlation between plasma LRG-1 levels and age, but also with body mass index, cholesterol levels, and endothelial function [51]. Therefore, it is likely that LRG-1 is modulated by metabolic alterations as well. Additionally, in our study, LRG-1 serum levels were significantly elevated in postmenopausal patients. Given that active hormone therapy involving estrogen and progestin in postmenopausal women decreased LRG-1 proteins, this potentially suggest a modulation of LRG-1 by sex hormones [52].
Our study has potential limitations. First, the study lacks an age-matched control group which would allow to compare LRG-1 levels between healthy patients and early BC patients. Likewise, sequential sampling of LRG-1 serum levels at defined time points would provide more insights into the temporal LRG-1 distribution and the impact of tumor resection. This would also strengthen the rationale for LRG-1-targeted therapies and accurate prognosis in individual patients with varying histological and molecular types of BC. For example, neoadjuvant treatment with letrozole reduces gene expression levels of LRG-1 in patients with estrogen receptor-positive BC [53]. However, the large size of the well-characterized cohort, together with the DTC assessment and the detailed and long follow-up are strengths of our study. Nonetheless, this is the first study that identifies serum LRG-1 as an independent prognostic marker in early BC which confirms findings from previous histological analyses of LRG-1 expression levels [34]. Our findings might translate into novel diagnostic approaches because the analysis of serum samples in patients with cancer are more readily available, cost-effective and easily implementable into routine clinical diagnostics.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Takahashi N, Takahashi Y, Putnam FW (1985) Periodicity of leucine and tandem repetition of a 24-amino acid segment in the primary structure of leucine-rich alpha 2-glycoprotein of human serum. Proc Natl Acad Sci 82:1906–1910
Camilli C, Hoeh AE, De Rossi G, Moss SE, Greenwood J (2022) LRG1: an emerging player in disease pathogenesis. J Biomed Sci 29:1–29
Ng A, Xavier RJ (2011) Leucine-rich repeat (LRR) proteins: integrators of pattern recognition and signaling in immunity. Autophagy 7:1082–1084
Shirai R, Hirano F, Ohkura N, Ikeda K, Inoue S (2009) Up-regulation of the expression of leucine-rich α2-glycoprotein in hepatocytes by the mediators of acute-phase response. Biochem Biophys Res Commun 382:776–779
O’Donnell LC, Druhan LJ, Avalos BR (2002) Molecular characterization and expression analysis of leucine-rich alpha2-glycoprotein, a novel marker of granulocytic differentiation. J Leukoc Biol 72:478–485
Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA-K, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist P-H, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F (2015) Tissue-based map of the human proteome. Science (80- ) 347(6220):1260419
Wang X, Abraham S, McKenzie JAG, Jeffs N, Swire M, Tripathi VB, Luhmann UFO, Lange CAK, Zhai Z, Arthur HM, Bainbridge JWB, Moss SE, Greenwood J (2013) LRG1 promotes angiogenesis by modulating endothelial TGF-β signalling. Nature 499:306–311
Mundo L, Tosi GM, Lazzi S, Pertile G, Parolini B, Neri G, Posarelli M, De Benedetto E, Bacci T, Silvestri E, Siciliano MC, Barbera S, Orlandini M, Greenwood J, Moss SE, Galvagni F (2021) LRG1 expression is elevated in the eyes of patients with neovascular age-related macular degeneration. Int J Mol Sci 22:1–12
Chen C, Chen X, Huang H, Han C, Qu Y, Jin H, Niu T, Zhang Y, Liu K, Xu X (2019) Elevated plasma and vitreous levels of leucine-rich-α2-glycoprotein are associated with diabetic retinopathy progression. Acta Ophthalmol 97:260–264
De Rossi G, Da Vitoria Lobo ME, Greenwood J, Moss SE (2022) LRG1 as a novel therapeutic target in eye disease. Eye 36:328–340
Hong Q, Zhang L, Fu J, Verghese DA, Chauhan K, Nadkarni GN, Li Z, Ju W, Kretzler M, Cai G-Y, Chen X-M, D’Agati VD, Coca SG, Schlondorff D, He JC, Lee K (2019) LRG1 promotes diabetic kidney disease progression by enhancing TGF-β-induced angiogenesis. J Am Soc Nephrol 30:546–562
Liu TT, Luo R, Yang Y, Cheng YC, Chang D, Dai W, Li YQ, Ge SW, Xu G (2021) LRG1 mitigates renal interstitial fibrosis through alleviating capillary rarefaction and inhibiting inflammatory and pro-fibrotic cytokines. Am J Nephrol 52:228–238
Serada S, Fujimoto M, Ogata A, Terabe F, Hirano T, Iijima H, Shinzaki S, Nishikawa T, Ohkawara T, Iwahori K, Ohguro N, Kishimoto T, Naka T (2010) iTRAQ-based proteomic identification of leucine-rich α-2 glycoprotein as a novel inflammatory biomarker in autoimmune diseases. Ann Rheum Dis 69:770–774
Serada S, Fujimoto M, Terabe F, Iijima H, Shinzaki S, Matsuzaki S, Ohkawara T, Nezu R, Nakajima S, Kobayashi T, Plevy SE, Takehara T, Naka T (2012) Serum leucine-rich alpha-2 glycoprotein is a disease activity biomarker in ulcerative colitis. Inflamm Bowel Dis 18:2169–2179
Zou Y, Xu Y, Chen X, Wu Y, Fu L, Lv Y (2022) Research progress on leucine-rich alpha-2 glycoprotein 1: a review. Front Pharmacol 12:1–11
Liu C, Teo MHY, Pek SLT, Wu X, Leong ML, Tay HM, Hou HW, Ruedl C, Moss SE, Greenwood J, Tavintharan S, Hong W, Wang X (2020) A multifunctional role of leucine-rich α-2-glycoprotein 1 in cutaneous wound healing under normal and diabetic conditions. Diabetes 69:2467–2480
Nakajima H, Nakajima K, Serada S, Fujimoto M, Naka T, Sano S (2021) The involvement of leucine-rich α-2 glycoprotein in the progression of skin and lung fibrosis in bleomycin-induced systemic sclerosis model. Mod Rheumatol 31:1120–1128
He L, Feng A, Guo H, Huang H, Deng Q, Zhao E, Yang M (2022) LRG1 mediated by ATF3 promotes growth and angiogenesis of gastric cancer by regulating the SRC/STAT3/VEGFA pathway. Gastric Cancer 25:527–541
Zhong B, Cheng B, Huang X, Xiao Q, Niu Z, Feng CY, Yu Q, Wang W, Wu XJ (2022) Colorectal cancer-associated fibroblasts promote metastasis by up-regulating LRG1 through stromal IL-6/STAT3 signaling. Cell Death Dis 13:1–15
Xie ZB, Zhang YF, Jin C, Mao YS, Fu DL (2019) LRG-1 promotes pancreatic cancer growth and metastasis via modulation of the EGFR/p38 signaling. J Exp Clin Cancer Res 38:1–12
Singhal M, Gengenbacher N, Pari AAA, Kamiyama M, Hai L, Kuhn BJ, Kallenberg DM, Kulkarni SR, Camilli C, Preuß SF, Leuchs B, Mogler C, Espinet E, Besemfelder E, Heide D, Heikenwalder M, Sprick MR, Trumpp A, Krijgsveld J, Schlesner M, Hu J, Moss SE, Greenwood J, Augustin HG (2021) Temporal multi-omics identifies LRG1 as a vascular niche instructor of metastasis. Sci Transl Med 13:1–14
Zhang J, Zhu L, Fang J, Ge Z, Li X (2016) LRG1 modulates epithelial–mesenchymal transition and angiogenesis in colorectal cancer via HIF-1α activation. J Exp Clin Cancer Res 35:1–11
Fan M, Li C, He P, Fu Y, Li M, Zhao X (2019) Knockdown of long noncoding RNA-taurine-upregulated gene 1 inhibits tumor angiogenesis in ovarian cancer by regulating leucine-rich α-2-glycoprotein-1. Anticancer Drugs 30:562–570
Zhang Q, Huang R, Tang Q, Yu Y, Huang Q, Chen Y, Wang G, Wang X (2018) Leucine-rich alpha-2-glycoprotein-1 is up-regulated in colorectal cancer and is a tumor promoter. Onco Targets Ther 11:2745–2752
Guldvik IJ, Zuber V, Braadland PR, Grytli HH, Ramberg H, Lilleby W, Thiede B, Zucknick M, Saatcioglu F, Gislefoss R, Kvåle R, George A, Grönberg H, Wiklund F, Neal DE, Gnanapragasam VJ, Taskén KA, Mills IG (2020) Identification and validation of leucine-rich α-2-glycoprotein 1 as a noninvasive biomarker for improved precision in prostate cancer risk stratification. Eur Urol Open Sci 21:51–60
Kwan YP, Teo MHY, Lim JCW, Tan MS, Rosellinny G, Wahli W, Wang X (2021) LRG1 promotes metastatic dissemination of melanoma through regulating EGFR/STAT3 signalling. Cancers (Basel) 13:1–16
Shinozaki E, Tanabe K, Akiyoshi T, Tsuchida T, Miyazaki Y, Kojima N, Igarashi M, Ueno M, Suenaga M, Mizunuma N, Yamaguchi K, Nakayama K, Iijima S, Yamaguchi T (2018) Serum leucine-rich alpha-2-glycoprotein-1 with fucosylated triantennary N-glycan: a novel colorectal cancer marker. BMC Cancer 18:1–9
Andersen JD, Boylan KLM, Jemmerson R, Geller MA, Misemer B, Harrington KM, Weivoda S, Witthuhn BA, Argenta P, Vogel RI, Skubitz APN (2010) Leucine-rich alpha-2-glycoprotein-1 is upregulated in sera and tumors of ovarian cancer patients. J Ovarian Res 3(1):1–14
Ladd JJ, Busald T, Johnson MM, Zhang Q, Pitteri SJ, Wang H, Brenner DE, Lampe PD, Kucherlapati R, Feng Z, Prentice RL, Hanash SM (2012) Increased plasma levels of the APC-interacting protein MAPRE1, LRG1, and IGFBP2 preceding a diagnosis of colorectal cancer in women. Cancer Prev Res 5:655–664
Zhou Y, Zhang X, Zhang J, Fang J, Ge Z, Li X (2017) LRG1 promotes proliferation and inhibits apoptosis in colorectal cancer cells via RUNX1 activation. PLoS ONE 12:1–14
Okano T, Kondo T, Kakisaka T, Fujii K, Yamada M, Kato H, Nishimura T, Gemma A, Kudoh S, Hirohashi S (2006) Plasma proteomics of lung cancer by a linkage of multi-dimensional liquid chromatography and two-dimensional difference gel electrophoresis. Proteomics 6:3938–3948
Kawakami T, Hoshida Y, Kanai F, Tanaka Y, Tateishi K, Ikenoue T, Obi S, Sato S, Teratani T, Shiina S, Kawabe T, Suzuki T, Hatano N, Taniguchi H, Omata M (2005) Proteomic analysis of sera from hepatocellular carcinoma patients after radiofrequency ablation treatment. Proteomics 5:4287–4295
Furukawa K, Kawamoto K, Eguchi H, Tanemura M, Tanida T, Tomimaru Y, Akita H, Hama N, Wada H, Kobayashi S, Nonaka Y, Takamatsu S, Shinzaki S, Kumada T, Satomura S, Ito T, Serada S, Naka T, Mori M, Doki Y, Miyoshi E, Nagano H (2015) Clinicopathological significance of leucine-rich α2-glycoprotein-1 in sera of patients with pancreatic cancer. Pancreas 44:93–98
Zhang YS, Han L, Yang C, Liu YJ, Zhang XM (2021) Prognostic value of LRG1 in breast cancer: a retrospective study. Oncol Res Treat 44(1):36–41
Jemmerson R, Staskus K, Higgins LA, Conklin K, Kelekar A (2021) Intracellular leucine-rich alpha-2-glycoprotein-1 competes with Apaf-1 for binding cytochrome c in protecting MCF-7 breast cancer cells from apoptosis. Apoptosis 26:71–82
Pantel K, Brakenhoff RH, Brandt B (2008) Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer 8:329–340
Hartkopf AD, Wallwiener M, Hahn M, Fehm TN, Brucker SY, Taran F-A (2016) Simultaneous detection of disseminated and circulating tumor cells in primary breast cancer patients. Cancer Res Treat 48:115–124
Friberg S, Nyström A (2015) Cancer metastases: early dissemination and late recurrences. Cancer Growth Metastasis 8:43–49
Hartkopf AD, Brucker SY, Taran F-A, Harbeck N, von Au A, Naume B, Pierga J-Y, Hoffmann O, Beckmann MW, Rydén L, Fehm T, Aft R, Solà M, Walter V, Rack B, Schuetz F, Borgen E, Ta M-H, Bittner A-K, Fasching PA, Fernö M, Krawczyk N, Weilbaecher K, Margelí M, Hahn M, Jueckstock J, Domschke C, Bidard F-C, Kasimir-Bauer S, Schoenfisch B, Kurt AG, Wallwiener M, Gebauer G, Klein CA, Wallwiener D, Janni W, Pantel K (2021) Disseminated tumour cells from the bone marrow of early breast cancer patients: results from an international pooled analysis. Eur J Cancer 154:128–137
Rachner TD, Göbel A, Hoffmann O, Erdmann K, Kasimir-Bauer S, Breining D, Kimmig R, Hofbauer LC, Bittner A-K (2020) High serum levels of periostin are associated with a poor survival in breast cancer. Breast Cancer Res Treat 180:515–524
Rachner TD, Kasimir-Bauer S, Gobel A, Erdmann K, Hoffmann O, Browne A, Wimberger P, Rauner M, Hofbauer LC, Kimmig R, Bittner A-K (2019) Prognostic value of RANKL/OPG serum levels and disseminated tumor cells in nonmetastatic breast cancer. Clin Cancer Res 25(4):1369–1378
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2006) REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 100:229–235
Kommission Mamma | Leitlinien & Empfehlungen | Leitlinien & Stellungnahmen | AGO – Die Arbeitsgemeinschaft Gynäkologische Onkologie [Internet]. [cited 2023 May 4]. Available from: https://www.ago-online.de/leitlinien-empfehlungen/leitlinien-empfehlungen/kommission-mamma
Kasimir-Bauer S, Reiter K, Aktas B, Bittner AK, Weber S, Keller T, Kimmig R, Hoffmann O (2016) Different prognostic value of circulating and disseminated tumor cells in primary breast cancer: influence of bisphosphonate intake? Sci Rep 6:1–10
Xiao S, Zhu H (2018) Leucine-rich alpha-2-glycoprotein1 gene interferes with regulation of apoptosis in leukemia KASUMI-1 cells. Med Sci Monit 24:8348–8356
O’Connor MN, Kallenberg DM, Camilli C, Pilotti C, Dritsoula A, Jackstadt R, Bowers CE, Watson HA, Alatsatianos M, Ohme J, Dowsett L, George J, Blackburn JWD, Wang X, Singhal M, Augustin HG, Ager A, Sansom OJ, Moss SE, Greenwood J (2021) LRG1 destabilizes tumor vessels and restricts immunotherapeutic potency. Med 2:1231-1252.e10
Javaid F, Pilotti C, Camilli C, Kallenberg D, Bahou C, Blackburn J, Baker R, Greenwood J, Moss SE, Chudasama V (2021) Leucine-rich alpha-2-glycoprotein 1 (LRG1) as a novel ADC target. RSC Chem Biol 2:1206–1220
Wang Y, Xu J, Zhang X, Wang C, Huang Y, Dai K, Zhang X (2017) TNF-α-induced LRG1 promotes angiogenesis and mesenchymal stem cell migration in the subchondral bone during osteoarthritis. Cell Death Dis 8:e2715
Druhan LJ, Lance A, Li S, Price AE, Emerson JT, Baxter SA, Gerber JM, Avalos BR (2017) Leucine rich α-2 glycoprotein: a novel neutrophil granule protein and modulator of myelopoiesis. Rishi A, editor. PLoS One 12:e0170261
Alberro A, Iribarren-Lopez A, Sáenz-Cuesta M, Matheu A, Vergara I, Otaegui D (2021) Inflammaging markers characteristic of advanced age show similar levels with frailty and dependency. Sci Rep 11:1–10
Pek SLT, Tavintharan S, Wang X, Lim SC, Woon K, Yeoh LY, Ng X, Liu J, Sum CF (2015) Elevation of a novel angiogenic factor, leucine-rich-α2-glycoprotein (LRG1), is associated with arterial stiffness, endothelial dysfunction, and peripheral arterial disease in patients with type 2 diabetes. J Clin Endocrinol Metab 100:1586–1593
Pitteri SJ, Hanash SM, Aragaki A, Amon LM, Chen L, Busald Buson T, Paczesny S, Katayama H, Wang H, Johnson MM, Zhang Q, McIntosh M, Wang P, Kooperberg C, Rossouw JE, Jackson RD, Manson JE, Hsia J, Liu S, Martin L, Prentice RL (2009) Postmenopausal estrogen and progestin effects on the serum proteome. Genome Med 1:121
Ramirez-Ardila DE, Ruigrok-Ritstier K, Helmijr JC, Look MP, van Laere S, Dirix L, Berns EMJJ, Jansen MPHM (2016) LRG1 mRNA expression in breast cancer associates with PIK3CA genotype and with aromatase inhibitor therapy outcome. Mol Oncol 10:1363–1373
Funding
Open Access funding enabled and organized by Projekt DEAL. The work was funded by individual grants and as part of the priority program (SPP-2084) μBONE by the Deutsche Forschungsgemeinschaft to AG (GO 3055/4-1), LCH (HO 1875/27-1), and to TDR (RA 2151/5-1, RA 2151/7-1) and by the Deutsche Krebshilfe to AG and TDR as part of the Mildred Scheel Early Career Center and individual funding (#70113573). The Article Processing Charges (APC) were funded by the joint publication funds of the TU Dresden, including Carl Gustav Carus Faculty of Medicine, and the SLUB Dresden as well as the Open Access Publication Funding of the DFG.
Author information
Authors and Affiliations
Contributions
Study design: AKB, OH and TDR. Study conduct: AG, AKB, OH and TDR. Data collection: AKB, OH, RK, SKB. Data analysis: AG, DK, AKB, OH, and TDR. Data interpretation: AG, DK, AKB, LCH, OH, SKB, and TDR. Drafting manuscript: AG, DK, AKB, OH, SKB, and TDR. Revising manuscript content: AG, DK, AKB, LCH, OH, RK, SKB, and TDR. Approving final version of manuscript: AG, DK, AKB, LCH, OH, RK, SKB, and TDR. TDR takes responsibility of the integrity of the data analysis.
Corresponding author
Ethics declarations
Conflicts of interest
SKB is a consultant for QIAGEN, Hilden, Germany. The authors have received honoraria, unrestricted educational grants and research funding from to the individual or the institution by Alexion (LCH), Amgen (AKB, LCH, OH), Ascendis (LCH), Astra Zeneca (AKB, OH), Daiichi Sankyo (AKB, OH), Eisai (OH), Gilead (AKB, OH), Hexal (AKB, OH), MSD (OH), Novartis (AKB, OH), Pharmacosmos (LCH), Pfizer (AKB, OH), Riemser (OH), Roche (OH), Seagen (OH), and UCB (LCH). All other authors declare no potential conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Samples of patients were obtained before therapy after written informed consent from all subjects using protocols approved by the clinical ethic committee of the University Hospital.
Essen (05/2856).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Göbel, A., Rachner, T.D., Hoffmann, O. et al. High serum levels of leucine-rich α-2 glycoprotein 1 (LRG-1) are associated with poor survival in patients with early breast cancer. Arch Gynecol Obstet (2024). https://doi.org/10.1007/s00404-024-07434-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00404-024-07434-0